Abstract
Sand nourishments and groynes as coastal protection measures (CPM) address similar challenges on sandy coasts but take different approaches: while groynes are intended to reduce alongshore sediment transport and erosion, nourishments add new sediment to the system to compensate for erosion. The aim of this study is to compare the ecological effects of such measures on the vegetation. To this end, nutrient analysis and botanical mappings were carried out on a site with installed groynes, a site where sand nourishments are regularly carried out, and a control site without any CPM. In addition to an increase in nutrient availability after the sand nourishment, significant changes in plant species diversity and composition were also measured. The number of higher plants, mosses, and lichen species was lower at the nourishment site. The opposite impacts were observed at the groyne site: an increase in sediment cover by higher plants and mosses and a distinct increase in lichen species. The results suggest that groynes lead to a stabilization of the coastal system and enable dense vegetation growth. In contrast, sand nourishments lead to nutrient input and unstable habitat conditions, attracting certain plant communities but preventing the establishment of ground-covering vegetation.
1. Introduction
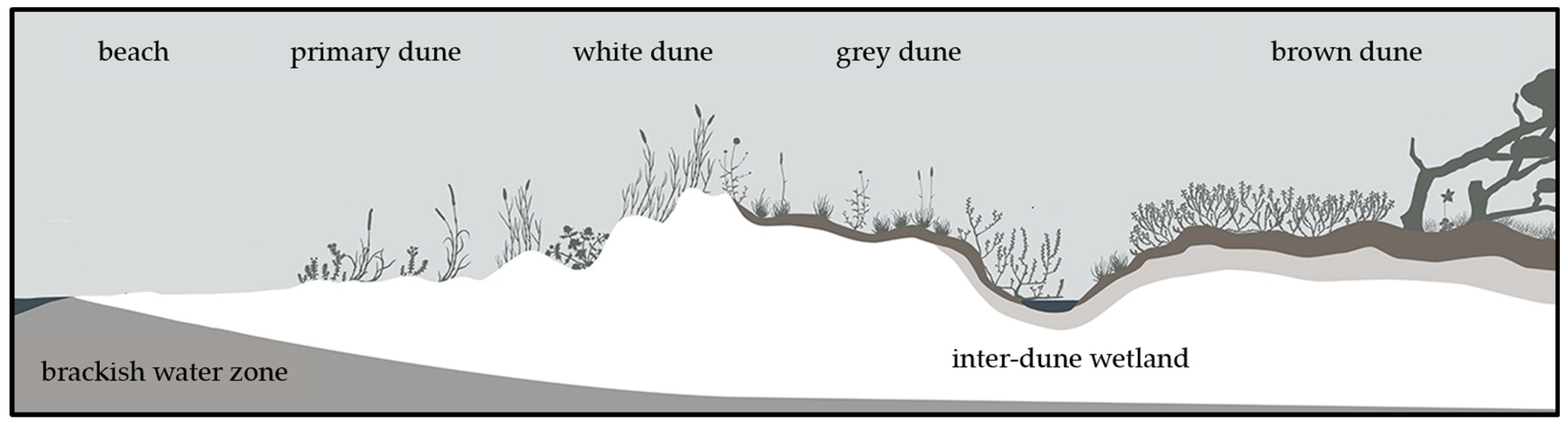
Coastal dunes are exposed to numerous ecological challenges, including nutrient and moisture deficiency, sediment transport, and salt spray [1]. These challenges create spatial dynamics within the dune: the influences of wind, sand movement, and salt stress tend to decrease with increasing distance from the sea [2]. This gradient leads to an ecological differentiation and manifests itself in a zonation pattern characterized by different vegetation types (Figure 1). Nutrient-rich, moist beachwrack piles near the waterline provide a specialized habitat for nitrophytes and create wind shelter for sand accretion. This process promotes the establishment of pioneer vegetation that marks the formation of primary dunes. Within these primary dunes, species such as Leymus arenarius (L.) HOCHST. and Elytrigia junceiformis (Á. Löve & D. Löve) HAND & BUTTLER, as well as summer annuals including Atriplex littoralis (L.), Cakile maritima SCOP., and Salsola kali (L.), find a niche. The stabilization of the sediment and the further accumulation of sand are facilitated by the rhizomes of these plants [3,4,5]. Wind drift transports sand towards the land and leads to the formation of secondary dunes, the foredunes. The sand is stabilized by the large rhizomes of Ammophila arenaria (L.) LINK and Elytrigia junceiformis. These plants can catch sand and even grow through up to 40 cm of sand if they are buried underneath [3,5]. The wind-exposed, secondary white dune is still influenced by the interplay of wind-driven sedimentation and erosion [5]. The sand is lime-rich and maintains a high pH, yet it lacks humus [2]. The wind-sheltered side of the dunes transitions into the grey dune, which is characterized by changing microclimates and soil conditions [5]. In this area, although still relatively dry and exposed to the wind, the soil is leached and humus accumulates, leading to a pH shift towards acidic conditions and an increase in soil fertility [2]. Sandy grasslands predominate here with species such as Corynephorus canescens (L.) P. BEAUV., Carex arenaria (L.), Jasione montana (L.), pioneer mosses, and lichens [3,5]. This zonation of coastal dunes is usually stable but dynamic changes and developments are possible over varying timescales. The transition from a primary dune to a white dune can occur within a few years, the shift to a grey dune can take up to 10–20 years, and further progression to a brown dune can span over 60–70 years [5]. While these dune ecosystems demonstrate resilience, interventions such as storm floods, land subsidence, and tread damage can trigger regression and alter the established zonation [5]. To a certain extent, these disturbances can maintain dune mobility and offer an ecological niche for rare pioneer species [6] but anthropogenic disturbances in particular often lead to a loss of biodiversity [7].
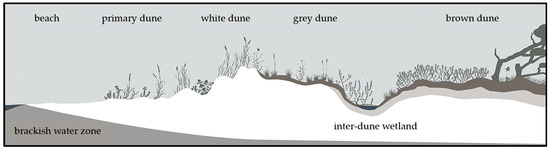
Figure 1.
Cross-sectional overview of the stages of dune development and the most common terminology at the German Baltic Sea Coast. Adapted with permission from [8]. 2024, Sarah Mamerow.
Coastal protection measures (CPM), including sand nourishments and groynes, are essential strategies employed to safeguard vulnerable coastal regions. However, the coastal environments, including sandy beaches and dunes, already face significant threats such as coastal squeeze [9]. CPM can add more challenges to these coastal systems, e.g., by the introduction of foreshore structures, particularly hard structures to stabilize the coastline. They can affect sediment dynamics and hydrodynamic as well as depositional processes [10,11,12]. The consequences of the stabilization of formerly dynamic ecosystems are shrub invasion, increasing nutrient concentration, and a decrease in species variety [5]. Furthermore, ecosystem engineering processes by dune grasses are disturbed [13]. In contrast to those hard measures, soft measures were developed as more environmentally friendly techniques. Dunes, when appropriately managed, serve as a critical component of soft CPM too. However, it is important to recognize that the wear and erosion of these dunes are expected. Therefore, repeated nourishment becomes a necessary part of their maintenance. To extend the duration of sand nourishment projects, groynes or breakwaters are installed at the beach [14]. Although sand nourishments are considered as soft CPM, these actions can still affect the ecology of dunes starting with biota burial and habitat loss [10,12] leading to a reduction in the essential ecosystem functions [15]. Changes in plant diversity and coverage are believed to be the result of substantial anthropogenic action too [16]. Disturbances may lead to decreased plant coverage, but the implementation of CPM can also introduce nutrient inputs, fostering increased plant coverage by species adapted to nutrient-rich sediments [16], underlining the intricate ecological consequences of CPM in sandy beach and dune ecosystems.
The present study contains botanical mappings to study the following questions: (i) How is the vegetation composition changed due to the sand nourishment? (ii) Are there differences in the vegetation composition between nourished, groyne-protected, and unprotected coastal zones? (iii) How do species richness and biodiversity react to sand nourishments?
