Environmental Impact Assessment of Anti-Corrosion Coating Life Cycle Processes for Marine Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Life Cycle Assessment (LCA) Methodology
2.1.2. Life Cycle Inventory (LCI) for Production of Carbon Steel (System 1) and Steel Substrate Coated with Anti-Corrosion Coating (System 2)
3. Results and Discussion
3.1. LCIA
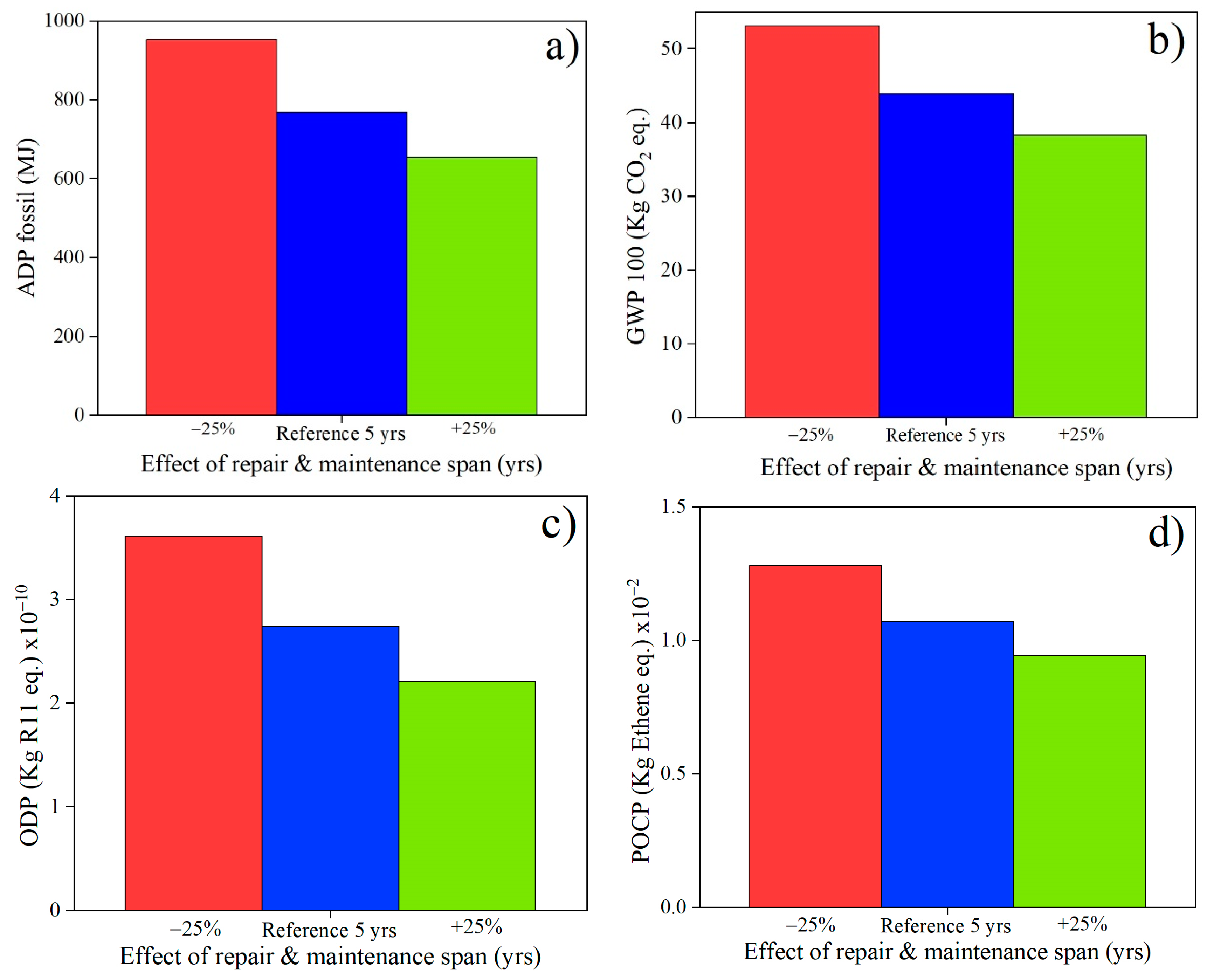
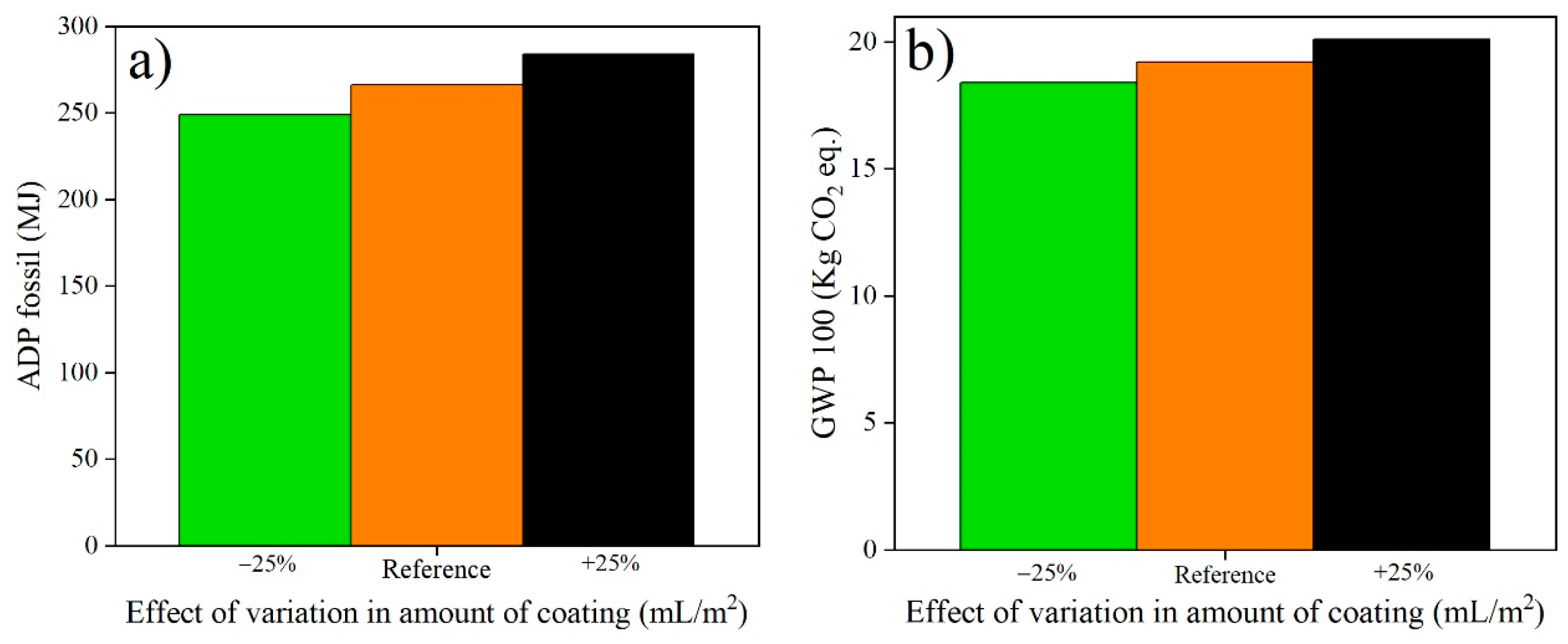
3.2. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LCA | Life cycle assessment | EP | Eutrophication |
| LCIA | Life cycle impact assessment | FAETP | Freshwater aquatic ecotoxicity |
| LCC | Life cycle cost | GWP | Global warming (100) |
| LCI | Life cycle inventory | HTP | Human toxicity |
| CML | Centrum voor Milieukunde Leiden | MAETP | Marine aquatic ecotoxicity |
| ADE | Abiotic depletion (elements) | ODP | Ozone depletion |
| ADF | Abiotic depletion (fossil) | POCP | Photochemical oxidation |
| AP | Acidification | TETP | Terrestrial ecotoxicity |
| GPTMS | 3-Glycidyloxypropyltrimethoxysilane | TTIP | Titanium isopropoxide |
| GHG | Greenhouse gas |
References
- Wei, Y.; Hongtao, L.; Wei, Z. Preparation of anti-corrosion superhydrophobic coatings by an Fe-based micro/nano composite electro-brush plating and blackening process. RSC Adv. 2015, 5, 103000–103012. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Frankel, G.S. The carbon footprint of steel corrosion. NPJ Mater. Degrad. 2022, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Popoola, L.T.; Grema, A.S.; Latinwo, G.K.; Gutti, B.; Balogun, A.S. Corrosion problems during oil and gas production and its mitigation. Int. J. Ind. Chem. 2013, 4, 35. [Google Scholar] [CrossRef]
- Momber, A.W.; Plagemann, P.; Stenzel, V. Performance and integrity of protective coating systems for offshore wind power structures after three years under offshore site conditions. Renew. Energy 2015, 74, 606–617. [Google Scholar] [CrossRef]
- Momber, A.W.; Marquardt, T. Protective coatings for offshore wind energy devices (OWEAs): A review. J. Coat. Technol. Res. 2018, 15, 13–40. [Google Scholar] [CrossRef]
