Plant Antioxidants Affect Human and Gut Health, and Their Biosynthesis Is Influenced by Environment and Reactive Oxygen Species
Abstract
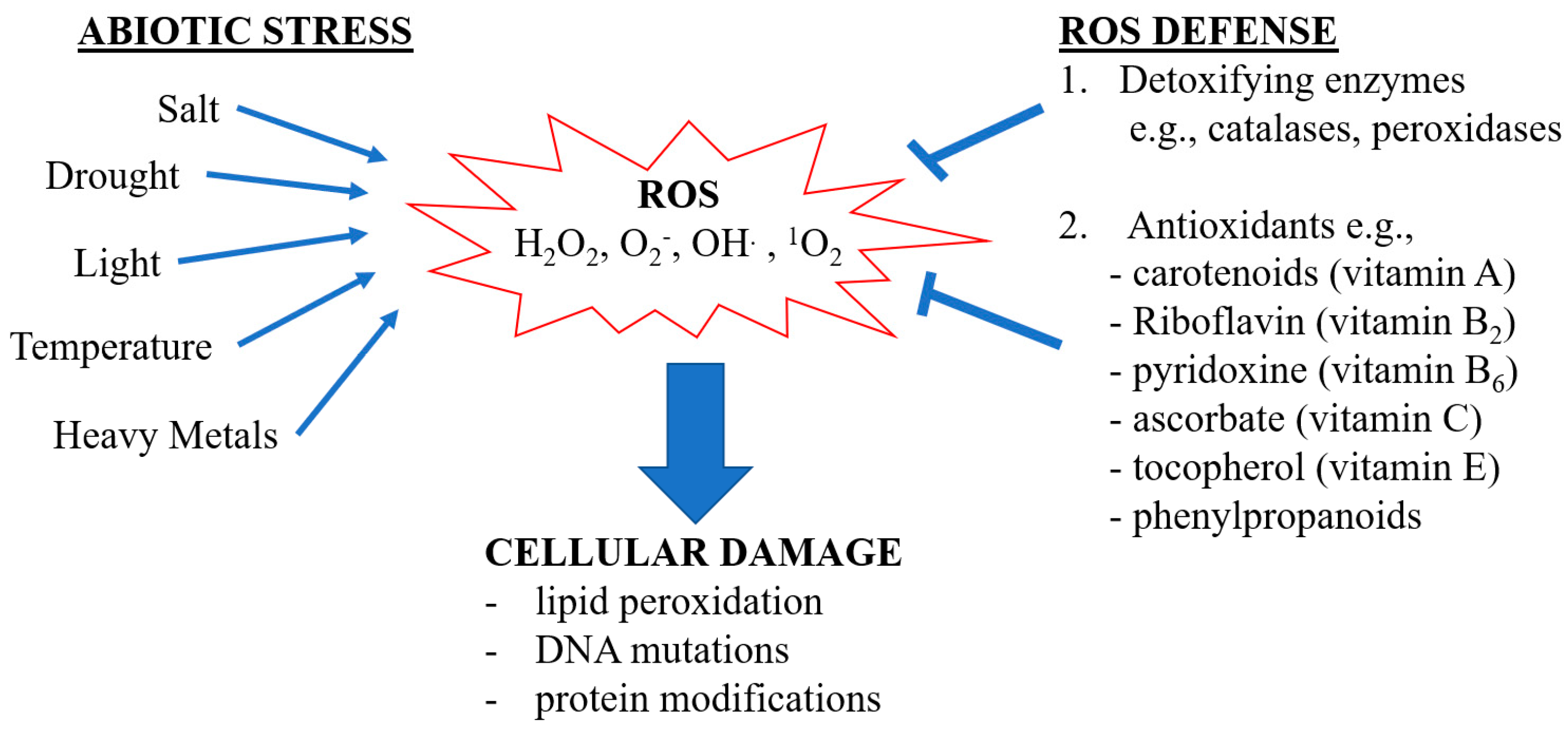
:1. Introduction
2. Vitamins
2.1. Vitamin C
2.2. Vitamin E
2.3. Vitamin B2
2.4. Vitamin B6
3. Phytonutrients
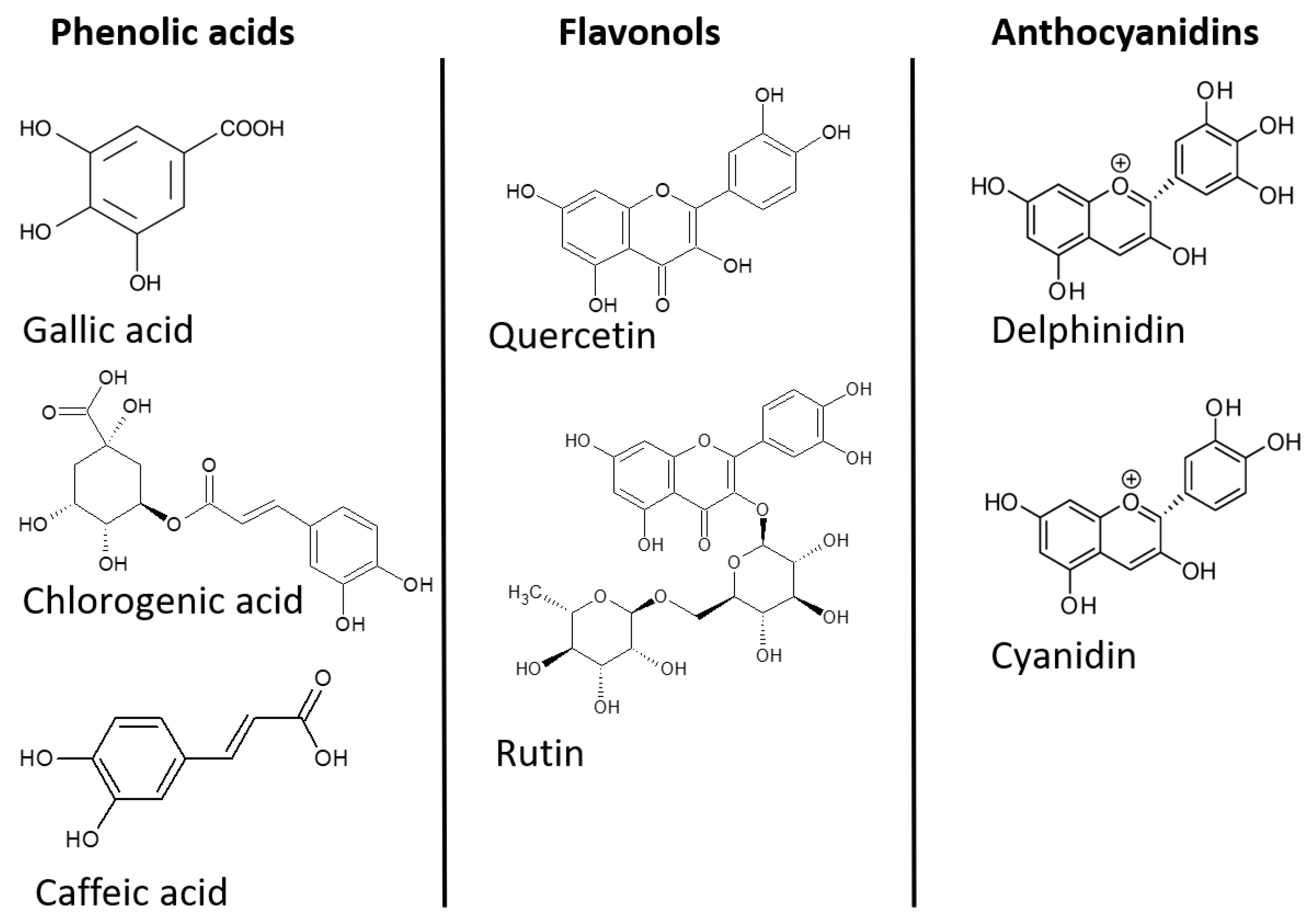
3.1. Phenylpropanoids
3.2. Carotenoids
4. Effect of Plant Foods on Gut Health from an Antioxidant Perspective
4.1. Oxidative Stress, Inflammation, and Gut Health
4.2. Plant Antioxidants in Alleviating Gut Epithelial Barrier Disruption, Inflammation, and Oxidative Stress
4.3. Plant Antioxidants Shape Gut Microbiota
4.3.1. Gut Microbiota and Gut Health
4.3.2. Plant Antioxidants and Gut Microbiota
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thornton, P.K.; Ericksen, P.; Herrero, M.; Challinor, A. Climate variability and vulnerability to climate change: A review. Glob. Chang. Biol. 2014, 20, 3313–3328. [Google Scholar] [CrossRef] [PubMed]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Tong, T.; Chen, X.; Deng, F.; Zeng, F.; Pan, R.; Zhang, W.; Chen, G.; Chen, Z.-H. Molecular response and evolution of plant anion transport systems to abiotic stress. Plant Mol. Biol. 2021, 1–16. [Google Scholar] [CrossRef]
- Bassi, R.; Dall’Osto, L. Dissipation of Light Energy Absorbed in Excess: The Molecular Mechanisms. Annu. Rev. Plant Biol. 2021, 72, 47–76. [Google Scholar] [CrossRef]
- Pascual, J.; Rahikainen, M.; Kangasjärvi, S. Plant Light Stress; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 1–6. [Google Scholar]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Singh, A.; Mehta, S.; Yadav, S.; Nagar, G.; Ghosh, R.; Roy, A.; Chakraborty, A.; Singh, I.K. How to Cope with the Challenges of Environmental Stresses in the Era of Global Climate Change: An Update on ROS Stave off in Plants. Int. J. Mol. Sci. 2022, 23, 1995. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9, plx009. [Google Scholar] [CrossRef] [PubMed]
- Baniulis, D.; Hasan, S.S.; Stofleth, J.T.; Cramer, W.A. Mechanism of Enhanced Superoxide Production in the Cytochrome b(6)f Complex of Oxygenic Photosynthesis. Biochemistry 2013, 52, 8975–8983. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive Oxygen Species (ROS) Generation and Detoxifying in Plants. J. Plant Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Pathan, S.; Siddiqui, R.A. Nutritional Composition and Bioactive Components in Quinoa (Chenopodium quinoa Willd.) Greens: A Review. Nutrients 2022, 14, 558. [Google Scholar] [CrossRef]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Gruber, J.; Schaffer, S.; Halliwell, B. The mitochondrial free radical theory of ageing—Where do we stand? Front Biosci. 2008, 13, 6554–6579. [Google Scholar] [CrossRef]
