Comparing the Effects of Different Unsaturated Fatty Acids on Fermentation Performance of Saccharomyces cerevisiae and Aroma Compounds during Red Wine Fermentation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yeast Growth and Fermentation Profiles
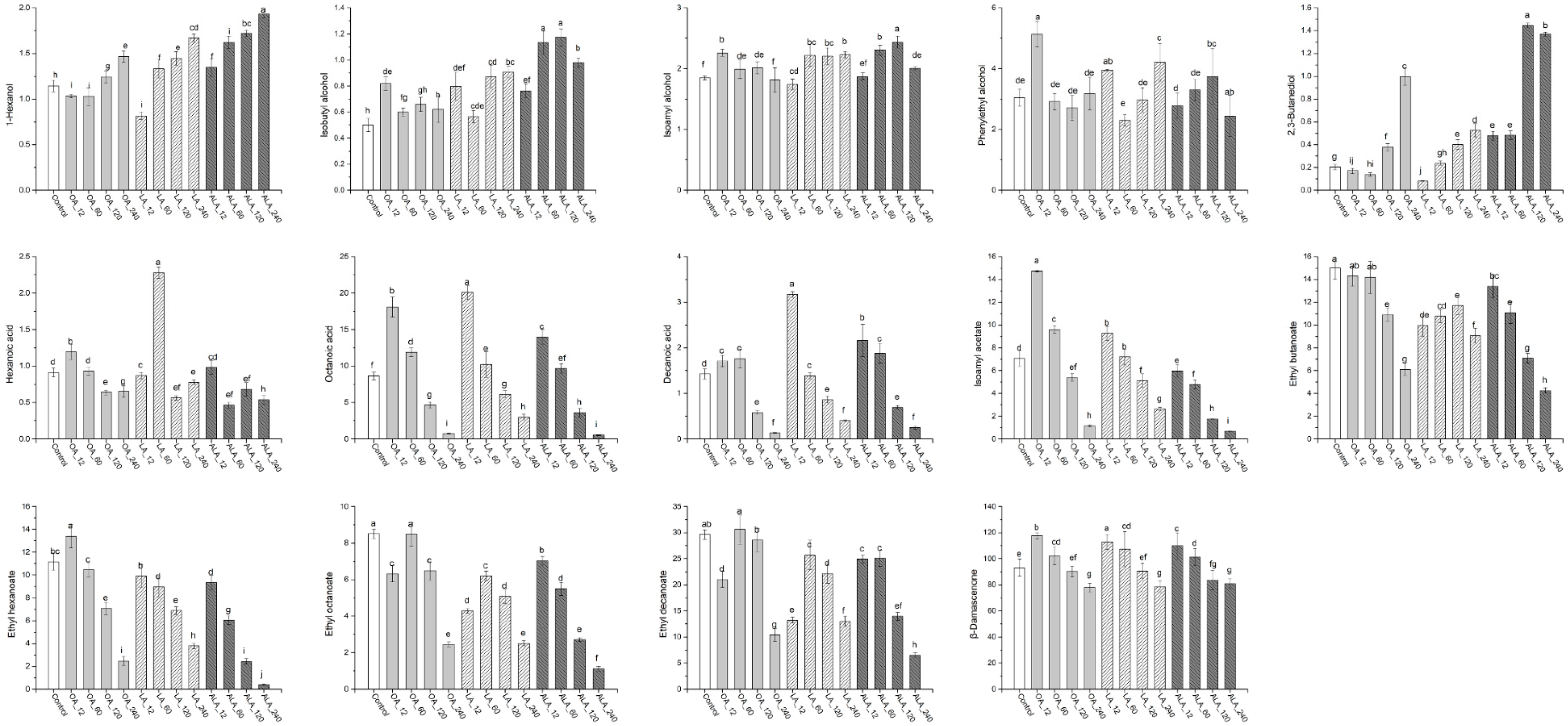
2.2. Volatile Compounds in the Wines
2.2.1. C6 Alcohols and Higher Alcohols
2.2.2. Medium-Chain Fatty Acids
2.2.3. Esters
2.2.4. Norisoprenoids and Terpenes
2.3. Expression of Genes Related to Volatile Compounds Formation
3. Materials and Methods
3.1. Yeast Strain and Culture Medium
3.2. Fermentation Conditions and Sampling
3.3. Analysis of Basic Wine Compounds
3.4. Analysis of Fatty Acids and Volatile Compounds
3.5. RNA Extraction and Real-Time qPCR Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Z.; Ough, C.S. Amino acid profiles of commercial grape juices and wines. Am. J. Enol. Viticult. 1991, 42, 261–267. [Google Scholar]
- Mato, I.; Suarez-Luque, S.; Huidobro, J.F. Simple determination of main organic acids in grape juice and wine by using capillary zone electrophoresis with direct UV detection. Food Chem. 2007, 102, 104–112. [Google Scholar] [CrossRef]
- Bell, S.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Deed, N.K.; Van Vuuren, H.J.J.; Gardner, R.C. Effects of nitrogen catabolite repression and di-ammonium phosphate addition during wine fermentation by a commercial strain of S. cerevisiae. Appl. Microbiol. Biot. 2011, 89, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microb. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Calderbank, J.; Keenan, M.H.; Rose, A.H. Plasma-membrane phospholipid unsaturation affects expression of the general amino-acid permease in Saccharomyces cerevisiae Y185. J. Gen. Microbiol. 1985, 131, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Trotter, P.J. The genetics of fatty acid metabolism in Saccharomyces cerevisiae. Annu. Rev. Genet. 2001, 21, 97–119. [Google Scholar]
- Gallander, J.F.; Peng, A.C. Lipid and fatty acid compositions of different grape types. Am. J. Enol. Viticult. 1980, 31, 24–27. [Google Scholar]
- Ancín, C.; Ayestarán, B.; García, A.; Garrido, J. Evolution of fatty acid contents in garnacha and viura musts during fermentation and the aging of wine. Zeitschrift für Lebensmitteluntersuchung und Forschung A 1998, 206, 143–147. [Google Scholar] [CrossRef]
- Yunoki, K.; Yasui, Y.; Hirose, S.; Ohnishi, M. Fatty acids in must prepared from 11 grapes grown in Japan: Comparison with wine and effect on fatty acid ethyl ester formation. Lipids 2005, 40, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Valero, E.; Millán, C.; Ortega, J.M. Influence of pre-fermentative treatment on the fatty acid content of Saccharomyces cerevisiae (M330-9) during alcoholic fermentation of grape must. J. Biosci. Bioeng. 2001, 91, 117–122. [Google Scholar] [CrossRef]