- Cheng, J.; Feng, Y.; Yan, C.; Hu, X.; Li, R.; Liang, X. Development and characterization of Al-based amorphous coating. JOM 2020, 72, 745–753. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, B.; Liu, Q.; Liang, X. In-situ synthesis of novel Al-Fe-Si metallic glass coating by arc spraying. J. Alloys Compd. 2017, 716, 88–95. [Google Scholar] [CrossRef]
- Qian, H.; Xu, D.; Du, C.; Zhang, D.; Li, X.; Huang, L.; Deng, L.; Tu, Y.; Mol, J.M.C.; Terryn, H.A. Dual-action smart coatings with a self-healing superhydrophobic surface and anti-corrosion properties. J. Mater. Chem. A 2017, 5, 2355–2364. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Zhang, W.; Yu, X.; Lin, L.; Guo, X.; Liu, M.; Jiang, L. Corrosion-resistant superhydrophobic coatings on Mg alloy surfaces inspired by lotus seedpod. Adv. Funct. Mater. 2017, 27, 1605446. [Google Scholar] [CrossRef]
- Xiang, T.; Han, Y.; Guo, Z.; Wang, R.; Zheng, S.; Li, S.; Li, C.; Dai, X. Fabrication of Inherent Anticorrosion Superhydrophobic Surfaces on Metals. ACS Sustain. Chem. Eng. 2018, 6, 5598–5606. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, H.; Zhong, M. Triple-Scale Superhydrophobic Surface with excellent anti-icing and icephobic performance via ultrafast laser hybrid fabrication. ACS Appl. Mater. Interfaces 2020, 13, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Y.; Jiang, Z.; Li, Y.; Wen, C.; Zhang, D.; Lian, J.; Zhang, Z. Improvement of corrosion resistance of H59 brass through fabricating superhydrophobic surface using laser ablation and heating treatment. Corros. Sci. 2021, 180, 109186. [Google Scholar] [CrossRef]
- Guo, F.; Duan, S.; Wu, D.; Matsuda, K.; Wang, T.; Zou, Y. Facile etching fabrication of superhydrophobic 7055 aluminum alloy surface towards chloride environment anticorrosion. Corros. Sci. 2021, 182, 109262. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Dehghani, K.; Rezaei, M.; Mahidashti, Z. Effect of stearic acid as a low cost and green material on the self-cleaning and anti-corrosion behavior of anodized titanium. Colloids Surf. A 2019, 583, 123971. [Google Scholar] [CrossRef]
- Khan, S.A.; Boltaev, G.S.; Iqbal, M.; Kim, V.; Ganeev, R.A.; Alnaser, A.S. Ultrafast fiber laser-induced fabrication of superhydrophobic and self-cleaning metal surfaces. Appl. Surf. Sci. 2021, 542, 148560. [Google Scholar] [CrossRef]
- Kim, W.; Eun, J.; Jeon, S. Anti-splashing properties of sticky superhydrophobic surfaces. Appl. Surf. Sci. 2021, 542, 148617. [Google Scholar] [CrossRef]