2. Materials and Methods
2.1. Study Site
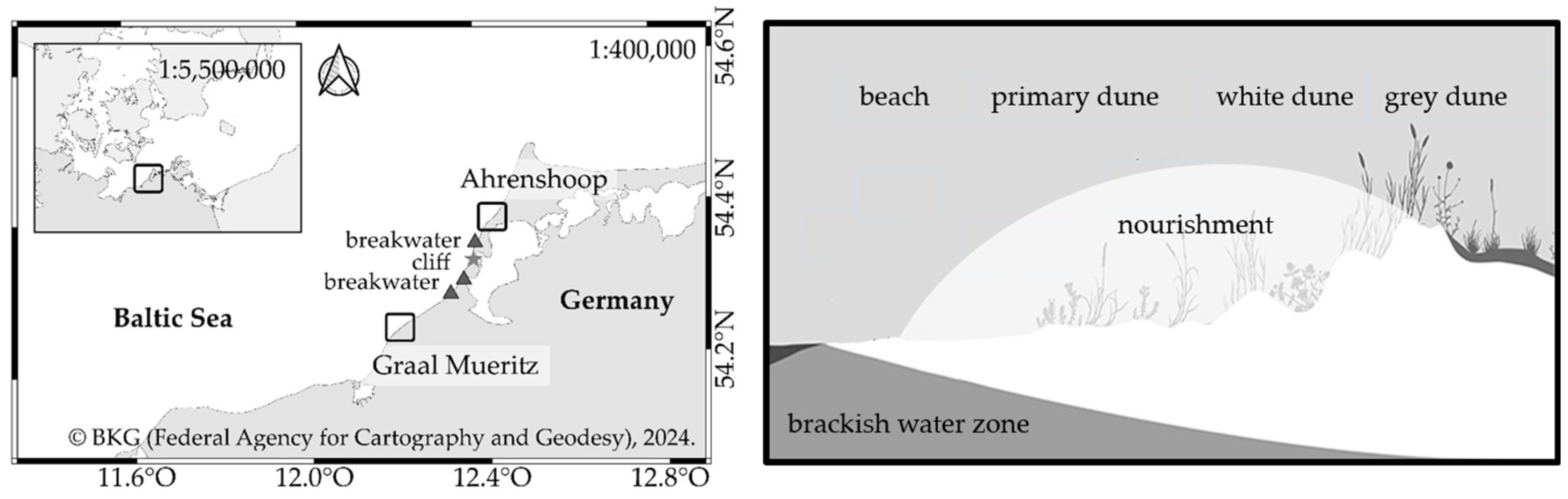
The data were collected in Ahrenshoop and Graal Mueritz, two municipalities at the German Baltic Sea Coast (Figure 2, Ahrenshoop: 54°22′50.573″ N 12°25′16.506″ E and Graal-Mueritz: 54°15′34.412″ N 12°14′30.692″ E). Both municipalities are located on the Bodden compensation coast, which consists of different islands and spits. Hilltops of moraines are connected by narrow land bridges. These land bridges were formed by currents that eroded material from the island cores. The most important feature of this geographical region is the highly structured shape of the coast [17,18]. While the sediment supply for Graal Mueritz is ensured by sand transport from more western sections of the coast, the sediment supply for Ahrenshoop was originally ensured by cliff retreat. These cliffs are located in the 15 km coast section between the two municipalities and are actually protected by three breakwaters to prevent a retreat of the coast [18]. They are used as sediment traps to stabilize the shore but prevent the alongshore sediment transport from feeding the adjacent beaches. Through the missing sediment supply by cliff retreat, these high alongshore sand transport rates weaken the protective function of the dunes in Ahrenshoop and storm surges threaten the municipality. Therefore, sand nourishments are carried out every five to ten years [19]. The last nourishment in winter 2021/22 consisted of 600,000 cubic meters nourishing almost 4.5 km of coastline. The sand for the nourishment was extracted approximately 10 km from the coast from a sand deposit that had previously been inspected for suitability by the construction supervisors. The nourished zone in Ahrenshoop included parts of the beach, as well as the primary dune and the white dune (Figure 2). The coastal dynamic location in Ahrenshoop is representative of many places on the German Baltic Sea coast and was therefore chosen for the analyses and comparison. Furthermore, the timing of the coastal protection measure (CPM) was another reason why this site was chosen for the analysis: sand nourishments only happen at selected sites in Germany, and for the year 2021, only the coast of Ahrenshoop was nourished. Graal Mueritz has an almost identical geographic orientation compared to Ahrenshoop and is therefore suitable for comparison with the coast in Ahrenshoop. As CPM, wooden groynes are normally installed on the beach in some parts of the coasts. There is also a protected nature reserve near Graal Mueritz, in which coastal protection is not carried out. This offers the opportunity to sample an unprotected section of coastline.
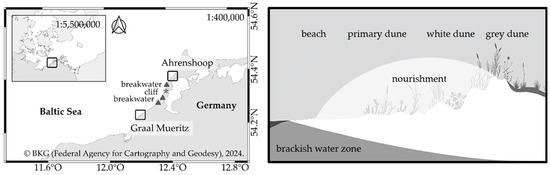
Figure 2.
On the left side, sampling sites Ahrenshoop and Graal-Mueritz at the Baltic Sea, Germany. In Ahrenshoop, a nourishment took place in winter 2021/2022. In Graal Mueritz, wooden groynes are installed in some parts of the beach. Another part of the beach is without any coastal protection (=control site). On the right side is a cross-sectional overview of the placement of the nourished material at the coast in Ahrenshoop. Adapted with permission from [8]. 2024, Sarah Mamerow.
2.2. Performed Samplings
The sampling plan consisted of taking sediment samples in Ahrenshoop before and after the sand nourishment to determine nutrient concentrations in the sediment and botanical mappings to determine the biodiversity on the beach and dune (GPS coordinates of all sampling sites are shown in Table A1). The sediment cores were driven 20 cm deep into the sediment to determine sediment conditions within the germination and root growth depth of most seeds. For the results of the sediment characteristics (mean grain size, sorting, water, and organic and carbonate content), see [20]. For botanical mappings, all higher plants and mosses were determined. Lichen sampling was only performed once at the site. The sediment samples and botanical mappings were performed on the dates according to the following table (Table 1):

Table 1.
Sampling plan for the performed sediment samplings and botanical mappings at the sites (Graal Mueritz and Ahrenshoop).
The concentrations of selected nutrients (nitrate, nitrite, phosphate, and ammonia) in each sediment sample were analyzed using the spectrophotometric method by Lambda 2, UV/VIS Spectrometer, and Perkin Elmer [21]. For this, 10 g of sediment was suspended in 50 mL of distilled water for one hour and then filtered through a glass fiber filter (pore size 0.45 µm, number of replicates n = 5).
Each botanical mapping consisted of 2 m wide belt transects (n = 5) from the beach to the end of the grey dune to determine species composition and soil coverage by higher plants. For this, long measuring tapes were placed vertically to the water line crossing all habitats from the beach to the coastal forest. On both sides of the measuring line, another line was placed in parallel at a distance of one meter. Within this 2 m wide area spanning from the waterline to the coastal forest, the plants were determined following Jäger et al. [22]. The percentage cover of each species was estimated using a modified and combined Braun–Blanquet abundance dominance scale (Table 2, [23]). Changes in species number and abundance were calculated with the Shannon Index H [24]. All lichens detected in the sampling area were collected by hand and stored in paper bags. The lichens were air-dried after collection and determined using a microscope with a maximum magnification of 400. The morphological identification of lichens followed Wirth et al. [25] including the nomenclature concept provided by Printzen et al. [26]. Some species of the genus Cladonia were analyzed using thin-layer chromatography according to Culberson and Amman [27] in solvent system A.

Table 2.
Braun–Blanquet table [28] with determination of the degrees of vegetation coverage in percent and transformation in accordance with Ellenberg [23] coverage. With ind. = individual.
For the statistical analysis, we used the R statistical software Version 4.0.3. The sediment samples were analyzed after checking for normality and variance homogeneity using an ANOVA or the Kruskal–Wallis test with the sampling site as the factor. The additional test was necessary since the requirements for the ANOVA were not met by all parameters. Post-hoc pairwise multiple comparisons were carried out using Dunn’s Test against an alpha level of 0.05 to identify differences between the sampling times. For multidimensional scaling, the Bray–Curtis distance was used.
3. Results
3.1. Nutrient Concentration in the Sediment
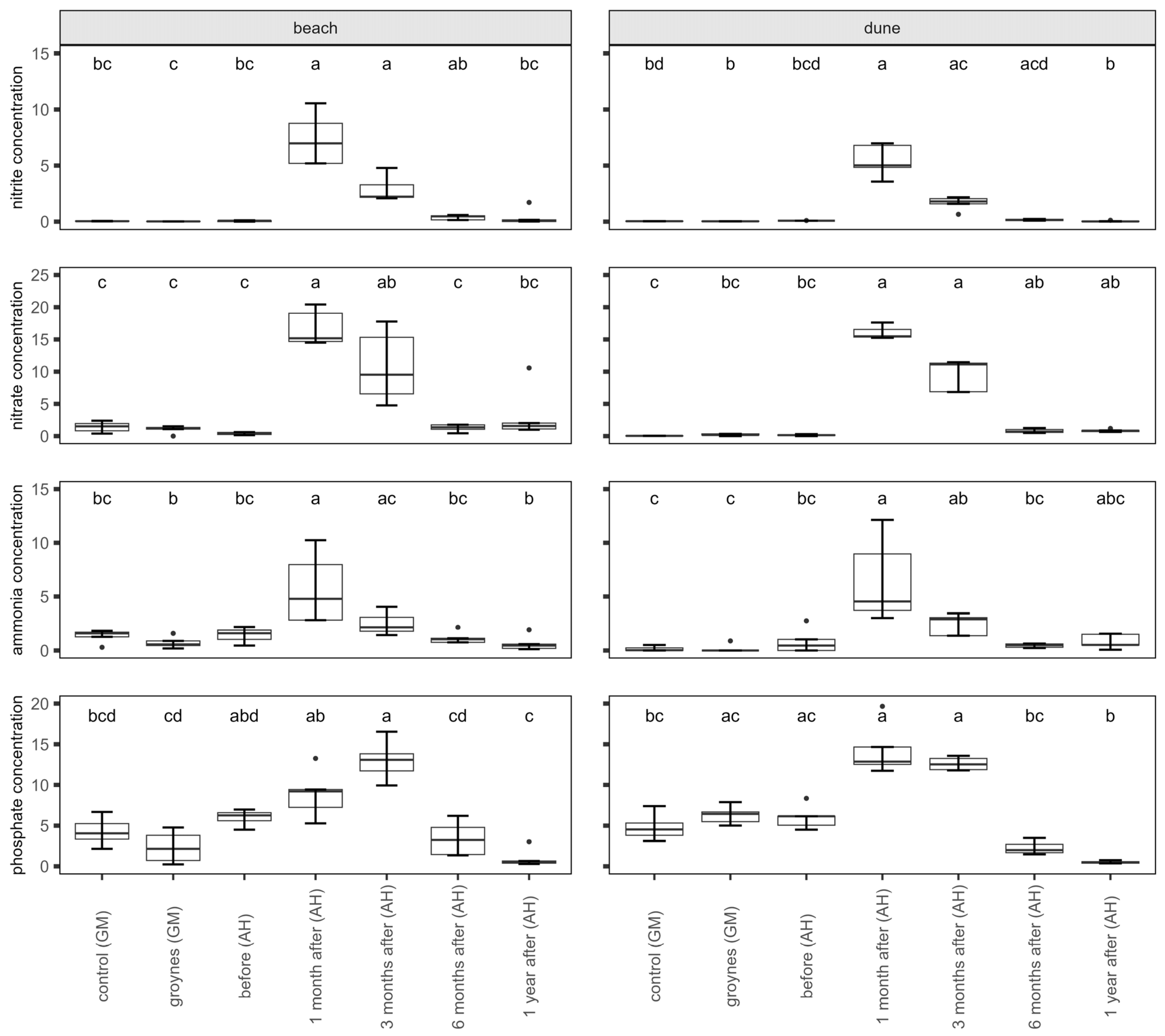
There were significant changes in nutrient concentrations within the samples (Figure 3). The nitrate, nitrite, and ammonia concentrations increased after the nourishment at both the beach and the dune. The highest values were measured one month after nourishment. After that, the concentration started to decrease again. The phosphate concentration in the sediment increased after nourishment too. The highest values were measured after nourishment at both habitats. The concentration did not decrease between one and three months after nourishment. Six months after nourishment, the concentration had decreased again.