- Casu, F.; Pinu, F.R.; Fedrizzi, B.; Greenwood, D.R.; Villas-Boas, S.G. The effect of linoleic acid on the Sauvignon Blanc fermentation by different wine yeast strains. FEMS Yeast Res. 2016, 16, fow050. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.L.; Shi, Y.; Jiang, R.; Yang, Q.; Wang, Y.Q.; Liu, P.T.; Duan, C.Q.; Yan, G.L. Effects of adding unsaturated fatty acids on fatty acid composition of Saccharomyces cerevisiae and major volatile compounds in wine. S. Afr. J. Enol. Vitic. 2015, 36, 285–295. [Google Scholar] [CrossRef]
- Tumanov, S.; Pinu, F.R.; Greenwood, D.R.; Villas-Boas, S.G. Effect of free fatty acids and lipolysis on Sauvignon Blanc fermentation. Aust. J. Grape Wine Res. 2018, 24, 398–405. [Google Scholar] [CrossRef]
- Mauricio, J.C.; Moreno, J.; Zea, L.; Ortega, J.M.; Medina, M. The effects of grape must fermentation conditions on volatile alcohols and esters formed by Saccharomyces cerevisiae. J. Sci. Food Agric. 1997, 75, 155–160. [Google Scholar] [CrossRef]
- Thurston, P.A.; Quain, D.E.; Tubb, R.S. Lipid metabolism and the regulation of volatile ester synthesis in Saccharomyces cerevisiae. J. Inst. Brew. 1982, 88, 90–94. [Google Scholar] [CrossRef]
- Varela, C.; Torrea, D.; Schmidt, S.A.; Ancin-Azpilicueta, C.; Henschke, P.A. Effect of oxygen and lipid supplementation on the volatile composition of chemically defined medium and Chardonnay wine fermented with Saccharomyces cerevisiae. Food Chem. 2012, 135, 2863–2871. [Google Scholar] [CrossRef]
- Rosa, M.F.; Sá-Correia, I. Ethanol tolerance and activity of plasma membrane ATPase in Kluyveromyces marxianus and Saccharomyces cerevisiae. Enzyme Microb. Technol. 1992, 14, 23–27. [Google Scholar] [CrossRef]
- Redón, M.; Guillamón, J.M.; Mas, A.; Rozèe, N. Effect of lipid supplementation upon Saccharomyces cerevisiae lipid composition and fermentation performance at low temperature. Eur. Food Res. Technol. 2009, 228, 833–840. [Google Scholar] [CrossRef]
- Mejía-Barajas, J.; Montoya-Pérez, R.; Manzo-Avalos, S.; Cortés-Rojo, C.; Riveros-Rosas, H.; Cervantes, C.; Saavedra-Molina, A. Fatty acid addition and thermotolerance of Kluyveromyces marxianus. FEMS Microbiol. Lett. 2018, 365, fny043. [Google Scholar] [CrossRef] [PubMed]
- Redón, M.; Guillamón, J.M.; Mas, A.; Rozèe, N. Effect of growth temperature on yeast lipid composition and alcoholic fermentation at low temperature. Eur. Food Res. Technol. 2011, 232, 517–527. [Google Scholar] [CrossRef]