- Bresciani, L.; Martini, D.; Mena, P.; Tassotti, M.; Calani, L.; Brigati, G.; Brighenti, F.; Holasek, S.; Malliga, D.-E.; Lamprecht, M.; et al. Absorption Profile of (Poly)Phenolic Compounds after Consumption of Three Food Supplements Containing 36 Different Fruits, Vegetables, and Berries. Nutrients 2017, 9, 194. [Google Scholar] [CrossRef]
- Vicente, A.R.; Manganaris, G.A.; Darre, M.; Ortiz, C.M.; Sozzi, G.O.; Crisosto, C.H. Chapter 19—Compositional determinants of fruit and vegetable quality and nutritional value. In Postharvest Handling, 4th ed.; Florkowski, W.J., Banks, N.H., Shewfelt, R.L., Prussia, S.E., Eds.; Academic Press: San Diego, CA, USA, 2022; pp. 565–619. [Google Scholar]
- Moser, M.A.; Chun, O.K. Vitamin C and Heart Health: A Review Based on Findings from Epidemiologic Studies. Int. J. Mol. Sci. 2016, 17, 1328. [Google Scholar] [CrossRef]
- Pearson, J.F.; Pullar, J.M.; Wilson, R.; Spittlehouse, J.K.; Vissers, M.C.M.; Skidmore, P.M.L.; Willis, J.; Cameron, V.A.; Carr, A.C. Vitamin C Status Correlates with Markers of Metabolic and Cognitive Health in 50-Year-Olds: Findings of the CHALICE Cohort Study. Nutrients 2017, 9, 831. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Hampl, J.S.; Taylor, C.A.; Johnston, C.S. Vitamin C Deficiency and Depletion in the United States: The Third National Health and Nutrition Examination Survey, 1988 to 1994. Am. J. Public Health 2004, 94, 870–875. [Google Scholar] [CrossRef]
- Granger, M.; Eck, P. Chapter Seven—Dietary Vitamin C in Human Health. In Advances in Food and Nutrition Research; Eskin, N.A.M., Ed.; Academic Press: San Diego, CA, USA, 2018; Volume 83, pp. 281–310. [Google Scholar]
- National Institutes of Health. Vitamin C. Office of Dietary Supplements. 2022. Available online: https://ods.od.nih.gov/factsheets/Vitaminc-Healthprofessional/ (accessed on 20 June 2022).
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Saga, G.; Giorgetti, A.; Fufezan, C.; Giacometti, G.M.; Bassi, R.; Morosinotto, T. Mutation Analysis of Violaxanthin De-epoxidase Identifies Substrate-binding Sites and Residues Involved in Catalysis. J. Biol. Chem. 2010, 285, 23763–23770. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Miyaji, T.; Kuromori, T.; Takeuchi, Y.; Yamaji, N.; Yokosho, K.; Shimazawa, A.; Sugimoto, E.; Omote, H.; Ma, J.F.; Shinozaki, K.; et al. AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nat. Commun. 2015, 6, 5928. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Laing, W.A.; Wright, M.A.; Cooney, J.; Bulley, S.M. The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proc. Natl. Acad. Sci. USA 2007, 104, 9534–9539. [Google Scholar] [CrossRef]
- Smirnoff, N. BOTANICAL BRIEFING: The Function and Metabolism of Ascorbic Acid in Plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef]
- Gest, N.; Gautier, H.; Stevens, R. Ascorbate as seen through plant evolution: The rise of a successful molecule? J. Exp. Bot. 2012, 64, 33–53. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Food Data Central. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 20 June 2022).