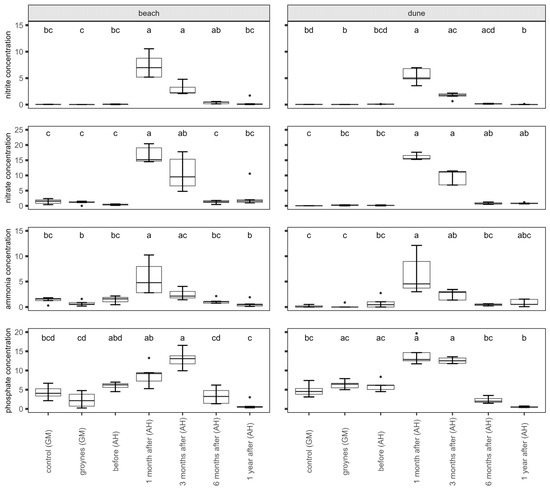
Figure 3.
Nutrient concentrations (nitrate, nitrite, ammonia, and phosphate) of the sediment at the beach and dune in µmol per L. Shown are sediment samples from Ahrenshoop (AH) before, one month, three months, six months, and one year after nourishment. For comparison, the species richness of two dunes in Graal Mueritz (GM, control, and groyne sites) is pictured. Values presented by Box plots are median (middle bar), and the lower and upper hinges correspond to the first and third quartiles (the 25th and 75th percentiles) from 5 replicates. The Box plots also include whiskers (representing 5–95% of variability) and outliers (points). Different letters indicate significant differences between sampling times (Dunn’s Test, p ˂ 0.05).
3.2. Botanical Mappings
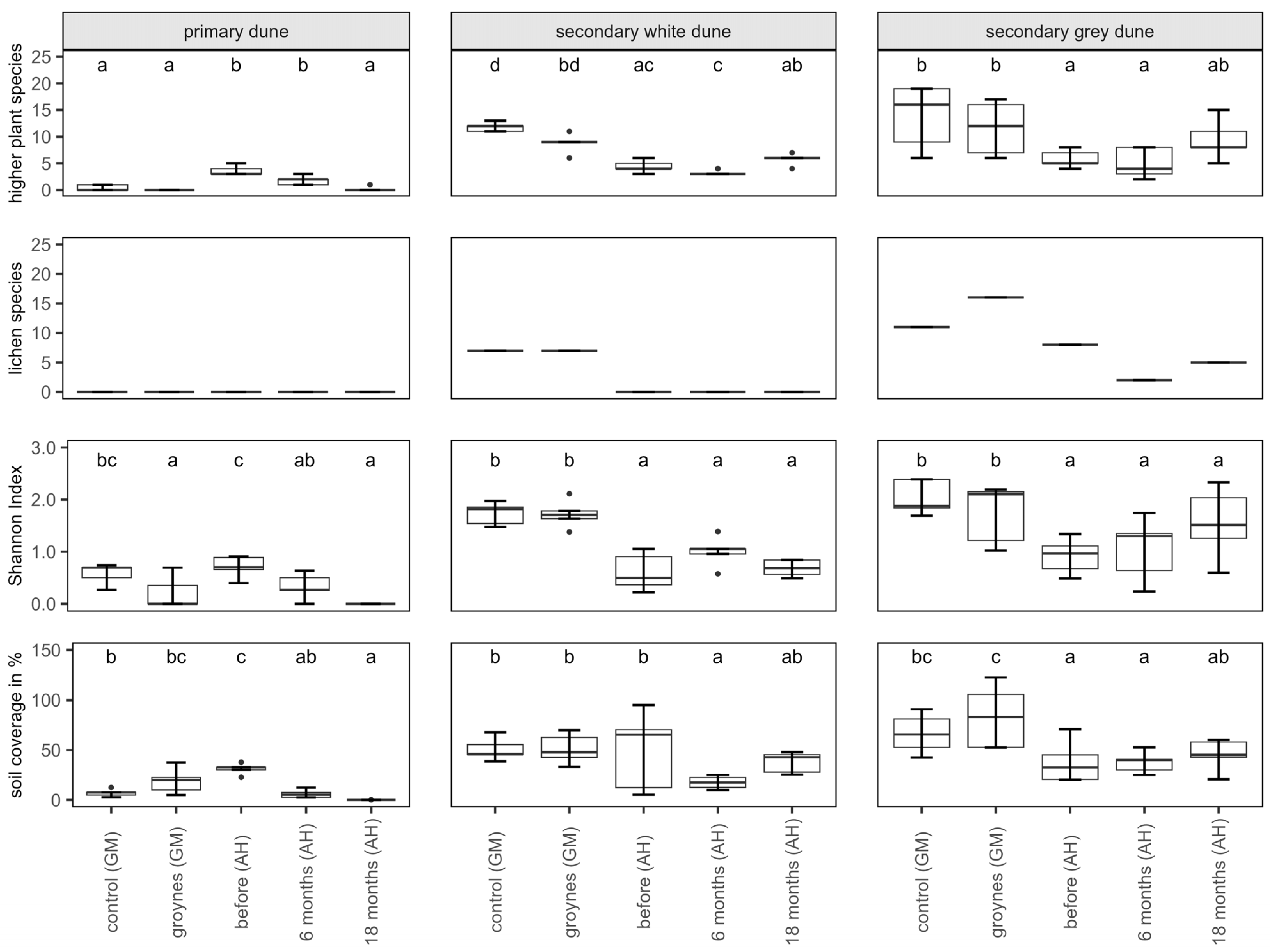
Several botanical mappings were conducted to determine species richness as well as soil coverage. There were significant differences between the control sampling sites, groynes, and the sampling times at the nourishment site (Figure 4). There are two different trends visible: on the secondary dunes at the nourishment site, the species richness was generally lower than at the other two sites. There was a slight increase in species richness in the second year after nourishment. There were also significant differences between the Shannon Index H of the sampling sites. Just like the species richness, at the secondary dunes, the Shannon Index H was slightly lower at the nourishment site than at the control and groyne sites.
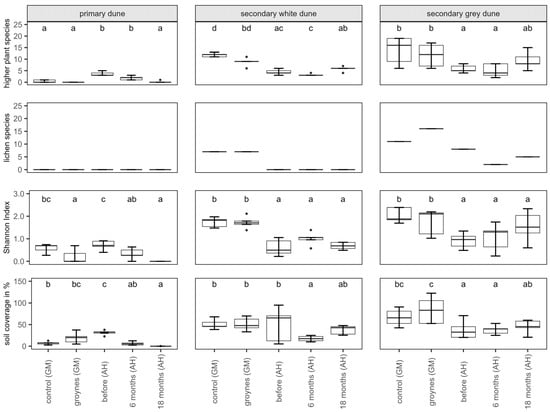
Figure 4.
Number of higher plants and lichens, Shannon Index H, and soil coverage for each habitat (primary, secondary white, and secondary grey dune) in Ahrenshoop (AH) before, 6 months, and 18 months after the nourishment. For comparison, the species richness of two dunes in Graal-Mueritz (GM, control site, and groyne site) is pictured. Values presented by Box plots are medians (middle bar), and the lower and upper hinges correspond to the first and third quartiles (the 25th and 75th percentiles) from 5 replicates. The Box plots also include whiskers (representing 5–95% of variability) and outliers (points). Different letters indicate significant differences between sampling times (Dunn’s Test, p ˂ 0.05).
These results of species number, soil coverage, and Shannon Index H are directly linked to the occurrence of the higher plant and lichen species in the mapped ecosystems. In the following table (Table 3), all detected species are listed, and the ecosystems where they were found are marked.

Table 3.