- Forde, C.G.; Cox, A.; Williams, E.R.; Boss, P.K. Associations between the sensory attributes and volatile composition of Cabernet Sauvignon wines and the volatile composition of the grapes used for their production. J. Agric. Food Chem. 2011, 59, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine Science: Principles and Applications, 3rd ed.; Elsevier-Academic Press: Oxford, UK, 2008; p. 310. [Google Scholar]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 2017, 41, S95–S128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, A.B.; Dufour, J. Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast 2000, 16, 1287–1298. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Jin, G.; Mei, W.; Li, T.; Tao, Y. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Verstrepen, K.J.; van Laere, S.D.M.; Voet, A.R.D.; van Dijck, P.; Delvaux, F.R.; Thevelein, J.M. The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J. Biol. Chem. 2006, 281, 4446–4456. [Google Scholar] [CrossRef]
- Barbosa, C.; Mendes-Faia, A.; Lage, P.; Mira, N.P.; Mendes-Ferreira, A. Genomic expression program of Saccharomyces cerevisiae along a mixed-culture wine fermentation with Hanseniaspora guilliermondii. Microb. Cell Fact. 2015, 14, 124. [Google Scholar] [CrossRef]
- Rossouw, D.; Naes, T.; Bauer, F.F. Linking gene regulation and the exo-metabolome: A comparative transcriptomics approach to identify genes that impact on the production of volatile aroma compounds in yeast. BMC Genom. 2008, 9, 530–548. [Google Scholar] [CrossRef]
- Furukawa, K.; Yamada, T.; Mizoguchi, H.; Hara, S. Increased ethyl caproate producitong by inositol limitation in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2003, 95, 448–454. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Pretorius, I.S.; Thevelein, J.M.; Delvaux, F.R. The Saccharomyces cerevisiae alcohol acetyl transferase gene ATF1 is a target of the cAMP/PKA and FGM nutrient-signalling pathways. FEMS Yeast Res. 2003, 4, 285–296. [Google Scholar] [CrossRef]
- Holt, S.; de Carvalho, B.T.; Foulquié-Moreno, M.R.; Thevelein, J.M. Polygenic analysis in absence of major effector ATF1 unveils novel components in yeast flavor ester biosynthesis. mBio 2018, 9, e01279-18. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Kobayashi, O.; Yoshimoto, H.; Furukawa, S.; Tamai, Y. Effect of aeration and unsaturated fatty acids on expression of the Saccharomyces ceresvisiae alcohol acetyltransgerase gene. Appl. Environ. Microb. 1997, 63, 910–915. [Google Scholar]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yu, K.; Li, Y.; Duan, C.; Yan, G. The content of linoleic acid in grape must influences the aromatic effect of branched-chain amino acids addition on red wine. Food Res. Int. 2018, 114, 214–222. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.; Wang, Y.; Lu, L.; Lan, Y.; Reeves, M.J.; Duan, C. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef]