- Pakzad, H.; Liravi, M.; Moosavi, A.; Nouri-Borujerdi, A.; Najafkhani, H. Fabrication of durable superhydrophobic surfaces using PDMS and beeswax for drag reduction of internal turbulent flow. Appl. Surf. Sci. 2020, 513, 145754. [Google Scholar] [CrossRef]
- Zhou, Y.; Rossi, B.; Zhou, Q.; Hihara, L.H.; Foster, M. Thin Plasma-Polymerized Coatings as a Primer with Polyurethane Topcoat for Improved Corrosion Resistance. Langmuir 2020, 36, 837–843. [Google Scholar] [CrossRef]
- Song, G.L.; Feng, Z. Modification, degradation and evaluation of a few organic coatings for some marine applications. Corros. Mater. Degrad. 2020, 1, 408–442. [Google Scholar] [CrossRef]
- Pathak, S.S.; Khanna, A.S. Sol–gel nanocoatings for corrosion protection. In Corrosion Protection and Control Using Nanomaterials; Saji, V.S., Cook, R., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 304–329. [Google Scholar]
- Ulaeto, S.B.; Ravi, R.P.; Udoh, I.I.; Mathew, G.M.; Rajan, T.P. Polymer-Based Coating for Steel Protection, Highlighting Metal–Organic Framework as Functional Actives: A Review. Corros. Mater. Degrad. 2023, 4, 284–316. [Google Scholar] [CrossRef]
- Nanvaee, A.A.; Yahya, R.; Gan, S.N. Cleaner production through using by-product palm stearin to synthesis alkyd resin for coating applications. J. Clean. Prod. 2013, 54, 307–314. [Google Scholar] [CrossRef]
- Denis, M.; Paré, A.; Le Borgne, D.; Caillol, S.; Negrell, C. Hydroxyurethane modifiers (HUM): An environmentally friendly route to improve chemical resistance of alkyd coatings. Prog. Org. Coat. 2023, 183, 107734. [Google Scholar] [CrossRef]
- Nguyen, T.T. Study on Manufacturing Environmentally Friendly Alkyd Paint. VNU J. Sci. Nat. Sci. Technol. 2021, 38, 45–60. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Laco, J.I.I.; Villota, F.C.; Mestres, F.L. Corrosion protection of carbon steel with thermoplastic coatings and alkyd resins containing polyaniline as conductive polymer. Prog. Org. Coat. 2005, 52, 151–160. [Google Scholar] [CrossRef]
- Kowalski, D.; Grzyl, B.; Kristowski, A. The cost analysis of corrosion protection solutions for steel components in terms of the object life cycle cost. Civ. Environ. Eng. Rep. 2017, 26, 5–13. [Google Scholar] [CrossRef]