Higher plant species and mosses (a) and lichen species (b) identified in the botanical mappings at Ahrenshoop and Graal Mueritz. Identified higher plant species are marked with grey shade. Light grey indicates a sediment coverage ≤10% and dark grey indicates a sediment coverage >10%. Marking X indicates the presence of a species without information about sediment coverage. Mapings were performed before sand nourishment in Ahrenshoop, as well as 6 months and 18 months after. In Graal Mueritz, the same mappings were performed on a site with groynes and on a site without any coastal protection (control). The mappings covered primary (P), secondary white (W), and secondary grey (G) dunes.
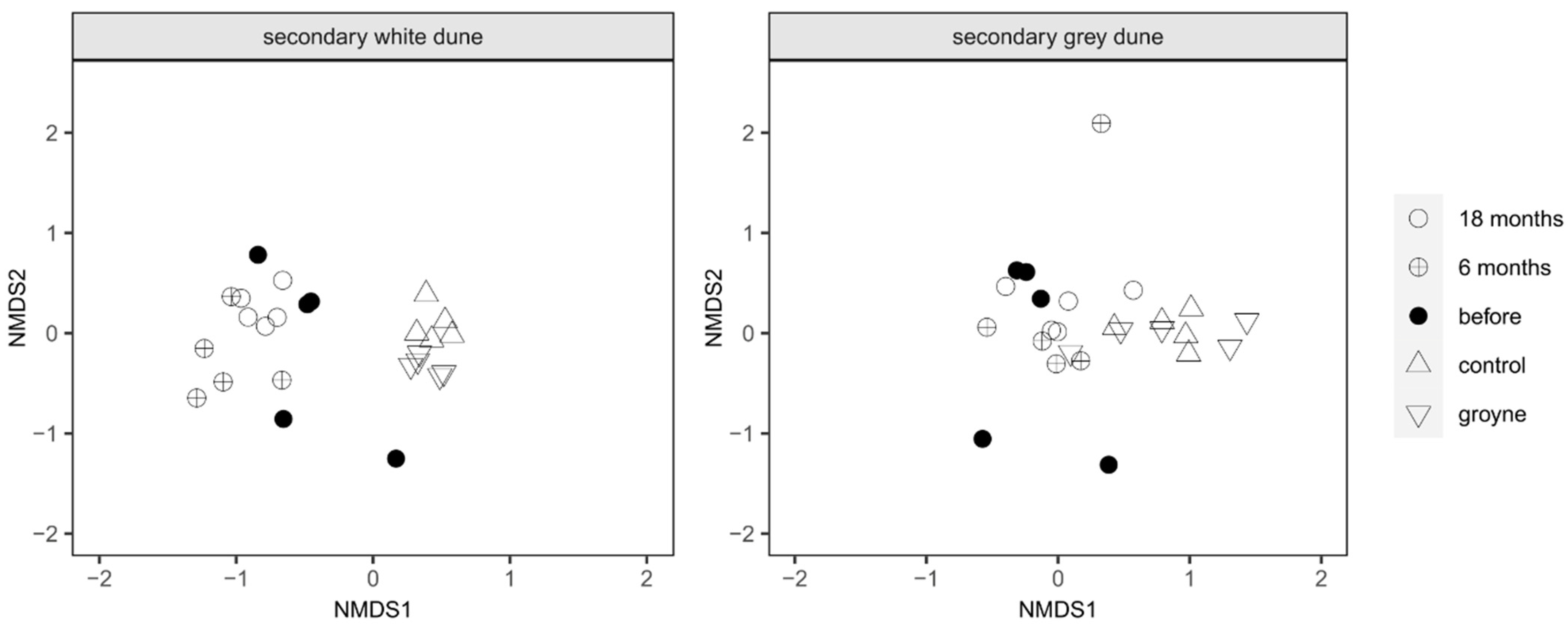
These differences in species composition and vegetation coverage between the sampling sites can be used to illustrate similarities between ecosystems for both secondary white and grey dunes. In the following figure (Figure 5), neighboring points indicate a similar species composition and coverage. Increasing distance between points indicates increasing differences. For the secondary white dune, all points from the nourishment site are quite far away from the control and groyne sites while the data points representing the two sites are close to each other. The points representing the different sampling times at nourishment are close to each other as well. Similar, albeit not quite as pronounced, trends are also evident in the secondary grey dune: again, points representing the species composition at the control and groyne sites are distant from the ones at the nourishment site. In general, the points are closer to each other than at the white dune. The stress value for the calculation of the multidimensional scaling (MDS) was 0.1260, and the stress plot showed R2 values of 0.984 (non-metric fit) and 0.901 (linear fit).
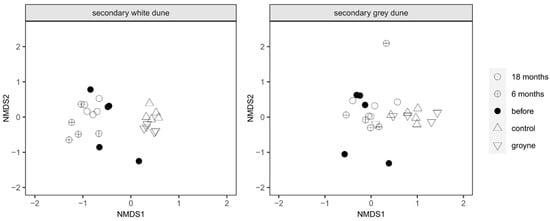
Figure 5.
Multidimensional scaling (MDS) of the vegetation coverage on the white and grey secondary dunes at the three experimental sites and times: the nourishment site before, 6 months after, and 18 months after nourishment, with one site protected by groynes and another one without any coastal protection (control site).
4. Discussion
4.1. Nutrient Availability
The nutrient availability in sediments in dune ecosystems is very low compared to ecosystems with more fertile soils [29,30]. This low phosphate availability, but also the high buffering capacity, favors plant species that have adapted to these conditions [31]. Therefore, nutrient additions to these ecosystems can be seen as a major interference with the natural nutrient conditions. Seedlings growing in coastal ecosystems are able to bypass nutrient deficiency due to the availability of nutrients from the salt spray [32]. Another natural source of nutrient input is beachwrack, which washes up on the shore and then is decomposed by microbial communities and invertebrates leading to a release of organically bound nutrients [33]. During sand nourishment, nutrient-rich sediments taken from deeper sediment layers were exposed to the coast and nutrient concentrations increased [34]. Changes in the wave climate or meteorological climate were not responsible for the input of nutrients [34]. Therefore, an effect of nutrient addition on plant communities can be expected, e.g., a shift in species composition or increased plant biomass after the addition of nitrogen [35]. The germination and growth of seedlings can be significantly increased by fertilization too [36]. Nevertheless, this effect does not seem to occur everywhere in the same way or reacts only on larger time scales since there are reports that even after four years of increased nitrogen availability, no effects on dune plant species were measured [31]. Since the nutrient input after sand nourishment in Ahrenshoop was only temporary and decreased within six months, a direct effect could be limited.
4.2. Single Species Analysis
Most species that were found at the three sites are common dune species according to Bakker [3]. However, the changing abiotic habitat conditions are reflected in the abundance and distribution of plant species along transects at the coast. At the nourishment site, additional nutrient loads are a dominant factor influencing the performance of the plant species. An increased moisture content, which was detected during and after nourishment, could also have an influence on this [20].
Plant species profit from nourishment – One of those species is Artemisia maritima, which is known to profit from fertilization in salt marshes [35]. Cakile maritima is also known to grow best under high-nutrient and -moisture conditions, e.g., through beach wrack [33,37]. Both were found more frequently after nourishment, especially at the white dune. Lathyrus japonicas might have also profited as this species is not negatively affected by nutrient addition [38,39]. Similar results were noticed for Sedum acre, which has already shown increased growth after fertilization of the soil [40], Honckenya peploides, which has a broad spectrum for nutrient concentration in substrates [41], Tripleurospermum maritimum, which prefers nitrogen-rich substrates and shows increased growth after adding fertilizer [42], and Salix repens, known to grow better under increased nutrient concentrations [43,44]. Additionally, arbuscular mycorrhiza [43] enables species such as Salix sp. or Rubus sp. to maintain nutrient uptake at a steady level [45,46]. Symbiotic nitrogen-fixating bacteria within their stem and rhizome tissue [47] enable Elymus athericus to increase growth under high-nutrient conditions [35,37,48,49]. Other species that profit from the nourishment are highly competitive r-strategists known to respond with high rates of growth after disturbances. Some fast-growing shrub species, e.g., Rosa rugosa, show this behavior, resulting in large-scale vegetation composition changes. As this species is a strong competitor for space and nutrients [50], an increase in the spreading after nourishment is also possible. However, large-scale distribution of this species is prevented by complete coverage of the plants by sand in the nourished area, which the plants cannot survive. This explains why Rosa rugosa is rarely found at the white dune at the nourishment site but is common and increasingly found at the grey dune. More competitive species are Hieracium umbellatum and Corynephorus canescens [51,52]. Enduring plant species able to withstand unsuitable conditions were also recorded, e.g., Jasione montana [53], Galium mollugo [54], and Crambe maritima [38,55]. At the control and groyne sites, these species were found on multiple transects, showing that the species is also able to establish a certain coverage under better conditions.
Plant species inhibited by the nourishment—There are multiple reasons why plant species did not emerge after nourishment or decreased in the months after nourishment activities. One of them is the distribution of seeds of annual plants. This is the case for Atriplex littoralis, which could benefit from additional nutrients [35] but the annual plant is limited in its spreading of seeds. Less competitive species, e.g., Cerastium holosteoides [56,57], Eryngium maritimum [38,58], and Rosa canina [59], are also inhibited after nourishment. Another reason for the inhibition of species distribution is the elevated moisture in the sediment, which not every species can cope with. Although the genus Taraxacum includes many different species, with some of them even preferring periodically flooded areas [60], the species did not emerge after the measure.
