Microbial Beta Glucosidase Enzymes: Recent Advances in Biomass Conversation for Biofuels Application
Abstract
1. Introduction
2. Industrial Importance of β-Glucosidase in Biofuels
3. Classification of β-Glucosidase
4. Catalytic Mechanism of β-Glucosidase Enzyme
5. Inhibition of Enzymes during Saccharification
6. Challenges and Future Prospects
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iea.org/renewables2018 (accessed on 4 June 2019).
- Available online: https://renewablesnow.com/news/renewables-supply-25-of-global-power-in-2017-iea-606070/ (accessed on 4 June 2019).
- Koupaie, E.H.; Dahadha, S.; Lakeh, A.B.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production—A review. J. Environ. Managem. 2019, 233, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, N.; Sivasankari, S.; Kiran, G.S.; Ninawe, A.; Selvin, J. Utilization of bioresources for sustainable biofuels: A Review. Renew. Sustain. Energy Rev. 2017, 73, 205–214. [Google Scholar] [CrossRef]
- Landis, D.A.; Gratton, C.; Jackson, R.D.; Gross, K.L.; Duncan, D.S.; Liang, C.; Meehan, T.D.; Robertson, B.A.; Schmidt, T.M.; Stahlheber, K.A.; et al. Biomass and biofuel crop effects on biodiversity and ecosystem services in the North Central US. Biomass Bioenergy 2018, 114, 18–29. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Azelee, I.W. Biogas as a renewable energy fuel. A review of biogas upgrading, utilization and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Lyytimaki, J. Renewable energy in the news. Environmental, economic policy and technology discussion of biogas. Sustain. Prod. Consum. 2018, 15, 65–73. [Google Scholar] [CrossRef]
- Frigon, J.C.; Mehta, P.; Gelot, S.R. Impact of mechanical, chemical and enzymatic pretreatments on the methane yield from the anaerobic digestion of switch grass. Biomass Energy 2012, 36, 1–11. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O.; Singh, N. Energy crops in sustainable phytoremediation. Renew. Sustain. Energy Rev. 2016, 54, 58–73. [Google Scholar] [CrossRef]
- Takellapati, S.; Li, T.; Gozalez, M.A. An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Brethauer, S.; Studer, M.H. Biochemical conversion process of lignocellulosic biomass to fuel and chemicals—A review. Chim. Int. J. Chem. 2015, 69, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansaw, J.A.; Mohd Falik, A.A.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. 2017, 10, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.; Gränitz, K.; Adamsen, A.P.; Møller, H.B. Extrusionasapretreatmentto increasebiogasproduction. Bioresour. Technol. 2011, 102, 4989–4994. [Google Scholar] [CrossRef] [PubMed]
- José, M.O.; María, J.N.; Paloma, M.; Ignacio, B.; Miguel, Á.C.; Felicia, S.; Mercedes, B.; Antonio, D.M. A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective. Fermentation 2017, 3, 15. [Google Scholar] [CrossRef]
- Patil, P.N.; Gogate, P.R.; Csoka, L.; DregelyiKiss, A.; Horvath, M. Intensification of biogas production using pretreatment based on hydrodynamic cavitation. Ultrason. Sonochem. 2016, 30, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ruly, T.H.; Lucas, R.; da Silva, S.S.; Giuliano, D.; Solange, I.M.; dos Santos, J.C. Hydrodynamic cavitation as a strategy to enhance the efficiency of lignocellulosic biomass pretreatment. Crit. Rev. Biotechnol. 2018, 38, 483–493. [Google Scholar] [CrossRef]
- Talha, Z.; Ding, W.; Mehryar, E.; Hassan, M.; Bi, J. Alkaline pretreatment of sugarcane bagasse and filtermud co-digested to improve biomethane production. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shetty, D.J.; Kshirsagar, P.; Tapadia-Maheshwari, S.; Lanjekar, V.; Singh, S.K.; Dhakephalkar, P.K. Alkali pretreatment at ambient temperature: A promising method to enhance biomethanation of rice straw. Bioresour. Technol. 2017, 226, 80–88. [Google Scholar] [CrossRef]