- Ingham, R.B.W. Life Cycle Assessment of Marine Coatings; MDPI: Basel, Switzerland, 2022; pp. 1–25. [Google Scholar]
- Qian, Y.; Li, Y.; Jungwirth, S.; Seely, N.; Fang, Y.; Shi, X. The application of anti-corrosion coating for preserving the value of equipment asset in chloride-laden environments: A review. Int. J. Electrochem. Sci. 2015, 10, 10756–10780. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, K.; Feng, Z.; Li, C.; Xie, J.; Ma, J.; Zhang, X.; Wang, X.; Xu, K.; Li, C.; et al. Corrosion Behavior of Alkyd-Resin-Coated Carbon Steel under Cathodic Polarization in Both Static and Flowing Seawater. Coatings 2023, 13, 1296. [Google Scholar] [CrossRef]
- Seddik, N.B.; Chraka, A.; Zarki, Y.; Idriss, H.O.; Rami, S.; Janoub, F.; Raissouni, I.; Draoui, K.; Aghzzaf, A.A.; Bouchta, D. Reinforcement of an alkyd resin coating incorporating a swelling clay encapsulated with L-cysteine molecules: Characterization and corrosion inhibition of Cu-36Zn alloy. J. Alloys Compd. 2023, 960, 171052. [Google Scholar] [CrossRef]
- Suer, J.; Traverso, M.; Jäger, N. Review of life cycle assessments for steel and environmental analysis of future steel production scenarios. Sustainability 2022, 14, 14131. [Google Scholar] [CrossRef]
- Adsetts, J.R.; Ebrahimi, N.; Zhang, J.; Jalaei, F.; Noël, J.J. Steel Bridge-Coating Systems and Their Environmental Impacts: Current Practices and Future Trends. Coatings 2023, 13, 850. [Google Scholar] [CrossRef]
- Agustín-Sáenz, C.; Santa Coloma, P.; Fernández-Carretero, F.J.; Brusciotti, F.; Brizuela, M. Design of corrosion protective and antistatic hybrid sol-gel coatings on 6XXX AlMgSi alloys for aerospace application. Coatings 2020, 10, 441. [Google Scholar] [CrossRef]
- Oner, D.D.; McCarthy, T.J. Ultrahydrophobic Surfaces. Effects of Topography Length Scales on Wettability. Langmuir 2000, 16, 7777–7782. [Google Scholar] [CrossRef]
- Curran, M.A. Life Cycle Assessment Handbook: A Guide for Environmentally Sustainable Products; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 304–329. [Google Scholar] [CrossRef]
- PE International. GaBi Databases 2022 Editions—Upgrades and Improvements. 24 February 2022. Available online: https://gabi.sphera.com/international/support/gabi/gabi-2022-edition-upgrades-improvements/ (accessed on 6 June 2024).