Plant species not affected by the nourishment—Plant species that neither increased nor decreased were Carex arenaria and Leymus arenarius. Two years after nourishment, both species were still present on the white and grey dunes. A possible reason for the fast recovery is their root system and ability to acquire nutrients: Carex arenaria is a fast-growing species with roots up to four meters in length [61], and there have been reports of increased growth under high-nutrient conditions [54,62], as well as indirect evidence for nutrient transfer through clones [63]. For the dune species Leymus arenarius, arbuscular mycorrhiza has been reported [64]. Additionally, a wide-ranging root system enables plant species to acquire nutrients from adjacent areas [65], helping them to profit from increased nutrient loads, which can result in increased seedling establishment [66].
Plant and moss species detected only/mostly at the control and groyne site—There were several species occurring at both groyne and control sites but not or rarely at the nourishment site, indicating that this site is unsuitable. One of those species is Brachythecium albicans, a widely spread moss species that can be found on sandy substrates [67]. The moss species Ceratodon purpureus and Dicranum scoparium are also pioneer mosses appearing mostly in later successional dune stages [68]. These species did not appear on the freshly nourished dune but did at control and groyne sites. Festuca sp. and Senecio vulgaris dominate stabilized dunes under low-nutrient conditions [69,70,71]. Other species rely on already-developed soil to establish a population, which is only provided at the groyne and control sites, e.g., Hypochaeris radicata [72], or developed dune landscapes with depressions and increased soil moisture, e.g., Juncus sp. [73].
Melampyrum pratense and Sonchus arvensis are common plants growing on fixed dunes and contribute to sand-catching [3,74]. The species Puccinellia distans and Poa nemoralis are not common dune species but can survive if the ecosystem enables soil development [75,76]. This composition of different species in dense vegetation stands offers a habitat to other plants, such as Nardus stricta [77], Ilex aquifolium [78], and Quercus robur [79]. It is therefore not surprising to see those species appear on the stabilized dune. Low sediment dynamics can lead to increased coverage by lichens.
In contrast to species that rely on stable conditions, some plant species need a certain amount of disruptions and cannot establish themselves on completely fixed dunes, such as Anthriscus sylvestris [80], Pinus sylvestris [81], Bromus sp. [82], and Thes tricolor [83].
Lichen species–Lichen species, which are able to grow on Ammophila arenaria [68,84] on white dunes, were only present at the control and groyne sites. Species of the genera Cladonia and Peltigera, as well as the fruticose lichens Evernia prunastri and Hypogymnia physodes and the foliose lichen Parmelia sulcata, were only growing at these two sites, too. The species of the genera Cladonia and Peltigera occur on more or less acidic, rather nutrient-poor sand [25,68,84]. The other three lichen species usually grow epihytically [25,84], but they can also settle on rather nutrient-poor sand [68,84]. The settlement of all these species will only be possible if sand transport is reduced and the conditions are more stable. They mark the beginning of the development of grey dunes [2]. The contents of nutrient elements and organic matter in grey dunes are already higher than in white dunes [68,84]. The lichen species Cladonia rangifera has, in previous experiments, also reacted well to increased nutrients up to a certain level as a consequence of soil development [85], causing vegetation composition through soil coverage [86].
The number of lichen species was generally higher at the control site, and especially at the groyne site, than at the nourishment site. That means that the lower the sand dynamics, the greater the lichen diversity.
4.3. Vegetation Composition and Biodiversity
The biodiversity of coastal dune ecosystems is similar in most habitats. Embryo (primary) dunes, main (secondary white) dunes, and transition (secondary grey) dunes have similar species numbers [87]. To maintain this high coastal biodiversity, it is therefore important to conserve all habitats in the coastal system since all of them can contribute evenly to the biodiversity. Mobile dunes can have a higher plant biodiversity [6,88]. Indicators for a mobile dune are natural disturbances, e.g., regular wash-overs by waves or blow-outs by wind [88,89]. CPMs are usually opportunistic to these disturbances, either inhibiting them by stabilizing the dune or forcing oversized disturbances. Moreover, the replantation of certain species, e.g., Ammophila arenaria, used on a global scale for the reforestation of dunes [90], can lead to a reduction or disappearance of certain dune species [91].
Differences in species composition on the dunes are visible in this study in the results of the different CPMs: At the groyne site, the adverse effects of nourishment on plant diversity were only minimal, enabling lichen and moss species to persist. No additional sediment was flushed onto the dune, so no burial of existing vegetation happened, and the vegetation cover was able to develop. At the nourishment site, the additional sediment first led to the burial and destruction of the existing vegetation. In the second step, only young individuals of Ammophila arenaria were replanted. These changes caused a setback in the dune succession to a less stabilized dune more similar to embryo dunes or early white dunes. Increased eolian sediment transport can promote this even further and mainly reinforces the establishment of plants adapted to sediment movement. Moreover, although nourishment was limited to the beach and white dunes, the grey dune was also affected by these processes.
Active dune management, and primarily the removal of certain plant species, affects the vegetation composition as well. This influences shrub species the most as shrub invasion in dune systems causes the loss of grassland and leads to the enrichment of the soil and therefore is not a desired goal for dune development [60,92]. Both species of Hippophae rhamnoides and Populus tremula were affected by this removal at the nourishment site.
In general, the species detected at all sites are in the wide range of species numbers already published by other researchers for this region [7]. The number of species at the nourishment site is at the lower limit of this range. A low species richness is an indicator of missing habitat stability concerning abiotic and biotic conditions [93]. The lichen species numbers were also lower at the nourishment site. Lichen settlement starts only under stable habitat conditions, e.g., lower sediment dynamics, since they are not able to survive burial by sand. The soil coverage follows the same trend, although replantation of marram grass was performed. However, the necessary soil coverage of 30% to stabilize the sediment and inhibit eolian erosion was achieved [94]. In general, this study shows that the soil coverage by plants increases with increasing stabilization of the dune. While the coverage by marram grass decreases, the increase in heath and shrub species progresses even faster [90]. This supports the higher soil coverage measured at the groyne and control sites than at the nourishment site. The overall reduced species numbers are also shown by a decrease in plant diversity measured by Shannon Index H. While the plant diversity at the groyne and control sites is similar to earlier reports, the nourishment site again shows lower values than at other sites at the Baltic Sea, normally ranging between one and three [15]. On other coasts, this can be even higher. Reports from the Mediterranean Coast usually show diversity indices of around three to four with an increased species number [95]. Index H includes both the number of species and abundance to make an estimation of the diversity at the site, with a higher index indicating higher diversity.
Another factor that can influence the vegetation composition at the nourished dune is sediment characteristics changes after nourishment. Plant seedlings show both avoidance and tolerance strategies to withstand sediment stress, e.g., sediment moisture. However, especially on the dune, seedling establishment coincided with high moisture conditions [1]. During sand nourishment, additional moisture enters the sediment by flushing the sediment onto the coastal area. This was shown at the nourishment site in Ahrenshoop too, but like most other changes in sediment conditions, this was only temporary and was not measured after six months [20]. Therefore, the biggest influence on vegetation composition might not be the sediment characteristic changes, but rather the movement of sediment. This includes the burial of seedlings and seeds under nourished sediment that can significantly influence the emergence of seedlings [1]. In addition, it must also be noted that the overall sediment dynamics might have changed due to the regular nourishments [19] at the nourishment site for quite some time now. During normal dune succession, the replenishing sand for the dune originates from the beach and is moved in small quantities over a longer period of time [6]. In contrast, sand nourishments move large quantities of sediment over a small period of time leading to an overstabilized dune. These sand nourishments can increase eolian sand transport as well [96], and increased sediment movement can affect the plant species composition [97,98]. There are also demands for a change in nourishment practices, asking for either concentrated nourishment with larger amounts of sand instead of multiple repeated nourishment (e.g., sand motor in the Netherlands) to reduce the initial burial area [99] responsible for large biodiversity loss as a consequence of burial of all immobile and slow-moving organism [100] or mosaic nourishments to enhance the chances for natural recolonization [99].
5. Conclusions
Coastal protection measures (CPMs) are implemented with good reason and pursue a specific goal at their implementation site, e.g., erosion protection and flood reduction.
However, while doing this, they affect their surrounding ecosystems, which are already under pressure. Whereas the predominant practice was initially to stabilize the dune and beach and decrease erosion, there is now an increase in evidence supporting further effects. The species composition on nourished dunes suggests higher sediment mobility, while the more lichen- and moss-dominated vegetation at groyne and control sites indicate a stabilized habitat. Especially at the groyne site, the lichen species suggest a more mature dune system. Nutrient input by sand nourishment was only short term and the overall nutrient concentrations at the site were not increased by the regular repeated nourishments. These research findings can improve the current practice of coastal management: implemented CPMs should be closely monitored after installation, especially when they include repeated disturbances of the coastal system. Performed sand nourishments leading to reduced dune development and decreased soil coverage need to be compensated. This could include plant reforestation at the nourished dunes that include more diverse dune species increasing both sediment stability and biodiversity in these habitats. While the current practice is to restore only marram grass Ammophila arenaria, this could be extended to Leymus arenarius and Corynephorus canescens or other species. Other coastal protection measures should also be considered to counteract the cause of coastal retreat at this point. These could be, for example, additional, carefully placed breakwaters.
The decision for specific shore management is characterized by the available resources, littoral sediment transport, and also benefits such as recreational uses of the beach and dunes. The two fundamentally different coastal protection measures, repeated sand nourishment and groynes, were developed on the basis of these principles by coastal protection authorities ensuring either a dynamic or more stable coastal zone. The conflict of use in this heavily utilized environment will continue to have a significant impact on coastal protection in the future.