- Reeta, R.S.; Anil, K.P.; Rajeev, K.; Sukuamaran, C.L.; Ashok, P. Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour. Tech. 2013, 127, 500–507. [Google Scholar] [CrossRef]
- Satyamurthy, P.; Jain, P.; Balasubramanya, R.H.; Vigneshwaran, N. Preparation and characterization of cellulasenanowhiskers from cotton fibres by controlled microbial hydrolysis. Carbohydr. Polym. 2011, 83, 122–129. [Google Scholar] [CrossRef]
- Binod, P.; Gnansounou, E.; Sindhu, R.; Pandey, A. Enzymes for second generation biofuels: Recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 5, 317–325. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Zhang, Y.-H.P. Cellulases: Characteristics, Sources, Production, and Applications. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers, 1st ed.; Yang, S.-T., Hesham, A.E.-E., Nuttha, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 131–146. [Google Scholar]
- Bhatia, Y.; Mishra, S.; Bisaria, V. Microbial b-glucosidases: Cloning, properties, and applications. Crit. Rev. Biotechnol. 2002, 22, 375–407. [Google Scholar] [CrossRef]
- Tiwari, R.; Singh, S.; Shukla, P.; Nain, L. Novel cold temperature active b-glucosidase from Pseudomonas lutea BG8 suitable for simultaneous saccharification and fermentation. RSC Adv. 2014, 4, 58108–58115. [Google Scholar] [CrossRef]
- Cairns, J.R.K.; Esen, A. β-Glucosidase. Cell. Mol. Life Sci. 2010, 67, 3389–3405. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, X.; Dang, W.; Tran, P.L.; Park, S.; Oh, B.; Hong, W.; Lee, J.; Park, K. Characterization and application of an acidophilic and thermostable b-glucosidase from Thermofilumpendens. J. Biosci. Bioeng. 2013, 115, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Nigam, D.; Asthana, M.; Kumar, A. Penicillium: A Fungus in the Wine and Beer Industries. In New and Future Developments in Microbial Biotechnology and Bioengineering; Vijai, K.G., Susana, R.-C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 187–200. [Google Scholar]
- Harhangi, H.R.; Steenbappers, P.J.M.; Akhmanova, A.; Jetten, M.S.M.; Van der Drift, C.; Op den Camp, H.J.M. A highly expressive family 1 β-glucosidase with transglycosylation capacity from the anaerobic fungus Piromyces sp. E2. Biochem. Biophys. Acta 2002, 1574, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Min, Z.; Qin, Y.; Ye, D.-Q.; Song, Y.; Liu, Y.-L. Efficient Display of Aspergillus niger β-glucosidase on Saccharomyces cerevisiae cell wall for aroma enhancement in wine. J. Agric. Food Chem 2019, in press. [Google Scholar] [CrossRef]
- Wilkowska, A.; Pogorzelski, E. Aroma enhancement of cherry juice and wine using exogenous glycosidases from mould, yeast and lactic acid bacteria. Food Chem. 2017, 237, 282–289. [Google Scholar] [CrossRef]
- Keerti, G.A.; Kumar, V.; Dubey, A.; Verma, A.K. Kinetic Characterization nd effect of immobilized thermostable β-glucosidase in alginate gel beads on sugarcane juice. ISRN Biochem. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Rani, V.; Mohanram, S.; Tiwari, R.; Nain, L.; Arora, A. Beta-Glucosidase: Key Enzyme in Determining Efficiency of Cellulase and Biomass Hydrolysis. J. Bioproces Biotech. 2014, 5, 197. [Google Scholar] [CrossRef]
- Mark, J.; Dwight, E.T. Mechanism for β-glucosidase release into cellulose-grown Trichoderma reesei culture supernatants. Exp. Mycol. 1988, 12, 203–216. [Google Scholar] [CrossRef]
- Bischof, R.H.; Ramoni, J.; Seiboth, B. Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microb. Cell Factories 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Lee, J.P.; Baek, S.C.; Jo, Y.D.; Kim, H. Characterization of Three Extracellular β-Glucosidases Produced by a Fungal Isolate Aspergillus sp. YDJ14 and Their Hydrolyzing Activity for a Flavone Glycoside. J. Microbiol. Biotechnol. 2018, 28, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Hugues, M.; Delphine, H.; Dominique, C.; Frédéric, M.; Nicolas, L.F. Comparative kinetic analysis of two fungal β-glucosidases. Biotechnol. Biofuels 2010, 3, 1–8. [Google Scholar] [CrossRef]