- Guinée, J.B. Handbook on Life Cycle Assessment: Operational Guide to the ISO Standards; Springer Science & Business Media: Dordrecht, The Netherlands, 2002; Volume 7. [Google Scholar]
- Hauschild, M.Z.; Rosenbaum, R.K.; Olsen, S.I. Life Cycle Assessment; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Klöpffer, W.; Grahl, B. Life Cycle Assessment (LCA): A Guide to Best Practice; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- European Commission—Joint Research Centre—Institute for Environment and Sustainability. International Reference Life Cycle Data System (ILCD) Handbook—General Guide for Life Cycle Assessment—Detailed Guidance; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Chilkoor, G.; Upadhyayula, V.K.; Gadhamshetty, V.; Koratkar, N.; Tysklind, M. Sustainability of renewable fuel infrastructure: A screening LCA case study of anticorrosive graphene oxide epoxy liners in steel tanks for the storage of biodiesel and its blends. Environ. Sci. Process. Impacts 2017, 19, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.; Napolano, L.; Matteucci, G. Biotoxicity and life cycle assessment of two commercial antifouling coatings in marine systems. Chemosphere 2019, 237, 124475. [Google Scholar] [CrossRef]
- Eyres, D.J.; Bruce, G.J. Ship Construction, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2012; pp. 1–368. [Google Scholar]
- Papavinasam, S. Corrosion Control in the Oil and Gas Industry; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhang, H.; Sun, W.; Wang, L.; Wang, J.; Wang, S.; Liu, G. A mechanically and chemically stable superhydrophobic coating for preventing marine atmospheric corrosion. Surf. Interface Anal. 2021, 27, 101537. [Google Scholar] [CrossRef]
- Peng, S.; Li, T.; Shi, J.; Zhang, H. Simplified life cycle assessment and analysis of remanufacturing cleaning technologies. Procedia CIRP 2015, 29, 810–815. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Golden, R. What’s the Score? A Method for Quantitative Estimation of Energy Use and Emissions Reductions for UV/EB Curing. In Proceedings of the RADTECH USA Conference, Chicago, IL, USA, 30 April–2 May 2012; pp. 1–10. [Google Scholar]
- Usino, D. Life Cycle Assessment of the Use of Marine Biocides in Antifouling Paint. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2014. [Google Scholar]
- Moribe, T. Advanced intermeshing mixers for energy saving and reduction of environmental impact. Mitsubishi Heavy Ind. Tech. Rev. 2012, 49, 38–43. [Google Scholar]
- Norspray. RPR Method: Removal of Coating/Paints with Use of Induction. Available online: https://rprinduktion.se/wp-content/uploads/2016/04/RPR_Info_Brochure_English_version.pdf (accessed on 6 June 2024).
- López, C.; Peña, C.; Muñoz, E. Impact of the Secondary Steel Circular Economy Model on Resource Use and the Environmental Impact of Steel Production in Chile. In IOP Conference Series: Earth and Environmental Science, Proceedings of the IOP Conference, Temuco, Chile, 16–18 October 2019; IOP Publishing: Bristol, UK, 2020; Volume 503, p. 012024. [Google Scholar] [CrossRef]
- Prado, V.; Cinelli, M.; Ter Haar, S.F.; Ravikumar, D.; Heijungs, R.; Guinée, J.; Seager, T.P. Sensitivity to Weighting in Life Cycle Impact Assessment (LCIA). Int. J. Life Cycle Assess. 2020, 25, 2393–2406. [Google Scholar] [CrossRef]
- WSA. LCI Data for Steel Products; World Steel Association: Brussels, Belgium, 2016; pp. 1–8. Available online: https://www.worldsteel.org/publications/bookshop/product-details~LCI-data-for-steel-products~PRODUCT~LCI~.html (accessed on 6 June 2024).
