Versatile and Automated 3D Polydimethylsiloxane (PDMS) Patterning for Large-Scale Fabrication of Organ-on-Chip (OOC) Components †
Abstract
:1. Introduction
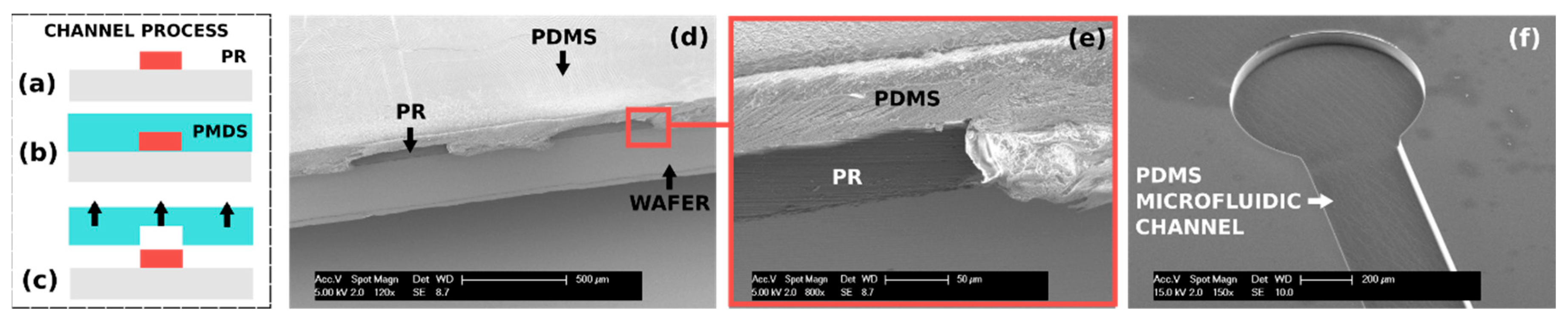
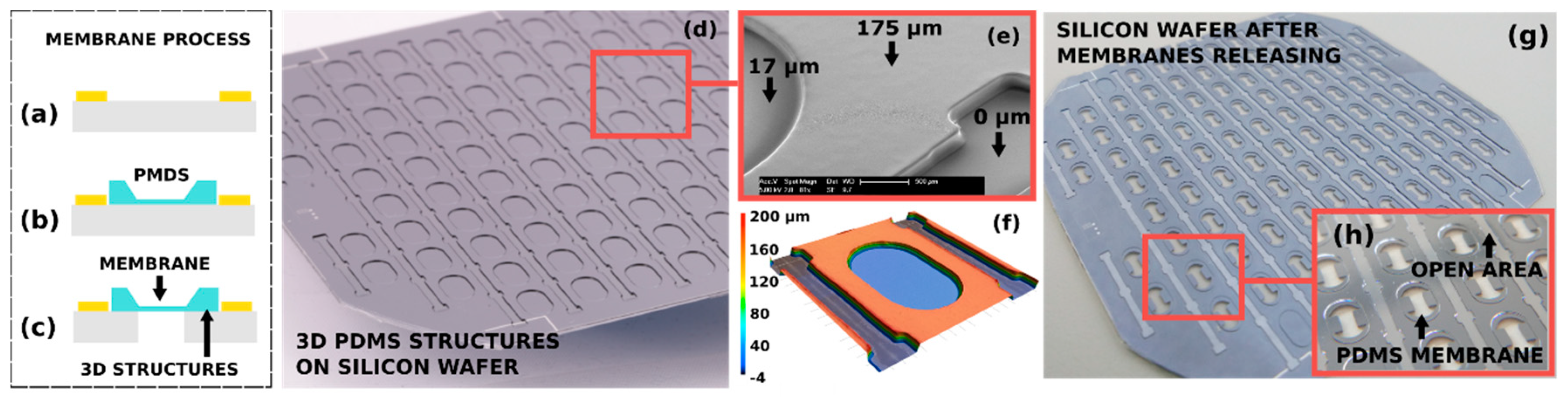
2. Automated 3D Molding on Silicon Wafers
3. Fabrication of Organ-on-Chip Components
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Beißner, N.; Lorenz, T.; Reichl, S. Organ on chip. In Microsystems for Pharmatechnology; Springer: Cham, Switzerland, 2016; pp. 299–339. [Google Scholar]
- Van de Stolpe, A.; den Toonder, J. Workshop meeting report Organs-on-Chips: Human disease models. Lab Chip 2013, 13, 3449–3470. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Gaio, N.; van Meer, B.; Quirós Solano, W.; Bergers, L.; van de Stolpe, A.; Mummery, C.; Sarro, P.M.; Dekker, R. Cytostretch, an organ-on-chip platform. Micromachines 2016, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Kim, H.J.; Fraser, J.P.; Shea, D.E.; Khan, M.; Bahinski, A.; Hamilton, G.A.; Ingber, D.E. Microfabrication of human organs-on-chips. Nat. Protoc. 2013, 8, 2135–2157. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaio, N.; Kersjes, S.; Solano, W.Q.; Sarro, P.; Dekker, R. Versatile and Automated 3D Polydimethylsiloxane (PDMS) Patterning for Large-Scale Fabrication of Organ-on-Chip (OOC) Components. Proceedings 2018, 2, 873. https://doi.org/10.3390/proceedings2130873
Gaio N, Kersjes S, Solano WQ, Sarro P, Dekker R. Versatile and Automated 3D Polydimethylsiloxane (PDMS) Patterning for Large-Scale Fabrication of Organ-on-Chip (OOC) Components. Proceedings. 2018; 2(13):873. https://doi.org/10.3390/proceedings2130873
Chicago/Turabian StyleGaio, Nikolas, Sebastiaan Kersjes, William Quiros Solano, Pasqualina Sarro, and Ronald Dekker. 2018. "Versatile and Automated 3D Polydimethylsiloxane (PDMS) Patterning for Large-Scale Fabrication of Organ-on-Chip (OOC) Components" Proceedings 2, no. 13: 873. https://doi.org/10.3390/proceedings2130873
APA StyleGaio, N., Kersjes, S., Solano, W. Q., Sarro, P., & Dekker, R. (2018). Versatile and Automated 3D Polydimethylsiloxane (PDMS) Patterning for Large-Scale Fabrication of Organ-on-Chip (OOC) Components. Proceedings, 2(13), 873. https://doi.org/10.3390/proceedings2130873