- Love, S.L.; Pavek, J.J. Positioning the Potato as a Primary Food Source of Vitamin C. Am. Potato J. 2008, 85, 277–285. [Google Scholar] [CrossRef]
- Love, S.L.; Salaiz, T.; Shafii, B.; Price, W.J.; Mosley, A.R.; Thornton, R.E. Stability of Expression and Concentration of Ascorbic Acid in North American Potato Germplasm. HortScience 2004, 39, 156–160. [Google Scholar] [CrossRef]
- Nassar, A.M.K.; Kubow, S.; Leclerc, Y.; Donnelly, D.J. Somatic mining for phytonutrient improvement of ’Russet Burbank’ potato. Am. J. Potato Res. 2014, 91, 89–100. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Cunha-Santos, E.C.E.; Viganó, J.; Neves, D.A.; Martínez, J.; Godoy, H.T. Vitamin C in camu-camu [Myrciaria dubia (H.B.K.) McVaugh]: Evaluation of extraction and analytical methods. Food Res. Int. 2019, 115, 160–166. [Google Scholar] [CrossRef]
- Shigeoka, S.; Maruta, T. Cellular redox regulation, signaling, and stress response in plants. Biosci. Biotechnol. Biochem. 2014, 78, 1457–1470. [Google Scholar] [CrossRef]
- Zhou, W.L.; Liu, W.K.; Yang, Q.C. Quality changes in hydroponic lettuce grown under pre-harvest short-duration continuous light of different intensities. J. Hortic. Sci. Biotechnol. 2012, 87, 429–434. [Google Scholar] [CrossRef]
- Yabuta, Y.; Mieda, T.; Rapolu, M.; Nakamura, A.; Motoki, T.; Maruta, T.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 2007, 58, 2661–2671. [Google Scholar] [CrossRef]
- Li, M.; Ma, F.; Shang, P.; Zhang, M.; Hou, C.; Liang, D. Influence of light on ascorbate formation and metabolism in apple fruits. Planta 2009, 230, 39–51. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.; Nicole, C.; Labrie, C.; Marcelis, L.F.M. Light regulation of vitamin C in tomato fruit is mediated through photosynthesis. Environ. Exp. Bot. 2019, 158, 180–188. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef]
- Fukunaga, K.; Fujikawa, Y.; Esaka, M. Light regulation of ascorbic acid biosynthesis in rice via light responsive cis-elements in genes encoding ascorbic acid biosynthetic enzymes. Biosci. Biotechnol. Biochem. 2010, 74, 888–891. [Google Scholar] [CrossRef]
- Hu, T.; Ye, J.; Tao, P.; Li, H.; Zhang, J.; Zhang, Y.; Ye, Z. The tomato HD-Zip I transcription factor Sl HZ 24 modulates ascorbate accumulation through positive regulation of the d-mannose/l-galactose pathway. Plant J. 2016, 85, 16–29. [Google Scholar] [CrossRef]
- Zhang, W.; Lorence, A.; Gruszewski, H.A.; Chevone, B.I.; Nessler, C.L. AMR1, an Arabidopsis gene that coordinately and negatively regulates the mannose/l-galactose ascorbic acid biosynthetic pathway. Plant Physiol. 2009, 150, 942–950. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yu, Y.; Zhang, Z.; Quan, R.; Zhang, H.; Ma, L.; Deng, X.W.; Huang, R. Arabidopsis CSN5B Interacts with VTC1 and Modulates Ascorbic Acid Synthesis. Plant Cell 2013, 25, 625–636. [Google Scholar] [CrossRef]
- Di Cagno, R.; Guidi, L.; De Gara, L.; Soldatini, G.F. Combined cadmium and ozone treatments affect photosynthesis and ascorbate-dependent defences in sunflower. New Phytol. 2001, 151, 627–636. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zhang, R.; Huang, R. The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012, 71, 273–287. [Google Scholar] [CrossRef]
- Sasaki-Sekimoto, Y.; Taki, N.; Obayashi, T.; Aono, M.; Matsumoto, F.; Sakurai, N.; Suzuki, H.; Hirai, M.Y.; Noji, M.; Saito, K.; et al. Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J. 2005, 44, 653–668. [Google Scholar] [CrossRef]
- Serio, F.; De Gara, L.; Caretto, S.; Leo, L.; Santamaria, P. Influence of an increased NaCl concentration on yield and quality of cherry tomato grown in posidonia(Posidonia oceanica(L) Delile). J. Sci. Food Agric. 2004, 84, 1885–1890. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Mena, P.; García-Viguera, C.; Moreno, D.A. Brassica Foods as a Dietary Source of Vitamin C: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1076–1091. [Google Scholar] [CrossRef]
- Dale, M.F.B.; Griffiths, D.W.; Todd, D.T. Effects of genotype, environment, and postharvest storage on the total ascorbate content of potato (Solanum tuberosum) tubers. J. Agric. Food Chem. 2003, 51, 244–248. [Google Scholar] [CrossRef]
- Keijbets, M.J.H.; Ebbenhorst-Seller, G. Loss of vitamin C (L-ascorbic acid) during long-term cold storage of Dutch table potatoes. Potato Res. 1990, 33, 125–130. [Google Scholar] [CrossRef]
- Licciardello, F.; Lombardo, S.; Rizzo, V.; Pitino, I.; Pandino, G.; Strano, M.G.; Muratore, G.; Restuccia, C.; Mauromicale, G. Integrated agronomical and technological approach for the quality maintenance of ready-to-fry potato sticks during refrigerated storage. Postharvest Biol. Technol. 2018, 136, 23–30. [Google Scholar] [CrossRef]
- Külen, O.; Stushnoff, C.; Holm, D.G. Effect of cold storage on total phenolics content, antioxidant activity and vitamin C level of selected potato clones. J. Sci. Food Agric. 2013, 93, 2437–2444. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Semchuk, N.M. Tocopherol biosynthesis: Chemistry, regulation and effects of environmental factors. Acta Physiol. Plant. 2012, 34, 1607–1628. [Google Scholar] [CrossRef]
- Fritsche, S.; Wang, X.; Jung, C. Recent Advances in our Understanding of Tocopherol Biosynthesis in Plants: An Overview of Key Genes, Functions, and Breeding of Vitamin E Improved Crops. Antioxidants 2017, 6, 99. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Trebst, A. Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J. Exp. Bot. 2006, 57, 1677–1684. [Google Scholar] [CrossRef]
- Salimath, S.S.; Romsdahl, T.B.; Konda, A.R.; Zhang, W.; Cahoon, E.B.; Dowd, M.K.; Wedegaertner, T.C.; Hake, K.D.; Chapman, K.D. Production of Tocotrienols in Seeds of Cotton (Gossypium hirsutum L.) Enhances Oxidative Stability and Offers Nutraceutical Potential. Plant Biotechnol. J. 2021, 19, 1268–1282. [Google Scholar] [CrossRef]
- Konda, A.R.; Nazarenus, T.J.; Nguyen, H.; Yang, J.; Gelli, M.; Swenson, S.; Shipp, J.M.; Schmidt, M.A.; Cahoon, R.E.; Ciftci, O.N.; et al. Metabolic engineering of soybean seeds for enhanced vitamin E tocochromanol content and effects on oil antioxidant properties in polyunsaturated fatty acid-rich germplasm. Metab. Eng. 2020, 57, 63–73. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Hajirezaei, M.; Hofius, D.; Sonnewald, U.; Voll, L.M. Specific roles of alpha- and gamma-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007, 143, 1720–1738. [Google Scholar] [CrossRef]
- Mangold, H.K. The Lipid Handbook, 2nd ed.; Gunstone, F.D., Harwood, J.L., Padley, F.B., Eds.; Chapman & Hall: London, UK, 1994; Volume 97, pp. 315–316. ISBN 0412433206. [Google Scholar]
- Liu, X.; Hua, X.; Guo, J.; Qi, D.; Wang, L.; Liu, Z.; Jin, Z.; Chen, S.; Liu, G. Enhanced tolerance to drought stress in transgenic tobacco plants overexpressing VTE1 for increased tocopherol production from Arabidopsis thaliana. Biotechnol. Lett. 2008, 30, 1275–1280. [Google Scholar] [CrossRef]
- Ouyang, S.; He, S.; Liu, P.; Zhang, W.; Zhang, J.; Chen, S. The role of tocopherol cyclase in salt stress tolerance of rice (Oryza sativa). Sci. China Life Sci. 2011, 54, 181–188. [Google Scholar] [CrossRef]
- Cheng, Z.; Sattler, S.; Maeda, H.; Sakuragi, Y.; Bryant, D.A.; DellaPenna, D. Highly Divergent Methyltransferases Catalyze a Conserved Reaction in Tocopherol and Plastoquinone Synthesis in Cyanobacteria and Photosynthetic Eukaryotes. Plant Cell 2003, 15, 2343–2356. [Google Scholar] [CrossRef]