Author Contributions
Conceptualization, D.G. and U.S.; data curation, D.G. and U.S.; formal analysis, D.G.; funding acquisition, H.S.; investigation, D.G. and U.S.; methodology, D.G. and U.S.; project administration, H.S.; resources, D.G.; software, D.G.; supervision, H.S.; validation, D.G.; visualization, D.G.; writing—original draft, D.G.; writing—review and editing, D.G. and U.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the BMBF (“Bundesministerium für Bildung und Forschung”/Federal Ministry of Education and Research), grant number FKZ 03F0860F as part of the project ECAS Baltic. The APC was funded by the Open Access Department University of Rostock, Germany.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors are thankful to the BMBF for funding the project. Special thanks go to the StALU MM (Staatliches Amt für Landwirtschaft und Umwelt Mittleres Mecklenburg), Germany, for exceptional communication and cooperation.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Coordinates of all sediment sampling stations in Ahrenshoop and Graal Mueritz. The botanical mappings were performed along transects, which span between the coordinates of each station.
Table A1.
Coordinates of all sediment sampling stations in Ahrenshoop and Graal Mueritz. The botanical mappings were performed along transects, which span between the coordinates of each station.
| Site | Station | Description | Longitude (Degrees East) | Latitude (Degrees North) |
|---|---|---|---|---|
| Ahrenshoop (sand nourishment) | 1 | Beach | 12.412062 | 54.379118 |
| 1 | Dune | 12.412390 | 54.378995 | |
| 2 | Beach | 12.423305 | 54.384627 | |
| 2 | Dune | 12.423598 | 54.384430 | |
| 3 | Beach | 12.430064 | 54.387775 | |
| 3 | Dune | 12.430459 | 54.387484 | |
| 4 | Beach | 12.436747 | 54.391684 | |
| 4 | Dune | 12.437031 | 54.391506 | |
| 5 | Beach | 12.443353 | 54.397647 | |
| 5 | Dune | 12.443826 | 54.397472 | |
| Graal Mueritz (groynes) | 1 | Beach | 12.272940 | 54.270792 |
| 1 | Dune | 12.273260 | 54.270476 | |
| 2 | Beach | 12.272162 | 54.270477 | |
| 2 | Dune | 12.272439 | 54.270236 | |
| 3 | Beach | 12.271260 | 54.270152 | |
| 3 | Dune | 12.271525 | 54.269905 | |
| 4 | Beach | 12.270729 | 54.269949 | |
| 4 | Dune | 12.271000 | 54.269687 | |
| 5 | Beach | 12.269996 | 54.269632 | |
| 5 | Dune | 12.270210 | 54.269451 | |
| Graal Mueritz (control) | 1 | Beach | 12.286490 | 54.275244 |
| 1 | Dune | 12.286903 | 54.274995 | |
| 2 | Beach | 12.285852 | 54.274992 | |
| 2 | Dune | 12.286066 | 54.274792 | |
| 3 | Beach | 12.285117 | 54.274749 | |
| 3 | Dune | 12.285370 | 54.274512 | |
| 4 | Beach | 12.284139 | 54.274428 | |
| 4 | Dune | 12.284340 | 54.274221 | |
| 5 | Beach | 12.283473 | 54.274172 | |
| 5 | Dune | 12.283702 | 54.273967 |
References
- Maun, M.A. Adaptations enhancing survival and establishment of seedlings on coastal dune systems. Vegetatio 1994, 111, 59–70. [Google Scholar] [CrossRef]
- Ellenberg, H.; Leuschner, C. Vegetation Mitteleuropas mit den Alpen in Ökologischer, Dynamischer und Historischer Sicht: 203 Tabellen, 6th ed.; Ulmer: Stuttgart, Germany, 2010. [Google Scholar]
- Bakker, J.P. Phytogeographical Aspects of the Vegetation of the Outer Dunes in the Atlantic Province of Europe. J. Biogeogr. 1976, 3, 85–104. [Google Scholar] [CrossRef]
- Van Puijenbroek, M.E.B.; Teichmann, C.; Meijdam, N.; Oliveras, I.; Berendse, F.; Limpens, J. Does salt stress constrain spatial distribution of dune building grasses Ammophila arenaria and Elytrichia juncea on the beach? Ecol. Evol. 2017, 7, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, J.; Kirmer, A.; Tischew, S.; Hölzel, N.; Kiehl, K. Renaturierungsökologie, 1st ed.; Springer: Berlin, Germany, 2019. [Google Scholar]
- Arens, S.M.; Mulder, J.P.; Slings, Q.L.; Geelen, L.H.; Damsma, P. Dynamic dune management, integrating objectives of nature development and coastal safety: Examples from the Netherlands. Geomorphology 2013, 199, 205–213. [Google Scholar] [CrossRef]
- Łabuz, T.A.; Grunewald, R. Studies on Vegetation Cover of the Youngest Dunes of the świna Gate Barrier (Western Polish Coast). J. Coast. Res. 2007, 231, 160–172. [Google Scholar] [CrossRef]
- Dunes of the Baltic Sea Coast, Botanical Garden, University of Rostock. Adapted with Permission from Dethardt Götze. 2024, Sarah Mamerow. Available online: https://www.garten.uni-rostock.de/studium-und-lehre/vertiefung-ausgewaehlter-themen/duenen-der-ostseekueste/ (accessed on 3 June 2024).
- Firth, L.B.; Knights, A.M.; Bridger, D.; Evans, A.J.; Mieszkowska, N.; Moore, P.; O’Connor, N.E.; Sheehan, E.; Thompson, R.C.; Hawkins, S.J. Ocean Sprawl: Challenges and opportunities for biodiversity management in a changing world. In Oceanography and Marine Biology: An Annual Review; Hughes, R.N., Hughes, D.J., Smith, I.P., Dale, A.C., Eds.; Taylor & Francis: London, UK, 2016; Volume 54, pp. 189–262. [Google Scholar]
- Dugan, J.E.; Airoldi, L.; Chapman, M.G.; Walker, S.J.; Schlacher, T. Estuarine and Coastal Structures. Treatise Estuar. Coast. Sci. 2011, 8, 17–41. [Google Scholar]
- Van Rijn, L.C. Design of Hard Coastal Structures against Erosion. Available online: www.leovanrijn-sediment.com (accessed on 17 October 2023).
- Schoonees, T.; Gijón Mancheño, A.; Scheres, B.; Bouma, T.J.; Silva, R.; Schlurmann, T.; Schüttrumpf, H. Hard Structures for Coastal Protection, Towards Greener Designs. Estuaries Coasts 2019, 42, 9–29. [Google Scholar] [CrossRef]
- de Groot, A.V.; Janssen, G.M.; Isermann, M.; Stock, M.; Glahn, M.; Elschot, K.; Hellwig, U.; Petersen, J.; Esselink, P.; van Duin, W.; et al. Beaches and dunes. In Wadden Sea Quality Status Report 2017; Kloepper, A., Ed.; Common Wadden Sea Secretariat: Wilhelmshaven, Germany, 2017. [Google Scholar]
- StAUN MM (State Agency for Environment and Nature Central Mecklenburg). Regelwerk Küstenschutz Mecklenburg-Vorpommern: Referenzhochwasserstand und Bemessungshochwasserstand; Ostseedruck Rostock: Rostock, Germany, 2022. [Google Scholar]
- Jordan, P.; Fröhle, P. Bridging the gap between coastal engineering and nature conservation? J. Coast. Conserv. 2022, 26, 4. [Google Scholar] [CrossRef]
- Grunewald, R. Assessment of Damages from Recreational Activities on Coastal Dunes of the Southern Baltic Sea. J. Coast. Res. 2006, 225, 45–57. [Google Scholar] [CrossRef]
- Leppäranta, M.; Myrberg, K. Physical Oceanography of the Baltic Sea; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Chichester, UK, 2009. [Google Scholar]
- StAUN MM (State Agency for Environment and Nature Central Mecklenburg). Regelwerk Küstenschutz Mecklenburg-Vorpommern: Übersichtsheft. Grundlagen, Grundsätze, Standortbestimmung und Ausblick; Ostseedruck Rostock: Rostock, Germany, 2009. [Google Scholar]
- Kortekaas, S.; Bagdanaviciute, I.; Gyssels, P.; Huerta, J.M.A.; Héquette, A. Assessment of the Effects of Marine Aggregate Extraction on the Coastline: An Example from the German Baltic Sea Coast. J. Coast. Res. 2010, 51, 205–214. [Google Scholar]
- Glueck, D. How sand nourishments can influence the ecology of coastal ecosystems: A case study. J. Coast. Conserv. 2024. manuscript submitted for publication. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis, 3rd ed.; Wiley VCH Verlag GmbH: Weinheim, Germany, 1999. [Google Scholar]
- Jäger, E.J.; Müller, F.; Ritz, C.; Welk, E.; Wesche, K. Gefäßpflanzen: Atlasband: Mit 3000 Abgebildeten Arten, 13th ed.; Springer Spektrum: Berlin, Germany, 2017. [Google Scholar]
- Ellenberg, H. Zeigerwerte von Pflanzen in Mitteleuropa, 2nd ed.; Goltze: Göttingen, Germany, 1992. [Google Scholar]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Wirth, V.; Hauck, M.; Schultz, M. Die Flechten Deutschlands; Eugen Ulmer UTB: Stuttgart, Germany, 2013. [Google Scholar]