- Lan, Y.B.; Qian, X.; Yang, Z.J.; Xiang, X.F.; Yang, W.X.; Liu, T.; Zhu, B.Q.; Pan, Q.H.; Duan, C.Q. Striking changes in volatile profiles at sub-zero temperatures during over-ripening of “Beibinghong” grapes in Northeastern China. Food Chem. 2016, 212, 172–182. [Google Scholar] [CrossRef]
- Ståhlberg, A.; Elbing, K.; Andrade-Garda, J.; Sjögreen, B.; Forootan, A.; Kubista, M. Multiway real-time PCR gene expression profiling in yeast Saccharomyces cerevisiae reveals altered transcriptional response of ADH-genes to glucose stimuli. BMC Genom. 2008, 9, 170. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |




| Time to Reach the End of Fermentation (h) | Maximum Biomass (g DCW·L−1) | Time to Reach Maximum Biomass (h) | Maximum Specific Growth Rate (h−1) | Maximum Fermentation Rate (g·L−1·h−1) | |
|---|---|---|---|---|---|
| Control | 250 | 2.15 ± 0.08 g | 84 | 0.008 ± 0.000 g | 2.77 |
| OA_12 | 108 | 2.96 ± 0.11 ab | 84 | 0.022 ± 0.001 b | 5.02 |
| OA_60 | 132 | 2.80 ± 0.24 bc | 84 | 0.015 ± 0.000 cd | 3.24 |
| OA_120 | 250 | 2.78 ± 0.10 bc | 72 | 0.009 ± 0.001 f | 3.73 |
| OA_240 | 444 | 2.34 ± 0.33 fg | 96 | 0.005 ± 0.000 h | 2.11 |
| LA_12 | 96 | 3.08 ± 0.11 ab | 60 | 0.023 ± 0.002 a | 7.97 |
| LA_60 | 132 | 3.00 ± 0.15 ab | 72 | 0.016 ± 0.001 c | 3.58 |
| LA_120 | 250 | 2.85 ± 0.14 abc | 72 | 0.01 ± 0.001 f | 3.49 |
| LA_240 | 444 | 2.53 ± 0.13 def | 108 | 0.005 ± 0.000 h | 2.6 |
| ALA_12 | 132 | 2.61 ± 0.18 cde | 84 | 0.015 ± 0.000 d | 3.71 |
| ALA_60 | 180 | 2.84 ± 0.05 abc | 84 | 0.014 ± 0.001 e | 3.35 |
| ALA_120 | 444 | 2.78 ± 0.14 bcd | 84 | 0.005 ± 0.000 h | 3.03 |
| ALA_240 | 444 | 2.39 ± 0.09 ef | 84 | 0.005 ± 0.000 h | 1.48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.-T.; Duan, C.-Q.; Yan, G.-L. Comparing the Effects of Different Unsaturated Fatty Acids on Fermentation Performance of Saccharomyces cerevisiae and Aroma Compounds during Red Wine Fermentation. Molecules 2019, 24, 538. https://doi.org/10.3390/molecules24030538
Liu P-T, Duan C-Q, Yan G-L. Comparing the Effects of Different Unsaturated Fatty Acids on Fermentation Performance of Saccharomyces cerevisiae and Aroma Compounds during Red Wine Fermentation. Molecules. 2019; 24(3):538. https://doi.org/10.3390/molecules24030538
Chicago/Turabian StyleLiu, Pei-Tong, Chang-Qing Duan, and Guo-Liang Yan. 2019. "Comparing the Effects of Different Unsaturated Fatty Acids on Fermentation Performance of Saccharomyces cerevisiae and Aroma Compounds during Red Wine Fermentation" Molecules 24, no. 3: 538. https://doi.org/10.3390/molecules24030538
APA StyleLiu, P.-T., Duan, C.-Q., & Yan, G.-L. (2019). Comparing the Effects of Different Unsaturated Fatty Acids on Fermentation Performance of Saccharomyces cerevisiae and Aroma Compounds during Red Wine Fermentation. Molecules, 24(3), 538. https://doi.org/10.3390/molecules24030538