- Grusak, M.A.; DellaPenna, D. Improving the nutrient composition of plants to enhance human nutrition and health. Annu. Rev. Plant Biol. 1999, 50, 133–161. [Google Scholar] [CrossRef]
- Traber, M.G.; Leonard, S.W.; Ebenuwa, I.; Violet, P.C.; Wang, Y.; Niyyati, M.; Padayatty, S.; Tu, H.; Courville, A.; Bernstein, S.; et al. Vitamin E absorption and kinetics in healthy women, as modulated by food and by fat, studied using 2 deuterium-labeled alpha-tocopherols in a 3-phase crossover design. Am. J. Clin. Nutr. 2019, 110, 1148–1167. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G. Vitamin E Inadequacy in Humans: Causes and Consequences. Adv. Nutr. Int. Rev. J. 2014, 5, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Berson, E.L.; Rosner, B.; Sandberg, M.A.; Hayes, K.C.; Nicholson, B.W.; Weigel-DiFranco, C.; Willett, W. A Randomized Trial of Vitamin A and Vitamin E Supplementation for Retinitis Pigmentosa-Reply. Arch. Ophthalmol. 1993, 111, 1463–1465. [Google Scholar] [CrossRef]
- Kalra, V.; Grover, J.K.; Ahuja, G.K.; Rathi, S.; Gulati, S.; Kalra, N. Vitamin E administration and reversal of neurological deficits in protein-energy malnutrition. J. Trop. Pediatr. 2001, 47, 39–45. [Google Scholar] [CrossRef]
- Di Donato, I.; Bianchi, S.; Federico, A. Ataxia with vitamin E deficiency: Update of molecular diagnosis. Neurol. Sci. 2010, 31, 511–515. [Google Scholar] [CrossRef]
- Upadhyaya, D.C.; Bagri, D.S.; Upadhyaya, C.P.; Kumar, A.; Thiruvengadam, M.; Jain, S.K. Genetic engineering of potato (Solanum tuberosum L.) for enhanced alpha-tocopherols and abiotic stress tolerance. Physiol. Plant. 2020, 173, 116–128. [Google Scholar]
- Crowell, E.F.; McGrath, J.M.; Douches, D.S. Accumulation of vitamin E in potato (Solanum tuberosum) tubers. Transgenic Res. 2008, 17, 205–217. [Google Scholar] [CrossRef]
- Karunanandaa, B.; Qi, Q.; Hao, M.; Baszis, S.R.; Jensen, P.K.; Wong, Y.-H.H.; Jiang, J.; Venkatramesh, M.; Gruys, K.J.; Moshiri, F.; et al. Metabolically engineered oilseed crops with enhanced seed tocopherol. Metab. Eng. 2005, 7, 384–400. [Google Scholar] [CrossRef]
- Raclaru, M.; Gruber, J.; Kumar, R.; Sadre, R.; Lühs, W.; Zarhloul, M.K.; Friedt, W.; Frentzen, M.; Weier, D. Increase of the tocochromanol content in transgenic Brassica napus seeds by overexpression of key enzymes involved in prenylquinone biosynthesis. Mol. Breed. 2006, 18, 93–107. [Google Scholar] [CrossRef]
- Chaudhary, N.; Khurana, P. Cloning, functional characterisation and transgenic manipulation of vitamin E biosynthesis genes of wheat. Funct. Plant Biol. 2013, 40, 1129–1136. [Google Scholar] [CrossRef]
- Wang, X.Q.; Yoon, M.Y.; He, Q.; Kim, T.S.; Tong, W.; Choi, B.W.; Lee, Y.S.; Park, Y.J. Natural variations in OsgammaTMT contribute to diversity of the alpha-tocopherol content in rice. Mol. Genet. Genom. 2015, 290, 2121–2135. [Google Scholar] [CrossRef] [PubMed]
- Wolak, N.; Zawrotniak, M.; Gogol, M.; Kozik, A.; Rapala-Kozik, M. Vitamins B1, B2, B3 and B9—Occurrence, Biosynthesis Pathways and Functions in Human Nutrition. Mini-Rev. Med. Chem. 2017, 17, 1075–1111. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Song, Y.H.; Imaizumi, T. LOV Domain-Containing F-Box Proteins: Light-Dependent Protein Degradation Modules in Arabidopsis. Mol. Plant 2012, 5, 573–582. [Google Scholar] [CrossRef]
- Roberts, D.; Pedmale, U.V.; Morrow, J.; Sachdev, S.; Lechner, E.; Tang, X.; Zheng, N.; Hannink, M.; Genschik, P.; Liscum, E. Modulation of Phototropic Responsiveness in Arabidopsis through Ubiquitination of Phototropin 1 by the CUL3-Ring E3 Ubiquitin Ligase CRL3NPH3. Plant Cell 2011, 23, 3627–3640. [Google Scholar] [CrossRef] [PubMed]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- De Gara, L.; de Pinto, M.C.; Tommasi, F. The antioxidant systems vis-à-vis reactive oxygen species during plant–pathogen interaction. Plant Physiol. Biochem. 2003, 41, 863–870. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Yuann, J.-M.P.; Cheng, C.-W.; Jian, H.-L.; Lin, C.-C.; Chen, L.-Y. Blue light induced free radicals from riboflavin on E. coli DNA damage. J. Photochem. Photobiol. B Biol. 2013, 119, 60–64. [Google Scholar] [CrossRef]
- Olfat, N.; Ashoori, M.; Saedisomeolia, A. Riboflavin is an antioxidant: A review update. Br. J. Nutr. 2022, 1–27. [Google Scholar] [CrossRef]
- Silva, R.; Aguiar, T.Q.; Oliveira, R.; Domingues, L. Light exposure during growth increases riboflavin production, reactive oxygen species accumulation and DNA damage in Ashbya gossypii riboflavin-overproducing strains. FEMS Yeast Res. 2019, 19, foy114. [Google Scholar]
- Toyosaki, T. Antioxidant Effect of Riboflavin in Enzymatic Lipid-Peroxidation. J. Agric. Food Chem. 1992, 40, 1727–1730. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, S.; Christodoulou, J.; Rahman, S. Disorders of riboflavin metabolism. J. Inherit. Metab. Dis. 2019, 42, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. An update on diagnosis and therapy of metabolic myopathies. Expert Rev. Neurother. 2018, 18, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Peechakara, B.V.; Gupta, M. Vitamin B2 (Riboflavin); StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sandoval, F.J.; Zhang, Y.; Roje, S. Flavin Nucleotide Metabolism in Plants: Monofunctional enzymes synthesize fad in plastids. J. Biol. Chem. 2008, 283, 30890–30900. [Google Scholar] [CrossRef]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B6 and Its Role in Cell Metabolism and Physiology. Cells 2018, 7, 84. [Google Scholar] [CrossRef]
- Tambasco-Studart, M.; Titiz, O.; Raschle, T.; Forster, G.; Amrhein, N.; Fitzpatrick, T.B. Vitamin B6 biosynthesis in higher plants. Proc. Natl. Acad. Sci. USA 2005, 102, 13687–13692. [Google Scholar] [CrossRef] [PubMed]
- Bilski, P.; Li, M.Y.; Ehrenshaft, M.; Daub, M.E.; Chignell, C.F. Symposium-in-Print Vitamin B6 (Pyridoxine) and Its Derivatives Are Efficient Singlet Oxygen Quenchers and Potential Fungal Antioxidants. Photochem. Photobiol. 2000, 71, 129–134. [Google Scholar] [CrossRef]
- Havaux, M.; Ksas, B.; Szewczyk, A.; Rumeau, D.; Franck, F.; Caffarri, S.; Triantaphylidès, C. Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biol. 2009, 9, 130. [Google Scholar] [CrossRef]
- Titiz, O.; Tambasco-Studart, M.; Warzych, E.; Apel, K.; Amrhein, N.; Laloi, C.; Fitzpatrick, T.B. PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J. 2006, 48, 933–946. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, L. Pyridoxine is required for post-embryonic root development and tolerance to osmotic and oxidative stresses. Plant J. 2005, 44, 396–408. [Google Scholar] [CrossRef]
- Czégény, G.; Kőrösi, L.; Strid, Å.; Hideg, É. Multiple roles for Vitamin B6 in plant acclimation to UV-B. Sci. Rep. 2019, 9, 1259. [Google Scholar] [CrossRef]
- Raschke, M.; Boycheva, S.; Crèvecoeur, M.; Nunes-Nesi, A.; Witt, S.; Fernie, A.R.; Amrhein, N.; Fitzpatrick, T.B. Enhanced levels of vitamin B6 increase aerial organ size and positively affect stress tolerance in Arabidopsis. Plant J. 2011, 66, 414–432. [Google Scholar] [CrossRef] [PubMed]
- Leuendorf, J.E.; Mooney, S.L.; Chen, L.; Hellmann, H.A. Arabidopsis thaliana PDX1.2 is critical for embryo development and heat shock tolerance. Planta 2014, 240, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Denslow, S.A.; Rueschhoff, E.E.; Daub, M.E. Regulation of the Arabidopsis thaliana vitamin B6 biosynthesis genes by abiotic stress. Plant Physiol. Biochem. 2007, 45, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, H.; Mooney, S. Vitamin B6: A molecule for human health? Molecules 2010, 15, 442–459. [Google Scholar] [CrossRef]