- Lemesle, C.; Frémiot, J.; Beaugendre, A.; Casetta, M.; Bellayer, S.; Duquesne, S.; Schuller, A.S.; Jimenez, M. Life cycle assessment of multi-step versus one-step coating processes using oil or bio-based resins. J. Clean. Prod. 2020, 242, 118527. [Google Scholar] [CrossRef]
- Backes, J.G.; Suer, J.; Pauliks, N.; Neugebauer, S.; Traverso, M. Life cycle assessment of an integrated steel mill using primary manufacturing data: Actual environmental profile. Sustainability 2021, 13, 3443. [Google Scholar] [CrossRef]
- Gratsos, G.A.; Zachariadis, P. Life Cycle Cost of Maintaining the Effectiveness of a Ship’s Structure and Environmental Impact of Ship Design Parameters. In Proceedings of the RINA International Conference: Design and Operation of Bulk Carriers, London, UK, 15–17 June 2005; RINA: London, UK, 2005; pp. 95–122. [Google Scholar]
- Ho, C.K.; Pacheco, J.E. Derivation of a Levelized Cost of Coating (LCOC) metric for evaluation of solar selective absorber materials. Energy Procedia. 2015, 69, 415–423. [Google Scholar] [CrossRef]
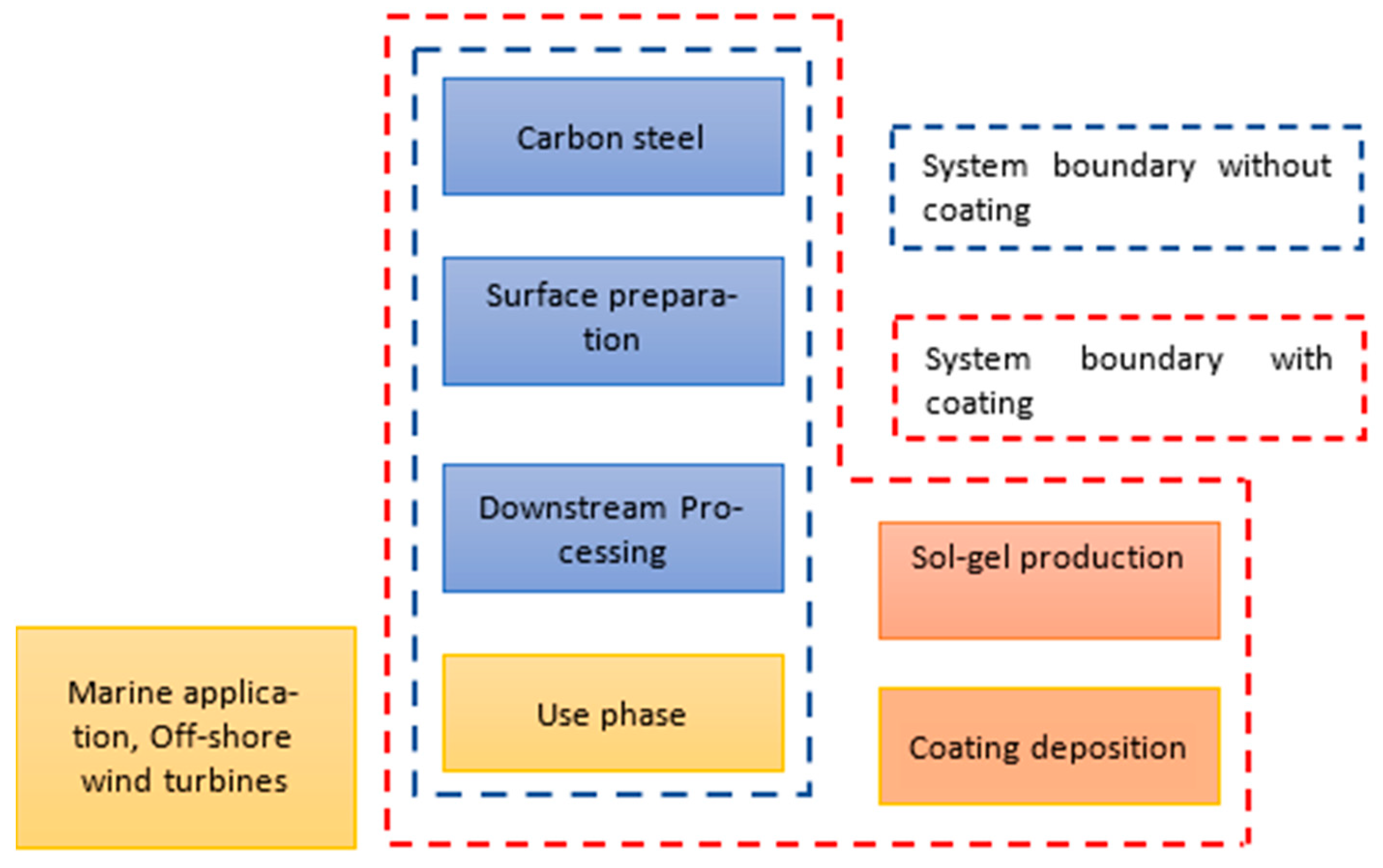



| Life Cycle Processes | Main Assumptions Made and Referred Data Sources |
|---|---|
| 1. Production of carbon steel substrate | LCI data for the production of carbon steel material were referenced from the GaBi database [38] |
| 2. Surface cleaning and preparation, shot blasting of cleaned surface | LCI data for the preparation of the surface (degreasing and cleaning) and the shot blasting process were referenced from the literature [47,48] |
| 3. Synthesis of alkyd coating | LCI data for the synthesis of the coating material were referenced from the literature [49] |
| 4. Application of coating by immersion (dipping process) | LCI data for the deposition of the coating material using the immersion method were referenced from the literature [50] |
| 5. Thermal curing for proper adhesion of coating | LCI data for thermal curing were referenced from an industrial process reported in the literature [50] |
| 6. Use of coated specimens under corrosive environments | The maximum life of the anti-corrosion coating was considered based on previously published literature [35,51] |