- Printzen, C.; von Brackel, W.; Bültmann, H.; Cezanne, R.; Dolnik, C.; Dornes, P.; Eckstein, J.; Eichler, M.; John, V.; Killmann, D.; et al. Die Flechten, flechtenbewohnenden und flechtenähnlichen Pilze Deutschlands—Eine überarbeitete Checkliste. Herzogia 2022, 35, 193–393. [Google Scholar] [CrossRef]
- Culberson, C.; Ammann, K. A standard method for analysing lichen substances with thin layer chromatography. Herzogia 1979, 5, 1–24. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie—Grundzüge der Vegetationskunde [Plant Socology—Principles of Vegetation Science]; Springer: Vienna, Austria, 1964. [Google Scholar]
- Kellman, M.; Roulet, N. Nutrient flux and retention in a tropical sand-dune succession. J. Ecol. 1990, 78, 664–676. [Google Scholar] [CrossRef]
- Olff, H.; Huisman, J.; van Tooren, B.F. Species dynamics and nutrient accumulation during early primary succession in coastal sand dunes. J. Ecol. 1993, 81, 693–706. [Google Scholar] [CrossRef]
- Pit, I.R.; Wassen, M.J.; Kooijman, A.M.; Dekker, S.C.; Griffioen, J.; Arens, S.M.; van Dijk, J. Can sand nourishment material affect dune vegetation through nutrient addition? Sci. Total Environ. 2020, 725, 138–233. [Google Scholar] [CrossRef] [PubMed]
- Holton, B. Some Aspects of Nitrogen Cycle in a Northern California Coastal Dune-Beach Ecosystem with Emphasis on Cakile maritima. Ph.D. Thesis, University of California, Davis, CA, USA, 1980. [Google Scholar]
- Van Egmond, E.M.; van Bodegom, P.M.; van Hal, J.R.; van Logtestijn, R.; Broekman, R.A.; Berg, M.P.; Aerts, R. Growth of pioneer beach plants is strongly driven by buried macroalgal wrack, whereas macroinvertebrates affect plant nutrient dynamics. J. Exp. Mar. Biol. Ecol. 2019, 514–515, 87–94. [Google Scholar] [CrossRef]
- Glueck, D. Comparison of high turbidity events: Sand nourishments and storm events on sandy beaches at the Baltic Sea, Germany. Mar. Pollut. Bull. 2023, 194 Pt A, 115389. [Google Scholar] [CrossRef]
- Van Wijnen, H.J.; Bakker, J.P. Nitrogen and phosphorus limitation in a coastal barrier salt marsh: The implications for vegetation succession. J. Ecol. 1999, 87, 265–272. [Google Scholar] [CrossRef]
- Van der Putten, W.H. Establishment of Ammophila arenaria (Marram Grass) from Culms, Seeds and Rhizomes. Br. Ecol. Soc. 1990, 27, 188–199. [Google Scholar]
- Del Vecchio, S.; Marbà, N.; Acosta, A.; Vignolo, C.; Traveset, A. Effects of Posidonia oceanica beach-cast on germination, growth and nutrient uptake of coastal dune plants. PLoS ONE 2013, 8, e70607. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, C.A.; Davy, A.J. The restoration of coastal shingle vegetation: Effects of substrate composition on the establishment of container-grown plants. J. Appl. Ecol. 1997, 34, 154–165. [Google Scholar] [CrossRef]
- Houle, G. Plant response to heterospecific neighbor removal and nutrient addition in a subarctic coastal dune system (northern Québec, Canada). Ecoscience 1998, 5, 526–533. [Google Scholar] [CrossRef]
- Clark, M.J.; Zheng, Y. Evaluating Fertilizer Influence on Overwintering Survival and Growth of Sedum Species in a Fall-installed Green Roof. HortScience 2012, 47, 1775–1781. [Google Scholar] [CrossRef]
- Houle, G. No evidence for interspecific interactions between plants in the first stage of succession on coastal dunes in subarctic Quebec, Canada. Can. J. Bot. 1997, 75, 902–915. [Google Scholar] [CrossRef]
- Kay, Q.O.N. Tripleurospermum inodorum (L.) Schultz Bip. J. Ecol. 1994, 82, 681–697. [Google Scholar]
- Van der Heijden, E.W.; Vosatka, M. Mycorrhizal associations of Salix repens L. communities in succession of dune ecosystems. II. Mycorrhizal dynamics and interactions of ectomycorrhizal and arbuscular mycorrhizal fungi. Can. J. Bot. 2000, 77, 1833–1841. [Google Scholar] [CrossRef]
- Sýkora, K.; van den Bogert, J.C.; Berendse, F. Changes in soil and vegetation during dune slack succession. J. Veg. Sci. 2004, 15, 209–218. [Google Scholar] [CrossRef]
- Andresen, L.C.; Michelsen, A. Off-season uptake of nitrogen in temperate heath vegetation. Oecologia 2005, 144, 585–597. [Google Scholar] [CrossRef]
- Lu, Q.; Bunn, R.; Whitney, E.; Feng, Y.; DeVetter, L.W.; Tao, H. Arbuscular mycorrhizae influence raspberry growth and soil fertility under conventional and organic fertilization. Front. Microbiol. 2023, 14, 1083319. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.A.; Kramer, S.; Azios, N.; Fusaro, S.; Cahill, E.; Kennedy, C. Endophytic nitrogen fixation in dune grasses (Ammophila arenaria and Elymus mollis) from Oregon. FEMS Microbiol. Ecol. 2004, 49, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Houle, G. Interactions between resources and abiotic conditions control plant performance on subarctic coastal dunes. Am. J. Bot. 1997, 84, 29–37. [Google Scholar] [CrossRef]
- Valéry, L.; Radureau, A.; Lefeuvre, J.-C. Spread of the native grass Elymus athericus in salt marshes of Mont-Saint-Michel bay as an unusual case of coastal eutrophication. J. Coast. Conserv. 2017, 21, 421–433. [Google Scholar] [CrossRef]
- Woch, M.W.; Kapusta, P.; Stanek, M.; Możdżeń, K.; Grześ, I.M.; Rożej-Pabijan, E.; Stefanowicz, A.M. Effects of invasive Rosa rugosa on Baltic coastal dune communities depend on dune age. NeoBiota 2023, 82, 163–187. [Google Scholar] [CrossRef]
- Weigelt, A.; Steinlein, T.; Beyschlag, W. Competition among three dune species: The impact of water availability on below-ground processes. Plant Ecol. 2005, 176, 57–68. [Google Scholar] [CrossRef]
- Bartelheimer, M.; Steinlein, T.; Beyschlag, W. Aggregative Root Placement: A Feature During Interspecific Competition in Inland Sand-Dune Habitats. Plant Soil 2006, 280, 104–114. [Google Scholar] [CrossRef]
- Parnell, J.A.N. Biological flora of the British Isles—Jasione montana L. J. Ecol. 1985, 73, 341–358. [Google Scholar] [CrossRef]
- Van den Berg, L.J.L.; Tomassen, H.B.M.; Roelofs, J.G.M.; Bobbink, R. Effects of nitrogen enrichment on coastal dune grassland: A mesocosm study. Environ. Pollut. 2005, 138, 77–85. [Google Scholar] [CrossRef]
- Christensen, J.; Lauridsen, U.B.; Andreasen, C.; Lütken, H. Influence of Temperature, Low Nutrient Supply, and Soil Composition on Germination and the Growth of Sea Kale (Crambe maritima L.). HortScience 2015, 50, 363–368. [Google Scholar]
- Bonis, A.; Grubb, P.J.; Coomes, D.A. Requirements of gap-demanding species in chalk grassland: Reduction of root competition versus nutrient-enrichment by animals. J. Ecol. 1997, 85, 625–633. [Google Scholar] [CrossRef]
- Luzuriaga, A.L.; Escudero, A. What determines emergence and net recruitment in an early succession plant community? Disentangling biotic and abiotic effects. J. Veg. Sci. 2008, 19, 445–456. [Google Scholar] [CrossRef]
- Isermann, M.; Rooney, P. Biological Flora of the British Isles: Eryngium maritimum. J. Ecol. 2014, 102, 789–821. [Google Scholar] [CrossRef]
- Grubb, P.J. Interactions of Irridiance and Soil Nutrient Supply on Growth of Seedlings of Ten European Tall-Shrub Species and Fagus sylvatica. J. Ecol. 1996, 84, 827–840. [Google Scholar] [CrossRef]
- Hofstra, J.; Zijlstra, O.G. Taraxacum frisicum Soest (syn. Taraxacum apiculatum Soest) op het vasteland van Friesland. Gorteria—Dutch Bot. Arch. 2023, 45, 64–80. [Google Scholar]
- Noble, J.C.; Marshall, C. The Population Biology of Plants with Clonar Growth: II. The Nutrient Strategy and Modular Physiology of Carex arenaria. J. Ecol. 1983, 71–73, 856–877. [Google Scholar]
- Mantilla-Contreras, J.; Schirmel, J.; Zerbe, S. Influence of soil and microclimate on species composition and grass encroachment in heath succession. J. Plant Ecol. 2012, 5, 249–259. [Google Scholar] [CrossRef]
- D’Hertefeldt, T.; Falkengren-Grerup, U.; Jónsdóttir, I.S. Responses to mineral nutrient availability and heterogeneity in physiologically integrated sedges from contrasting habitats. Plant Biol. (Stuttg.) 2011, 13, 483–492. [Google Scholar] [CrossRef]