- Annette, S.; Ahring, B.K.; Lübeck, M.; Ubhayasekera, W.; Bruno, K.S.; Culley, D.E.; Lübeck, P.S. Identifying and characterizing the most significant β-glucosidase of the novel species Aspergillus saccharolyticus. Can. J. Microbiol. 2012, 58, 1035–1046. [Google Scholar] [CrossRef]
- Bagudo, A.I.; Argungu, A.U.; Aliero, A.A.S.; Suleiman, N.; Kalpana, S. Bacillus subtilis as an Alternative Source of Beta-glucosidase. Int. J. Mod. Cell. Mol. Biotechnol. 2014, 3, 1–9. [Google Scholar]
- Li, Y.; Bu, M.; Chen, P.; Li, X.; Chen, C.; Gao, G.; Feng, Y.; Han, W.; Zhang, Z. Characterization of a Thermophilic Monosaccharide Stimulated β-Glucosidase from Acidothermus cellulolyticus. Chem. Res. Chin. Univ. 2018, 34, 212–220. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, T.; Li, Z.; Liu, P.; Wang, Y.; He, N.; Liang, D. Characterization of a beta-glucosidase from Bacillus licheniformis and its effect on bioflocculant degradation. AMB Express 2017, 7, 197. [Google Scholar] [CrossRef]
- José, C.S.S.; Luana, P.M.; Sibeli, C.; Ward, R.J. Glucose tolerant and glucose stimulated β-glucosidases—A review. Bioresour. Technol. 2018, 267, 704–713. [Google Scholar] [CrossRef]
- Tiwari, R.; Singh, P.K.; Singh, S.; Nain, P.K.S.; Nain, L.; Shukla, P. Bioprospecting of novel thermostable β-glucosidase from Bacillus subtilis RA10 and its application in biomass hydrolysis. Biotechnol. Biofuels 2017, 10, 246–263. [Google Scholar] [CrossRef]
- Treebupachatsakul, T.; Nakazawa, H.; Shinbo, H.; Fujikawa, H.; Nagaiwa, A.; Ochiai, N.; Kawaguchi, T.; Nikaido, N.; Totani, K.; Shioya, K.; et al. Heterologously expressed Aspergillus aculeatus β-glucosidase in Saccharomyces cerevisiae is a cost-effective alternative to commercial supplementation of β-glucosidase in industrial ethanol production using Trichoderma reesei cellulases. Biosci. Bioeng. 2016, 121, 27–35. [Google Scholar] [CrossRef]
- de França Passos, D.; Pereira, N., Jr.; de Castro, A.M. A comparative review of recent advances in cellulases production by Aspergillus, Penicillium and Trichoderma strains and their use for lignocellulose deconstruction. Curr. Opin. Green Sustain. Chem. 2018, 14, 60–66. [Google Scholar] [CrossRef]
- Li, C.; Lin, F.; Li, Y.; Wei, W.; Wang, H.; Qin, L.; Zhou, Z.; Li, B.; Wu, F.; Chen, Z. A β-glucosidase hyper production Trichodermareesei mutant reveals a potential role of cel3D in cellulase production. Microb. Cell Fact. 2016, 15, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Wu, Y.; Qin, X.; Ma, R.; Luo, H.; Su, X.; and Yao, B. Revisiting overexpression of a heterologous β-glucosidase in Trichoderma reesei: Fusion expression of the Neosartorya fischeri Bgl3A to cbh1 enhances the overall as well as individual cellulase activities. Microb. Cell Fact. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, H.; Kawai, T.; Ida, N.; Shida, Y.; Kobayashi, Y.; Okada, H.; Tani, S.; Sumitani, J.; Kawaguchi, T.; Morikawa, Y.; et al. Construction of a recombinant Trichodermareesei, strain expressing Aspergillusaculeatus β-glucosidase 1 for efficient biomass conversion. Biotechnol. Bioeng. 2012, 109, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Treebupachatsakul, T.; Shioya, K.; Nakazawa, H.; Kawaguchi, T.; Shioya, K.; Shida, Y.; Marikawa, Y.; Ogasawara, W.; Okada, H. Utilization of recombinant Trichodermareesei expressing Aspergillusaculeatus β-Glucosidase 1 (JN11) for a more economical production of ethanol from lignocellulosic biomass. J. Biosci. Bioeng. 