- Fudge, J.B.; Mangel, N.; Gruissem, W.; Vanderschuren, H.; Fitzpatrick, T.B. Rationalising vitamin B6 biofortification in crop plants. Curr. Opin. Biotechnol. 2017, 44, 130–137. [Google Scholar] [CrossRef]
- Vanderschuren, H.; Boycheva, S.; Li, K.-T.; Szydlowski, N.; Gruissem, W.; Fitzpatrick, T.B. Strategies for vitamin B6 biofortification of plants: A dual role as a micronutrient and a stress protectant. Front. Plant Sci. 2013, 4, 143. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New Concepts in Nutraceuticals as Alternative for Pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Chun, O.K.; Kim, D.-O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Croft, K.D. The chemistry and biological effects of flavonoids and phenolic acids a. Ann. N. Y. Acad. Sci. 1998, 854, 435–442. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. [Google Scholar]
- Hollman, P.C. Unravelling of the health effects of polyphenols is a complex puzzle complicated by metabolism. Arch. Biochem. Biophys. 2014, 559, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.; Alegre, L.; Van Breusegem, F.; Munné-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.; Guidi, L.; Sebastiani, F.; Tattini, M. Isoprenoids and phenylpropanoids are key components of the antioxidant defense system of plants facing severe excess light stress. Environ. Exp. Bot. 2015, 119, 54–62. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Campanella, A.; Pellegrini, E.; Lorenzini, G.; Nali, C.; Paoletti, E. Phenylpropanoids are key players in the antioxidant defense to ozone of European ash, Fraxinus excelsior. Environ. Sci. Pollut. Res. 2016, 25, 8137–8147. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Tohge, T.; Fernie, A.R. An Overview of Compounds Derived from the Shikimate and Phenylpropanoid Pathways and Their Medicinal Importance. Mini-Rev. Med. Chem. 2017, 17, 1013–1027. [Google Scholar] [CrossRef]
- Longo, V.D.; Antebi, A.; Bartke, A.; Barzilai, N.; Brown-Borg, H.M.; Caruso, C.; Curiel, T.J.; de Cabo, R.; Franceschi, C.; Gems, D.; et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell 2015, 14, 497–510. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Hubbard, B.P. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1209–1218. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, S. Biosynthesis and Regulation of Phenylpropanoids in Plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant Phenylalanine/Tyrosine Ammonia-lyases. Trends Plant Sci. 2019, 25, 66–79. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.-J. Multifaceted Regulations of Gateway Enzyme Phenylalanine Ammonia-Lyase in the Biosynthesis of Phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef]
- Hou, L.; Wang, L.; Wu, X.; Gao, W.; Zhang, J.; Huang, C. Expression patterns of two pal genes of Pleurotus ostreatus across developmental stages and under heat stress. BMC Microbiol. 2019, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Li, L.; Zhang, C.; Yang, C.; Chen, G.; Niu, Y.; Si, J.; Liu, T.; Sun, X.; Wang, S.; et al. Genome-Wide Analysis and Expression Profiling of the Phenylalanine Ammonia-Lyase Gene Family in Solanum tuberosum. Int. J. Mol. Sci. 2022, 23, 6833. [Google Scholar] [CrossRef] [PubMed]
- Christie, P.J.; Alfenito, M.R.; Walbot, V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 1994, 194, 541–549. [Google Scholar] [CrossRef]
- Lamb, C.J.; Rubery, P.H. Photocontrol of chlorogenic acid biosynthesis in potato tuber discs. Phytochemistry 1976, 15, 665–668. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Yoshioka, H.; Miyabe, M.; Hayakawa, Y.; Doke, N. Expression of Genes for Phenylalanine Ammonia-Lyase and 3-Hydroxy-3-Methylglutaryl CoA Reductase in Aged Potato Tubers Infected with Phytophthora infestans. Plant Cell Physiol. 1996, 37, 81–90. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Hughes, N.M.; Neufeld, H.S.; Burkey, K.O. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol. 2005, 168, 575–587. [Google Scholar] [CrossRef]
- An, J.-P.; Wang, X.-F.; Zhang, X.-W.; Xu, H.-F.; Bi, S.-Q.; You, C.-X.; Hao, Y.-J. An apple MYB transcription factor regulates cold tolerance and anthocyanin accumulation and undergoes MIEL1-mediated degradation. Plant Biotechnol. J. 2020, 18, 337–353. [Google Scholar] [CrossRef]
- Lin-Wang, K.; Micheletti, D.; Palmer, J.; Volz, R.; Lozano, L.; Espley, R.; Hellens, R.P.; Chagné, D.; Rowan, D.D.; Troggio, M.; et al. High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant Cell Environ. 2011, 34, 1176–1190. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, M.; Nicolas, P.; Kappel, C.; Dai, Z.W.; Hilbert, G.; Peccoux, A.; Lafontaine, M.; Ollat, N.; Gomès, E.; Delrot, S. Water limitation and rootstock genotype interact to alter grape berry metabolism through transcriptome reprogramming. Hortic. Res. 2015, 2, 15012. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; Rubio-Wilhelmi, M.D.M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef]
- Carmona, L.; Alquézar, B.; Marques, V.V.; Peña, L. Anthocyanin biosynthesis and accumulation in blood oranges during postharvest storage at different low temperatures. Food Chem. 2017, 237, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin-Wang, K.; Espley, R.; Wang, L.; Li, Y.; Liu, Z.; Zhou, P.; Zeng, L.; Zhang, X.; Zhang, J.; et al. StMYB44 negatively regulates anthocyanin biosynthesis at high temperatures in tuber flesh of potato. J. Exp. Bot. 2019, 70, 3809–3824. [Google Scholar] [CrossRef] [PubMed]
- Fogelman, E.; Oren-Shamir, M.; Hirschberg, J.; Mandolino, G.; Parisi, B.; Ovadia, R.; Tanami, Z.; Faigenboim, A.; Ginzberg, I. Nutritional value of potato (Solanum tuberosum) in hot climates: Anthocyanins, carotenoids, and steroidal glycoalkaloids. Planta 2019, 249, 1143–1155. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Postharvest UV-C irradiation increased the flavonoids and anthocyanins accumulation, phenylpropanoid pathway gene expression, and antioxidant activity in sweet cherries (Prunus avium L.). Postharvest Biol. Technol. 2021, 175, 111490. [Google Scholar] [CrossRef]
- Fischer, N.; Seo, E.-J.; Efferth, T. Prevention from radiation damage by natural products. Phytomedicine 2018, 47, 192–200. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under salinity stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of Flavonols over Hydroxycinnamic Acids Favors Oxidative Damage Protection under Abiotic Stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, M.N. Chlorogenic acids and other cinnamates–nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Malmberg, A.G.; Theander, O. Determination of chlorogenic acid in potato tubers. J. Agric. Food Chem. 1985, 33, 549–551. [Google Scholar] [CrossRef]
- Navarre, D.A.; Pillai, S.S.; Shakya, R.; Holden, M.J. HPLC profiling of phenolics in diverse potato genotypes. Food Chem. 2011, 127, 34–41. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Andre, C.M.; Oufir, M.; Guignard, C.; Hoffmann, L.; Hausman, J.-F.; Evers, D.; Larondelle, Y. Antioxidant profiling of native Andean potato tubers (Solanum tuberosum L.) reveals cultivars with high levels of β-carotene, α-tocopherol, chlorogenic acid, and petanin. J. Agric. Food Chem. 2007, 55, 10839–10849. [Google Scholar] [CrossRef]