- Stefansdottir, G.; Aradottir, A.L.; Sigurdsson, B.D. Accumulation of nitrogen and organic matter during primary succession of Leymus arenarius dunes on the volcanic island Surtsey, Iceland. Biogeosciences 2014, 11, 5763–5771. [Google Scholar] [CrossRef]
- Greipsson, S.; Davy, A.J. Responses of Leymus arenarius to nutrients: Improvement of seed production and seedling establishment for land reclamation. J. Appl. Ecol. 1997, 34, 1165–1176. [Google Scholar] [CrossRef]
- Isermann, M.; Diekmann, M.; Heemann, S. Effects of the expansion by Hippophaë rhamnoides on plant species richness in coastal dunes. Appl. Veg. Sci. 2007, 10, 33–42. [Google Scholar] [CrossRef]
- Meinunger, L.; Schröder, W. Verbreitungsatlas der Moose Deutschlands; Regensburgische Botanische Gesellschaft von 1790 e.V: Regensburg, Germany, 2007. [Google Scholar]
- Kammann, S.; Schiefelbein, U.; Dolnik, C.; Mikhailyuk, T.; Demchenko, E.; Karsten, U.; Glaser, K. Successional Development of the Phototrophic Community in Biological Soil Crusts on Coastal and Inland Dunes. Biology 2022, 12, 58. [Google Scholar] [CrossRef]
- Van Mierlo, J.E.; Wilms, Y.J.; Berendse, F. Effects of soil organic matter and nitrogen supply on competition between Festuca ovina and Deschampsia flexuosa during inland dune succession. Plant Ecol. 2000, 148, 51–59. [Google Scholar] [CrossRef]
- Vestergaard, P. Temporal development of vegetation and geomorphology in a man-made beach-dune system by natural processes. Nord. J. Bot. 2004, 24, 309–326. [Google Scholar] [CrossRef]
- Abbott, R.J. Edaphic ecotypic divergence in Senecio vulgaris and the evolutionary potential of predominantly self-fertilising species. Plant Ecol. Divers. 2023, 16, 29–44. [Google Scholar] [CrossRef]
- Jones, M.L.M.; Wallace, H.L.; Norris, D.; Brittain, S.A.; Haria, S.; Jones, R.E.; Rhind, P.M.; Reynolds, B.R.; Emmett, B.A. Changes in vegetation and soil characteristics in coastal sand dunes along a gradient of atmospheric nitrogen deposition. Plant Biol. 2004, 6, 598–605. [Google Scholar] [CrossRef]
- Álvarez-Rogel, J.; Martínez-Sánchez, J.J.; Blázquez, L.C.; Semitiel, C.M.M. A conceptual model of salt marsh plant distribution in coastal dunes of southeastern Spain. Wetlands 2006, 26, 703–717. [Google Scholar] [CrossRef]
- Tilk, M.; Tullus, T.; Ots, K. Effects of environmental factors on the species richness, composition and community horizontal structure of vascular plants in Scots pine forests on fixed sand dunes. Silva Fenn. 2017, 51, 6986. [Google Scholar] [CrossRef]
- Neuhaus, R. Mobile dunes and eroding salt marshes. Helgol. Meeresunters 1994, 48, 343–358. [Google Scholar] [CrossRef]
- Plue, J.; Cousins, S.A.O.; de Pauw, K.; Diekmann, M.; Hagenblad, J.; Helsen, K.; Hermy, M.; Liira, J.; Orczewska, A.; Vanneste, T.; et al. Biological Flora of the British Isles: Poa nemoralis. J. Ecol. 2020, 108, 1750–1774. [Google Scholar] [CrossRef]
- Hartley, S.E.; Amos, L. Competitive interactions between Nardus stricta L. and Calluna vulgaris (L.) Hull: The effect of fertilizer and defoliation on above- and below-ground performance. J. Ecol. 1999, 87, 330–340. [Google Scholar]
- Arrieta, S.; Suárez, F. Spatial dynamics of Ilex aquifolium populations seed dispersal and seed bank: Understanding the first steps of regeneration. Plant Ecol. 2005, 177, 237–248. [Google Scholar] [CrossRef]
- Muhamed, H.; Lingua, E.; Maalouf, J.-P.; Michalet, R. Shrub-oak seedling spatial associations change in response to the functional composition of neighbouring shrubs in coastal dune forest communities. Ann. For. Sci. 2015, 72, 231–241. [Google Scholar] [CrossRef]
- Austad, I.; Losvik, M.H. Changes in species composition following field and tree layer restoration and management in a wooded hay meadow. Nord. J. Bot. 1998, 18, 641–662. [Google Scholar] [CrossRef]
- Koprowski, M.; Winchester, V.; Zielski, A. Tree reactions and dune movements: Slowinski National Park, Poland. Catena 2010, 81, 55–65. [Google Scholar] [CrossRef]
- Griffith, A.B.; Ahmed, T.; Hildner, A.L.G.; Kuckreja, S.; Long, S. Constraints on coastal dune invasion for a notorious plant invader. AoB Plants 2015, 7, 126. [Google Scholar] [CrossRef]
- Lankinen, Å. Root competition influences pollen competitive ability in Viola tricolor effects of presence of a competitor beyond resource availability? J. Ecol. 2008, 96, 756–765. [Google Scholar] [CrossRef]
- Kammann, S.; Leinweber, P.; Glaser, K.; Schiefelbein, U.; Dolnik, C.; Mikhailyuk, T.; Demchenko, E.; Heilmann, E.; Karsten, U. Successional development of the phototrophic community in biological soil crusts, along with soil formation on Holocene deposits at the Baltic Sea coast. Front. Ecol. Evol. 2024, 11, 1266209. [Google Scholar] [CrossRef]
- Sparrius, L.B.; Kooijman, A.M.; Sevink, J. Response of inland dune vegetation to increased nitrogen and phosphorus levels. Appl. Veg. Sci. 2013, 16, 40–50. [Google Scholar] [CrossRef]
- Johnsen, I.; Christensen, S.N.; Riis-Nielsen, T. Nitrogen limitation in the coastal heath at Anholt, Denmark. J. Coast. Conserv. 2014, 18, 369–382. [Google Scholar] [CrossRef]
- Acosta, A.; Carranza, M.L.; Izzi, C.F. Are there habitats that contribute best to plant species diversity in coastal dunes? Biodivers. Conserv. 2009, 18, 1087–1098. [Google Scholar] [CrossRef]
- Ciccarelli, D.; Bacaro, G. Quantifying plant species diversity in coastal dunes: A piece of help from spatially constrained rarefaction. Folia Geobot. 2016, 51, 129–141. [Google Scholar] [CrossRef]
- Osswald, F.; Dolch, T.; Reise, K. Remobilizing stabilized island dunes for keeping up with sea level rise? J. Coast. Conserv. 2019, 23, 675–687. [Google Scholar] [CrossRef]
- Hertling, U.M.; Lubke, R.A. Use of Ammophila arenaria for Dune Stabilization in South Africa and Its Current Distribution—Perceptions and Problems. Environ. Manag. 1999, 24, 467–482. [Google Scholar] [CrossRef]
- Honrado, J.; Vicente, J.; Lomba, A.; Alves, P.; Macedo, J.A.; Henriques, R.; Granja, H.; Caldas, F.B. Fine-scale patterns of vegetation assembly in the monitoring of changes in coastal sand-dune landscapes. Web Ecol. 2010, 10, 1–14. [Google Scholar] [CrossRef]
- Richards, E.G.; Burningham, H. Hippophae rhamnoides on a coastal dune system: A thorny issue? J. Coast. Conserv. 2011, 15, 73–85. [Google Scholar] [CrossRef]
- Kuiters, A.T.; Kramer, K.; van der Hagen, H.G.J.M.; Schaminée, J.H.J. Plant diversity, species turnover and shifts in functional traits in coastal dune vegetation: Results from permanent plots over a 52-year period. J. Veg. Sci. 2009, 20, 1053–1063. [Google Scholar] [CrossRef]
- Arens, S.M. Aeolian Processes in the Dutch Foredunes. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 1994. [Google Scholar]
- Santoro, R.; Carboni, M.; Carranza, M.L.; Acosta, A.T. Focal species diversity patterns can provide diagnostic information on plant invasions. J. Nat. Conserv. 2012, 20, 85–91. [Google Scholar] [CrossRef]
- Hoonhout, B.; de Vries, S. Field measurements on spatial variations in aeolian sediment availability at the Sand Motor mega nourishment. Aeolian Res. 2017, 24, 93–104. [Google Scholar] [CrossRef]
- Maun, M.A. Adaptations of plants to burial in coastal sand dunes. Can. J. Bot. 1998, 76, 713–738. [Google Scholar]
- Levin, N.; Kidron, G.J.; Ben-Dor, E. A field quantification of coastal dune perennial plants as indicators of surface stability, erosion or deposition. Sedimentology 2008, 55, 751–772. [Google Scholar] [CrossRef]
- de Schipper, M.A.; Ludka, B.C.; Raubenheimer, B.; Luijendijk, A.P.; Schlacher, T.A. Beach nourishment has complex implications for the future of sandy shores. Nat. Rev. Earth Environ. 2021, 2, 70–84. [Google Scholar] [CrossRef]
- Staudt, F.; Gijsman, R.; Ganal, C.; Mielck, F.; Wolbring, J.; Hass, H.C.; Goseberg, N.; Schüttrumpf, H.; Schlurmann, T.; Schimmels, S. The sustainability of beach nourishments: A review of nourishment and environmental monitoring practice. J. Coast. Conserv. 2021, 25, 34. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).