2015, 120, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Dashtban, M.; Qin, W. Over expression of an exotic thermotolerant β-glucosidase in Trichodermareesei and its significant increase in cellulolytic activity and saccharification of barley straw. Microb. Cell Fact. 2012, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.S. Microbial Enzymes with Special Characteristics for Biotechnological Applications. Biomolecules 2013, 3, 597–611. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A.K.; Kumar, V. Catalytic properties, functional attributes and industrial applications of b-glucosidases. 3 Biotech. 2016, 6. [Google Scholar] [CrossRef]
- Ahmed, A.; Nasim, F.U.; Batool, K.; Bibi, A. Microbial β-Glucosidase: Sources, Production and Applications. J. Appl. Environ. Microbiol. 2017, 5, 31–46. [Google Scholar] [CrossRef]
- Chotinsky, D. The use of enzymes to improve utilization of nutrient in poultry diets. Bulg. J. Agric. Sci. 2015, 21, 429–435. [Google Scholar]
- de Carvalho, J.L.M.; de Castro, I.M.; da Silva, C.A.B. A study of retention of sugars in the process of clarification of pineapple juice (Ananas comosus, L. Merril) by micro-and ultra-filtration. J. Food Eng. 2008, 87, 447–454. [Google Scholar] [CrossRef]
- Ramadan, M.F. Enzymes in Fruit Juice Processing. In Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Kuddus, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-12-813280-7. [Google Scholar]
- Serra Colomer, M.; Funch, B.; Forster, J. The raise of Brettanomyces yeast species for beer production. Curr. Opin. Biotechnol. 2019, 56, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Annette, S.; Mette, L.; Peter, S.L.; Birgitte, K.A. Fungal Beta-Glucosidases: A Bottleneck in Industrial Use of Lignocellulosic Materials. Biomolecules 2013, 3, 612–631. [Google Scholar] [CrossRef]
- Abdellaa, A.; Mazeeda, T.E.-S.; -Baz, A.F.E.; Yang, S.-T. Production of β-glucosidase from wheat bran and glycerol by Aspergillus niger in stirred tank and rotating fibrous bed bioreactors. Process. Biochem. 2016, 51, 1331–1337. [Google Scholar] [CrossRef]
- Zhenming, C.; Zhe, C.; Guanglei, L.; Fang, W.; Liang, J.; Tong, Z. Saccharomycopsis fibuligera and its applications in biotechnology. Biotechnol. Adv. 2009, 27, 423–443. [Google Scholar] [CrossRef]
- Goswami, S.; Gupta, N.; Datta, S. Using the β-glucosidase catalyzed reaction product glucose to improve the ionic liquid tolerance of β-glucosidases. Biotechnol. Biofuels 2016, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Jalak, J.; Väljamäe, P. Mechanism of initial rapid rate retardation in cellobiohydrolase catalyzed cellulose hydrolysis. Biotechnol. Bioeng. 2010, 106, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Igarasi, K.; Uchihashi, T.; Koivula, A.; Wada, M.; Kimura, S.; Okamoto, T.; Penttila, M.; Ando, T.; Samejima, M. Traffic jams reduce hydrolytic efficiency of cellulase on cellulose surface. Science 2011, 333, 1279–1282. [Google Scholar] [CrossRef]
- Teugjas, H.; Väljamäe, P. Selecting β-glucosidases to support cellulases in cellulose saccharification. Biotechnol. Biofuels 2013, 6, 105. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Z.; Ren, G.; Kong, W.; Li, L.; Xie, W.; Liu, Y. Engineering a novel glucose-tolerant β-glucosidase as supplementation to enhance the hydrolysis of sugarcane bagasse at high glucose concentration. Biotechnol. Biofuels 2015, 8, 202. [Google Scholar] [CrossRef]
- Sharma, S.; Vaid, S.; Bhat, B.; Singh, S.; Bajaj, B.K. Thermostable Enzymes for Industrial Biotechnology. In Advances in Enzyme Technology; Singh, R., Singhania, R.R., Pandey, A., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 469–495. [Google Scholar]