- Jakobek, L.; Seruga, M.; Novak, I.; Medvidovic-Kosanovic, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensm. Rundsch. 2007, 103, 369–377. [Google Scholar]
- Crozier, A.; Lean, M.E.J.; McDonald, M.S.; Black, C. Quantitative Analysis of the Flavonoid Content of Commercial Tomatoes, Onions, Lettuce, and Celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Sampson, L.; Rimm, E.; Hollman, P.C.; de Vries, J.H.; Katan, M.B. Flavonol and Flavone Intakes in US Health Professionals. J. Am. Diet. Assoc. 2002, 102, 1414–1420. [Google Scholar] [CrossRef]
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.J.; Crozier, A. Occurrence of Flavonols in Tomatoes and Tomato-Based Products. J. Agric. Food Chem. 2000, 48, 2663–2669. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, A.M.; Velasco, R. Metabolite Profiling of Grape: Flavonols and Anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef]
- Zimmer, J.P.; Hammond, B.R., Jr. Possible influences of lutein and zeaxanthin on the developing retina. Clin. Ophthalmol. 2007, 1, 25. [Google Scholar]
- Al-Delaimy, W.K.; Van Kappel, A.L.; Ferrari, P.; Slimani, N.; Steghens, J.-P.; Bingham, S.; Johansson, I.; Wallström, P.; Overvad, K.; Tjønneland, A. Plasma levels of six carotenoids in nine European countries: Report from the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2004, 7, 713–722. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids—A Review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D. Do carotenoids serve as transmembrane radical channels? Free. Radic. Biol. Med. 2009, 47, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Chucair, A.J.; Rotstein, N.P.; Sangiovanni, J.P.; During, A.; Chew, E.Y.; Politi, L.E. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: Relation with docosahexaenoic acid. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5168–5177. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Wang, J.J.; Flood, V.; Rochtchina, E.; Smith, W.; Mitchell, P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: The Blue Mountains Eye Study. Ophthalmology 2008, 115, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci. Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S.; Daglia, M.; Rengasamy, K.R. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Iwanzik, W.; Tevini, M.; Stute, R.; Hilbert, R. Carotinoidgehalt und-zusammensetzung verschiedener deutscher Kartoffelsorten und deren Bedeutung für die Fleischfarbe der Knolle. Potato Res. 1983, 26, 149–162. [Google Scholar] [CrossRef]
- Brown, C.; Edwards, C.; Yang, C.-P.; Dean, B. Orange Flesh Trait in Potato: Inheritance and Carotenoid Content. J. Am. Soc. Hortic. Sci. 1993, 118, 145–150. [Google Scholar] [CrossRef]
- Burmeister, A.; Bondiek, S.; Apel, L.; Kühne, C.; Hillebrand, S.; Fleischmann, P. Comparison of carotenoid and anthocyanin profiles of raw and boiled Solanum tuberosum and Solanum phureja tubers. J. Food Compos. Anal. 2011, 24, 865–872. [Google Scholar] [CrossRef]
- Brown, C.R.; Culley, D.; Bonierbale, M.; Amorós, W. Anthocyanin, Carotenoid Content, and Antioxidant Values in Native South American Potato Cultivars. HortScience 2007, 42, 1733–1736. [Google Scholar] [CrossRef]
- Diretto, G.; Al-Babili, S.; Tavazza, R.; Papacchioli, V.; Beyer, P.; Giuliano, G. Metabolic Engineering of Potato Carotenoid Content through Tuber-Specific Overexpression of a Bacterial Mini-Pathway. PLoS ONE 2007, 2, e350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitchumroonchokchai, C.; Diretto, G.; Parisi, B.; Giuliano, G.; Failla, M.L. Potential of golden potatoes to improve vitamin A and vitamin E status in developing countries. PLoS ONE 2017, 12, e0187102. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Ji, C.Y.; Lee, C.-J.; Kim, S.-E.; Park, S.-C.; Kwak, S.-S. Orange: A target gene for regulating carotenoid homeostasis and increasing plant tolerance to environmental stress in marginal lands. J. Exp. Bot. 2018, 69, 3393–3400. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, Y.O.; Ahn, M.-J.; Jeong, J.C.; Lee, H.-S.; Kwak, S.-S. Cloning and characterization of an Orange gene that increases carotenoid accumulation and salt stress tolerance in transgenic sweetpotato cultures. Plant Physiol. Biochem. 2013, 70, 445–454. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.S.; Jung, Y.J.; Kim, S.H.; Ji, C.Y.; Wang, Z.; Jeong, J.C.; Lee, H.-S.; Lee, S.Y.; Kwak, S.-S. Orange protein has a role in phytoene synthase stabilization in sweetpotato. Sci. Rep. 2016, 6, 33563. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Kang, J.; Li, M.; Huang, J.; Ke, Q.; Kim, H.S.; Xu, B.; Kwak, S.-S. Overexpression of alfalfa Orange gene in tobacco enhances carotenoid accumulation and tolerance to multiple abiotic stresses. Plant Physiol. Biochem. 2018, 130, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Significance of Genetic, Environmental, and Pre- and Postharvest Factors Affecting Carotenoid Contents in Crops: A Review. J. Agric. Food Chem. 2018, 66, 5310–5324. [Google Scholar] [CrossRef] [PubMed]
- Payyavula, R.S.; Navarre, D.A.; Kuhl, J.C.; Pantoja, A.; Pillai, S.S. Differential effects of environment on potato phenylpropanoid and carotenoid expression. BMC Plant Biol. 2012, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef]
- Cheng, H.; Leblond, C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am. J. Anat. 1974, 141, 461–479. [Google Scholar] [CrossRef]
- Booth, C.; Potten, C.S. Gut instincts: Thoughts on intestinal epithelial stem cells. J. Clin. Investig. 2000, 105, 1493–1499. [Google Scholar] [CrossRef] [Green Version]
- Gehart, H.; Clevers, H. Tales from the crypt: New insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 19–34. [Google Scholar] [CrossRef]
- Crosnier, C.; Stamataki, D.; Lewis, J. Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nat. Rev. Genet. 2006, 7, 349–359. [Google Scholar] [CrossRef]
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Genet. 2011, 9, 356–368. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Suzuki, K.; Meek, B.; Doi, Y.; Muramatsu, M.; Chiba, T.; Honjo, T.; Fagarasan, S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc. Natl. Acad. Sci. USA 2004, 101, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Strowig, T.; Kau, A.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.; Gordon, J.I.; et al. NLRP6 Inflammasome Regulates Colonic Microbial Ecology and Risk for Colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.-J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef]
- Zhu, M.-J.; Sun, X.; Du, M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Hilsden, R.J.; Meddings, J.B.; Hardin, J.; Gall, D.G.; Sutherland, L.R. Intestinal permeability and postheparin plasma diamine oxidase activity in the prediction of Crohn’s disease relapse. Inflamm. Bowel. Dis. 1999, 5, 85–91. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Meerveld, B.G.-V.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Yu, L.C.-H. The Epithelial Gatekeeper Against Food Allergy. Pediatr. Neonatol. 2009, 50, 247–254. [Google Scholar] [CrossRef]
- Vaarala, O. Is the origin of type 1 diabetes in the gut? Immunol. Cell Biol. 2012, 90, 271–276. [Google Scholar] [CrossRef]
- Sun, X.; Du, M.; Navarre, D.A.; Zhu, M.-J. Purple Potato Extract Promotes Intestinal Epithelial Differentiation and Barrier Function by Activating AMP-Activated Protein Kinase. Mol. Nutr. Food Res. 2018, 62, 1700536. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Uhr, T. Induction of Protective IgA by Intestinal Dendritic Cells Carrying Commensal Bacteria. Science 2004, 303, 1662–1665. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V. Epithelial Cell Contributions to Intestinal Immunity. Adv. Immunol. 2015, 126, 129–172. [Google Scholar] [PubMed]