| System ID | Area of Steel | Mass (kg) |
|---|---|---|
| System 1 | 1 m2 | 15 |
| System 2 | 1 m2 | 15.2 |
| Materials | Quantities (kg) |
|---|---|
| n-Propanol | 0.286 |
| 3-Glycidyloxypropyltrimethoxysilane, (GPTMS, 98% purity) | 0.315 |
| 0.1 M H2SO4 in water (total) | 0.071 |
| Tetraethyl orthosilicate (TEOS, purity 98%) | 0.094 |
| Poly (bisphenol A-co-epichlorohydrin), glycidyl end-capped | 0.161 |
| Acetil acetone | 0.018 |
| Titanium isopropoxide | 0.051 |
| Electricity consumption | |
| Sand blasting of steel substrate | 3 kWh/m2 [52] |
| Stirring and Heating by ultrasound reactor (1 h at 40 °C) | 0.7 kWh/L [52] |
| Sol–gel deposition by dip coating | 0.44 kWh/m2 [52] |
| Curing | 0.126–0.1488 kWh/m2 [53] |
| Impact Category | Unit | System 1 | System 2 |
|---|---|---|---|
| ADE | kg Sb eq. | 0.001156 | 0.0004163 |
| ADF | MJ | 792 | 766.85 |
| AP | kg SO2 eq. | 0.2584 | 0.15231 |
| EP | kg Phosphate eq. | 0.02164 | 0.011957 |
| FAETP | kg DCB eq. | 0.3568 | 0.2024 |
| GHG | kg CO2 eq. | 64.4 | 43.896 |
| HTP | kg DCB eq. | 12.24 | 4.212 |
| MAETP | kg DCB eq. | 7440 | 3511 |
| ODP | kg R11 eq. | 8.12 × 10−11 | 2.74 × 10−10 |
| PCOP | kg Ethene eq. | 0.0172 | 0.010691 |
| TETP | kg DCB eq. | 0.2624 | 0.11458 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgaonkar, A.; McNamara, G. Environmental Impact Assessment of Anti-Corrosion Coating Life Cycle Processes for Marine Applications. Sustainability 2024, 16, 5627. https://doi.org/10.3390/su16135627
Borgaonkar A, McNamara G. Environmental Impact Assessment of Anti-Corrosion Coating Life Cycle Processes for Marine Applications. Sustainability. 2024; 16(13):5627. https://doi.org/10.3390/su16135627
Chicago/Turabian StyleBorgaonkar, Avinash, and Greg McNamara. 2024. "Environmental Impact Assessment of Anti-Corrosion Coating Life Cycle Processes for Marine Applications" Sustainability 16, no. 13: 5627. https://doi.org/10.3390/su16135627
APA StyleBorgaonkar, A., & McNamara, G. (2024). Environmental Impact Assessment of Anti-Corrosion Coating Life Cycle Processes for Marine Applications. Sustainability, 16(13), 5627. https://doi.org/10.3390/su16135627