- Pei, X.Q.; Yi, Z.L.; Tang, C.G.; Wu, Z.L. Three amino acid changes contribute markedly to the thermostability of β-glucosidase BglC from Thermobifida fusca. Bioresour. Technol. 2011, 102, 3337–3342. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Scott-Craig, J.S.; Walton, J.D. Improving enzymes for biomass conversion: A basic research perspective. Bioenergy Res. 2010, 3, 82–92. [Google Scholar] [CrossRef]
- Banerjee, S.; Mudliar, S.; Sen, R.; Giri, B.; Satpute, D.; Chakrabarti, T.; Pandey, R.A. Commercializing lignocellulosic bioethanol: Technology bottlenecks and possible remedies. Biofuels Bioprod. Biorefin. 2010, 4, 77–93. [Google Scholar] [CrossRef]
- Chen, H.L.; Chen, Y.C.; Lu, M.Y.; Chang, J.J.; Wang, H.T.; Ke, H.M.; Wang, T.Y.; Ruan, S.K.; Wang, T.Y.; Hung, K.Y.; et al. A highly efficient β-glucosidase from the buffalo rumen fungus Neocallimastix patriciarum W5. Biotechnol. Biofuels 2012, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Nan, H.; Kim, H.J.; Jin, Y.S. Simultaneous saccharification and fermentation by engineered Saccharomyces cerevisiae without supplementing extracellular β-glucosidase. J. Biotechnol. 2013, 167, 316–322. [Google Scholar] [CrossRef] [PubMed]
- da Gama Ferreira, R.; Azzoni, A.R.; Freitas, S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: The case of recombinant β-glucosidase. Biotechnol. Biofuels 2018, 11, 81. [Google Scholar] [CrossRef]
- Zahoor, S.; Javed, M.M.; Aftab, S.; Latif, F.; ul-Haq, I. Metabolic engineering and thermodynamic characterization of an extracellular β-glucosidase produced by Aspergillus niger. Afr. J. Biotechnol. 2011, 10, 8107–8116. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Wang, J.; Zhang, X.; Jia, R.; Gao, Y.; Peng, H. Increased enzymatic hydrolysis of sugarcane bagasse by a novel glucose- and xylose-stimulated β-glucosidase from Anoxybacillus flavithermus subsp. yunnanensis E13. BMC Biochem. 2017, 18, 4. [Google Scholar] [CrossRef]
- Yan, F.Y.; Xia, W.; Zhang, X.X.; Chen, S.; Nie, X.Z.; Qian, L. Characterization of β-glucosidase from Aspergillus terreus and its application in the hydrolysis of soybean isoflavones. J. Zhejiang Univ. Sci. B 2016, 17, 455–464. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, R.; Yang, X.; Zhang, Z.; Song, S.; Miao, Y.; Shen, Q. Characterization of a thermostable β-glucosidase from Aspergillus fumigatus Z5, and its functional expression in Pichia pastoris X33. Microb. Cell Factories 2012, 11, 25. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Gao, R.; Yu, X.; Chen, G. Synergistic effect of thermostable β-glucosidase TN0602 and cellulase on cellulose hydrolysis. 3 Biotech 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fusco, F.A.; Fiorentino, G.; Pedone, E.; Contursi, P.; Bartolucci, S.; Limauro, D. Biochemical characterization of a novel thermostable β-glucosidase from Dictyoglomus turgidum. Int. J. Biol. Macromol. 2018, 113, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Rapp, P. 1, 3-b-glucanase, 1, 6-b-glucanase and b-glucosidase activities of Sclerotium glucanicum, synthesis and properties. J. Gen. Microbiol. 1989, 135, 2847–2858. [Google Scholar] [CrossRef][Green Version]
- Zeng, Y.C.; Zhang, S.Z. Purification and properties of a b-glucosidase from Aspergillus phoenicis. Wei Sheng Wu Xue Bao 1989, 29, 195–199. [Google Scholar] [PubMed]
- Yeoman, C.J.; Han, Y.; Dodd, D.; Schroeder, C.M.; Mackie, R.I.; Cann, I.K.O. Thermostable Enzymes as Biocatalysts in the Biofuel Industry. Adv. Appl. Microbiol. 2010, 70, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Paramjeet, S.; Manasa, P.; Korrapati, N. Biofuels: Production of fungal-mediated ligninolytic enzymes and the modes of bioprocesses utilizing agro-based residues. Biocatal. Agric. Biotechnol. 2018, 14, 57–71. [Google Scholar] [CrossRef]
- Arnthong, J.; Chuaseeharonnachai, C.; Boonyuen, N.; Tachaapaikun, C.; Chimchana, D.; Eurwilaichitr, L.; Champreda, V.; Chantasingh, D. Cooperative Decomposition of Rice Straw by Co-cultivation of Cellulolytic Fungi. J. Sci. 2018, 45, 645–652. [Google Scholar]