- Meira, L.B.; Bugni, J.M.; Green, S.L.; Lee, C.-W.; Pang, B.; Borenshtein, D.; Rickman, B.H.; Rogers, A.B.; Moroski-Erkul, C.A.; McFaline, J.L.; et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Investig. 2008, 118, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Bibi, S.; Du, M.; Suzuki, T.; Zhu, M.-J. Regulation of the intestinal tight junction by natural polyphenols: A mechanistic perspective. Crit. Rev. Food Sci. Nutr. 2017, 57, 3830–3839. [Google Scholar] [CrossRef]
- Kisseleva, T.; Song, L.; Vorontchikhina, M.; Feirt, N.; Kitajewski, J.; Schindler, C. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J. Clin. Investig. 2006, 116, 2955–2963. [Google Scholar] [CrossRef]
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacol. Res. 2012, 66, 375–382. [Google Scholar] [CrossRef]
- Amasheh, M.; Fromm, A.; Krug, S.M.; Amasheh, S.; Andres, S.; Zeitz, M.; Fromm, M.; Schulzke, J.D. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J. Cell Sci. 2010, 123, 4145–4155. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J. Carbachol ameliorates lipopolysaccharide-induced intestinal epithelial tight junction damage by down-regulating NF-kappabeta and myosin light-chain kinase pathways. Biochem. Biophys. Res. Commun. 2012, 428, 321–326. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, J.X.; Hu, N.; Ren, J.; Du, M.; Zhu, M.J. Side-stream smoking reduces intestinal inflammation and increases expression of tight junction proteins. World J. Gastroenterol. 2012, 18, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Petecchia, L.; Sabatini, F.; Usai, C.; Caci, E.; Varesio, L.; Rossi, G.A. Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Lab. Investig. J. Tech. Methods Pathol. 2012, 92, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 401–407. [Google Scholar] [CrossRef]
- Chen, J.S.; Long, L.N.; Jiang, Q.; Kang, B.J.; Li, Y.H.; Yin, J. Effects of dietary supplementation ofLycium barbarumpolysaccharides on growth performance, immune status, antioxidant capacity and selected microbial populations of weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, G.; Hao, J.; Ma, J.; Wang, Y.; Jiang, X.; Jiang, H. Curcumin Ameliorates Hydrogen Peroxide-Induced Epithelial Barrier Disruption by Upregulating Heme Oxygenase-1 Expression in Human Intestinal Epithelial Cells. Am. J. Dig. Dis. 2012, 57, 1792–1801. [Google Scholar] [CrossRef]
- Watson, J.L.; Ansari, S.; Cameron, H.; Wang, A.; Akhtar, M.; McKay, D.M. Green tea polyphenol (-)-epigallocatechin gallate blocks epithelial barrier dysfunction provoked by IFN-gamma but not by IL-4. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G954–G961. [Google Scholar] [CrossRef]
- Yang, G.; Wang, H.; Kang, Y.; Zhu, M.-J. Grape seed extract improves epithelial structure and suppresses inflammation in ileum of IL-10-deficient mice. Food Funct. 2014, 5, 2558–2563. [Google Scholar] [CrossRef]
- Noda, S.; Tanabe, S.; Suzuki, T. Differential Effects of Flavonoids on Barrier Integrity in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2012, 60, 4628–4633. [Google Scholar] [CrossRef]
- Azuma, T.; Shigeshiro, M.; Kodama, M.; Tanabe, S.; Suzuki, T. Supplemental Naringenin Prevents Intestinal Barrier Defects and Inflammation in Colitic Mice. J. Nutr. 2013, 143, 827–834. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Ling, K.H.; Wang, M.F.; El-Nezami, H. Green tea polyphenol epigallocatechin-3-gallate improves epithelial barrier function by inducing the production of antimicrobial peptide pBD-1 and pBD-2 in monolayers of porcine intestinal epithelial IPEC-J2 cells. Mol. Nutr. Food Res. 2016, 60, 1048–1058. [Google Scholar] [CrossRef]
- Bibi, S.; Kang, Y.; Yang, G.; Zhu, M.-J. Grape seed extract improves small intestinal health through suppressing inflammation and regulating alkaline phosphatase in IL-10-deficient mice. J. Funct. Foods 2016, 20, 245–252. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Zhang, H.; Huang, Y.; Yang, G.; Du, M.; Zhu, M.-J. Dietary grape seed extract ameliorates symptoms of inflammatory bowel disease in IL10-deficient mice. Mol. Nutr. Food Res. 2013, 57, 2253–2257. [Google Scholar] [CrossRef] [PubMed]
- Cheah, K.Y.; Bastian, S.; Acott, T.M.V.; Abimosleh, S.M.; Lymn, K.A.; Howarth, G.S. Grape Seed Extract Reduces the Severity of Selected Disease Markers in the Proximal Colon of Dextran Sulphate Sodium-Induced Colitis in Rats. Am. J. Dig. Dis. 2013, 58, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.; Portillo, M.; Martínez, J.; Milagro, F. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, srep06234. [Google Scholar] [CrossRef]
- Kaspar, K.L.; Park, J.S.; Brown, C.R.; Mathison, B.D.; Navarre, D.A.; Chew, B.P. Pigmented Potato Consumption Alters Oxidative Stress and Inflammatory Damage in Men. J. Nutr. 2011, 141, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Grar, H.; Dib, W.; Gourine, H.; Negaoui, H.; Taleb, B.H.F.; Louaar, A.; Ouldhocine, S.; Kaddouri, H.; Kheroua, O.; Saidi, D. beta-Carotene improves intestinal barrier function by modulating proinflammatory cytokines and improving antioxidant capacity in beta-lactoglobulin-sensitized mice. J. Biol. Regul. Homeost. Agents 2020, 34, 1689–1697. [Google Scholar]
- Abdelhamid, L.; Luo, X.M. Retinoic Acid, Leaky Gut, and Autoimmune Diseases. Nutrients 2018, 10, 1016. [Google Scholar] [CrossRef]
- Sinisgalli, C.; Vezza, T.; Diez-Echave, P.; Ostuni, A.; Faraone, I.; Hidalgo-Garcia, L.; Russo, D.; Armentano, M.F.; Garrido-Mesa, J.; Rodriguez-Cabezas, M.E.; et al. The Beneficial Effects of Red Sun-Dried Capsicum annuum L. Cv Senise Extract with Antioxidant Properties in Experimental Obesity are Associated with Modulation of the Intestinal Microbiota. Mol. Nutr. Food Res. 2021, 65, e2000812. [Google Scholar] [CrossRef]
- Rajput, S.A.; Liang, S.J.; Wang, X.Q.; Yan, H.C. Lycopene Protects Intestinal Epithelium from Deoxynivalenol-Induced Oxidative Damage via Regulating Keap1/Nrf2 Signaling. Antioxidants 2021, 10, 1493. [Google Scholar] [CrossRef]
- Liu, K.Y.; Nakatsu, C.H.; Jones-Hall, Y.; Kozik, A.; Jiang, Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic. Biol. Med. 2021, 163, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Philippe, D.; Brahmbhatt, V.; Foata, F.; Saudan, Y.; Serrant, P.; Blum, S.; Benyacoub, J.; Vidal, K. Anti-inflammatory effects of Lacto-Wolfberry in a mouse model of experimental colitis. World J. Gastroenterol. 2012, 18, 5351–5359. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular Analysis of Commensal Host-Microbial Relationships in the Intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Stappenbeck, T.S.; Hooper, L.V.; Gordon, J.I. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc. Natl. Acad. Sci. USA 2002, 99, 15451–15455. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Hirano, A.; Umeno, J.; Okamoto, Y.; Shibata, H.; Ogura, Y.; Moriyama, T.; Torisu, T.; Fujioka, S.; Fuyuno, Y.; Kawarabayasi, Y.; et al. Comparison of the microbial community structure between inflamed and non-inflamed sites in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2018, 33, 1590–1597. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Takeshita, K.; Mizuno, S.; Mikami, Y.; Sujino, T.; Saigusa, K.; Matsuoka, K.; Naganuma, M.; Sato, T.; Takada, T.; Tsuji, H.; et al. A Single Species of Clostridium Subcluster XIVa Decreased in Ulcerative Colitis Patients. Inflamm. Bowel Dis. 2016, 22, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Kabeerdoss, J.; Sankaran, V.; Pugazhendhi, S.; Ramakrishna, B. Clostridium leptum group bacteria abundance and diversity in the fecal microbiota of patients with inflammatory bowel disease: A case–control study in India. BMC Gastroenterol. 2013, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Comalada, M.; Bailón, E.; De Haro, O.; Lara-Villoslada, F.; Xaus, J.; Zarzuelo, A.; Galvez, J. The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. J. Cancer Res. Clin. Oncol. 2006, 132, 487–497. [Google Scholar] [CrossRef]
- Klepinina, L.; Klepinin, A.; Truu, L.; Chekulayev, V.; Vija, H.; Kuus, K.; Teino, I.; Pook, M.; Maimets, T.; Kaambre, T. Colon cancer cell differentiation by sodium butyrate modulates metabolic plasticity of Caco-2 cells via alteration of phosphotransfer network. PLoS ONE 2021, 16, e0245348. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Li, T.; Li, M.; Huang, S.; Qiu, Y.; Feng, R.; Zhang, S.; Chen, M.; Xiong, L.; Zeng, Z. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Sun, J.; Chen, P.; Lakshman, S.; Molokin, A.; Harnly, J.M.; Vinyard, B.T.; Urban, J.F., Jr.; Davis, C.D.; Solano-Aguilar, G. Flavanol-Enriched Cocoa Powder Alters the Intestinal Microbiota, Tissue and Fluid Metabolite Profiles, and Intestinal Gene Expression in Pigs. J. Nutr. 2016, 146, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-N.; Guan, L.; Jiang, Y.-X.; Ma, S.-H.; Sun, Y.-N.; Lei, H.-T.; Yang, W.-F.; Wang, Q.-F. Microbiome and metabonomics study of quercetin for the treatment of atherosclerosis. Cardiovasc. Diagn. Ther. 2019, 9, 545–560. [Google Scholar] [CrossRef]
- Shi, T.; Bian, X.; Yao, Z.; Wang, Y.; Gao, W.; Guo, C. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. 2020, 11, 8003–8013. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Zheng, P.; Luo, Y.; Huang, Z.; Luo, J.; Mao, X.; Yu, J.; He, J. Changes of porcine gut microbiota in response to dietary chlorogenic acid supplementation. Appl. Microbiol. Biotechnol. 2019, 103, 8157–8168. [Google Scholar] [CrossRef]
- Xie, M.G.; Fei, Y.Q.; Wang, Y.; Wang, W.Y.; Wang, Z. Chlorogenic Acid Alleviates Colon Mucosal Damage Induced by a High-Fat Diet via Gut Microflora Adjustment to Increase Short-Chain Fatty Acid Accumulation in Rats. Oxidative Med. Cell. Longev. 2021, 2021, 18. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, B.; Zhao, X.; Lin, Y.; Wang, J.; Wang, X.; Hu, N.; Wang, S. Chlorogenic acid supplementation ameliorates hyperuricemia, relieves renal inflammation, and modulates intestinal homeostasis. Food Funct. 2021, 12, 5637–5649. [Google Scholar] [CrossRef]
- Miene, C.; Weise, A.; Glei, M. Impact of Polyphenol Metabolites Produced by Colonic Microbiota on Expression of COX-2 and GSTT2 in Human Colon Cells (LT97). Nutr. Cancer 2011, 63, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Wu, Z.; Weng, P. The Modulatory Effect of Anthocyanins from Purple Sweet Potato on Human Intestinal Microbiota in Vitro. J. Agric. Food Chem. 2016, 64, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- Reddivari, L.; Kim, S.W.; Radhakrishnan, S.; Yang, P.; Knight, R.; Vanamala, J. Purple-fleshed potatoes suppress colon-systemic oxidative stress/inflammatory markers via alternations in the gut bacterial signature. FASEB J. 2013, 27, 1056.9. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Lampakis, D.; Petrotos, K.; Giavasis, I. The Effect of Encapsulated Powder of Goji Berry (Lycium barbarum) on Growth and Survival of Probiotic Bacteria. Microorganisms 2020, 8, 57. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Dong, W.; Huang, K.; Ran, L.; Mi, J.; Lu, L.; Zeng, X.; Cao, Y. Effects of long-term intake of anthocyanins from Lycium ruthenicum Murray on the organism health and gut microbiota in vivo. Food Res. Int. 2020, 130, 108952. [Google Scholar] [CrossRef]
- Tian, B.M.; Zhao, J.H.; Zhang, M.; Chen, Z.F.; Ma, Q.Y.; Liu, H.C.; Nie, C.X.; Zhang, Z.Q.; An, W.; Li, J.X. Lycium ruthenicum Anthocyanins Attenuate High-Fat Diet-Induced Colonic Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Mol. Nutr. Food Res. 2021, 65, e2000745. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, G.; Zhang, S.; Ross, C.F.; Zhu, M.-J. Goji Berry Modulates Gut Microbiota and Alleviates Colitis in IL-10-Deficient Mice. Mol. Nutr. Food Res. 2018, 62, e1800535. [Google Scholar] [CrossRef]
- Wiese, M.; Bashmakov, Y.; Chalyk, N.; Nielsen, D.S.; Krych, L.; Kot, W.; Klochkov, V.; Pristensky, D.; Bandaletova, T.; Chernyshova, M.; et al. Prebiotic Effect of Lycopene and Dark Chocolate on Gut Microbiome with Systemic Changes in Liver Metabolism, Skeletal Muscles and Skin in Moderately Obese Persons. BioMed Res. Int. 2019, 2019, 4625279. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut–Brain Axis Balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef]
- Zhu, L.; Song, Y.; Liu, H.; Wu, M.; Gong, H.; Lan, H.; Zheng, X. Gut microbiota regulation and anti-inflammatory effect of beta-carotene in dextran sulfate sodium-stimulated ulcerative colitis in rats. J. Food Sci. 2021, 86, 2118–2130. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, L.; Hong, P.; Lang, W.; Hui, J.; Yang, Y.; Zheng, X. beta-Carotene prevents weaning-induced intestinal inflammation by modulating gut microbiota in piglets. Anim. Biosci. 2021, 34, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Otten, A.T.; Bourgonje, A.R.; Peters, V.; Alizadeh, B.Z.; Dijkstra, G.; Harmsen, H.J.M. Vitamin C supplementation in healthy individuals leads to shifts of bacterial ppulations in the gut-a pilot study. Antioxidants 2021, 10, 1278. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Dong, W.; Liu, W.; Yan, Y.; Wan, P.; Peng, Y.; Xu, Y.; Zeng, X.; Cao, Y. 2-O-β-d-Glucopyranosyl-l-ascorbic Acid, an Ascorbic Acid Derivative Isolated from the Fruits of Lycium Barbarum L., Modulates Gut Microbiota and Palliates Colitis in Dextran Sodium Sulfate-Induced Colitis in Mice. J. Agric. Food Chem. 2019, 67, 11408–11419. [Google Scholar] [CrossRef]
- Liu, F.; Cottrell, J.J.; Furness, J.B.; Rivera, L.R.; Kelly, F.W.; Wijesiriwardana, U.; Pustovit, R.V.; Fothergill, L.J.; Bravo, D.M.; Celi, P.; et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 2016, 101, 801. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarre, D.A.; Zhu, M.; Hellmann, H. Plant Antioxidants Affect Human and Gut Health, and Their Biosynthesis Is Influenced by Environment and Reactive Oxygen Species. Oxygen 2022, 2, 348-370. https://doi.org/10.3390/oxygen2030025
Navarre DA, Zhu M, Hellmann H. Plant Antioxidants Affect Human and Gut Health, and Their Biosynthesis Is Influenced by Environment and Reactive Oxygen Species. Oxygen. 2022; 2(3):348-370. https://doi.org/10.3390/oxygen2030025
Chicago/Turabian StyleNavarre, Duroy A., Meijun Zhu, and Hanjo Hellmann. 2022. "Plant Antioxidants Affect Human and Gut Health, and Their Biosynthesis Is Influenced by Environment and Reactive Oxygen Species" Oxygen 2, no. 3: 348-370. https://doi.org/10.3390/oxygen2030025
APA StyleNavarre, D. A., Zhu, M., & Hellmann, H. (2022). Plant Antioxidants Affect Human and Gut Health, and Their Biosynthesis Is Influenced by Environment and Reactive Oxygen Species. Oxygen, 2(3), 348-370. https://doi.org/10.3390/oxygen2030025