- Benoit-Gelber, I.; Gruntjes, T.; Vinck, A.; van Veluw, J.G.; Wösten, H.A.B.; Boeren, S.; Vervoort, J.J.M.; de Vries, R.P. Mixed colonies of Aspergillus niger and Aspergillus oryzae cooperatively degrading wheat bran. Fungal Genet. Biol. 2017, 102, 31–37. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, D.; Hao, F. Mixed culture of recombinant Trichodermareesei and Aspergillusniger for cellulase production to increase the cellulose degrading capability. Biomass Bioenergy 2018, 112, 93–98. [Google Scholar] [CrossRef]
- Hu, H.L.; van den Brink, J.; Gruben, B.S.; Wosten, H.A.B.; Gu, J.-D.W.; de Vries, R.P. Improved enzyme production by co-cultivation of Aspergillusniger and Aspergillusoryzae and with other fungi. Int. J. Biodeterior. Biodegrad. 2011, 65, 248–252. [Google Scholar] [CrossRef]
- Julieta, M.; Leandro, P.; Laura, L. Characterization of β-Glucosidase Produced by the White Rot Fungus Flammulina velutipes. J. Microbiol. Biotechnol. 2014, 25, 57–65. [Google Scholar] [CrossRef]
- Oh, J.M.; Lee, J.P.; Baek, S.C.; Kim, S.G.; Jo, Y.D.; Kim, J.; Kim, H. Characterization of two extracellular β-glucosidases produced from the cellulolytic fungus Aspergillus sp. YDJ216 and their potential applications for the hydrolysis of flavone glycosides. Int. J. Biol. Macromol. 2018, 111, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.; Guadamuro, L.; Flórez, A.B.; Vázquez, L.; Mayo, B. Fermentation of commercial soy beverages with lactobacilli and bifidobacteria strains featuring high β-glucosidase activity. Innov. Food Sci. Emerg. Technol. 2019, 51, 148–155. [Google Scholar] [CrossRef]
- Fan, G.; Xu, Y.; Zhang, X.; Lei, S.; Yang, S.; Pan, S. Characteristics of immobilised b-glucosidase and its effect on bound volatile compounds in orange juice. Int. J. Food Sci. Technol. 2011, 46, 2312–2320. [Google Scholar] [CrossRef]
- Su, E.; Xia, T.; Gao, L.; Dai, Q.; Zhang, Z. Immobilization of bglucosidase and its aroma-increasing effect on tea beverage. Food Bioprod. Process. 2010, 88, 83–89. [Google Scholar] [CrossRef]
- González-Pombo, P.; Fariña, L.; Carrau, F.; Batista-Viera, F.; Brena, B.M. A novel extracellular β-glucosidase from Issatchenkia terricola: Isolation, immobilization and application for aroma enhancement of white Muscat wine. Process Biochem. 2011, 46, 385–389. [Google Scholar] [CrossRef]
- Hati, S.; Vij, S.; Singh, B.P.; Mandal, S. b-Glucosidase activity and bioconversion of isoflavones during fermentation of soymilk. J. Sci. Food Agric. 2015, 95, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Marazza, J.A.; Garro, M.S.; Savoy de Giori, G. Aglycone production by Lactobacillus rhamnosus CRL981 during soymilk fermentation. Food Microbiol. 2009, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Wojtusik, M.; Yepes, C.M.; Villar, J.C.; Cordes, A.; Arroyo, M.; Garcia-Ochoa, F.; Ladero, M. Kinetic modeling of cellobiose by a β-glucosidase from Aspergillus fumigatus. Chem. Eng. Res. Des. 2018, 136, 502–512. [Google Scholar] [CrossRef]
- Terry, D.B. Iminosugar Inhibitors of Substrate Reduction Therapy for Lysosomal Glycosphingolipidoses. In Iminosugars: From Synthesis to Therapeutic Application; Compain, P., Martin, O.R., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 249–268. [Google Scholar]
- Schröder, C.; Elleuche, S.; Blank, S.; Antranikian, G. Characterization of a heat-active archaeal β-glucosidase from a hydrothermal spring metagenome. Enzym. Microb. Technol. 2014, 57, 48–54. [Google Scholar] [CrossRef]
- Elliston, A.; Collins, S.R.A.; Wilson, D.R.; Roberts, I.N.; Waldron, K.W. High concentrations of cellulosic ethanol achieved by fed batch semi simultaneous saccharification and fermentation of waste-paper. Bioresour. Technol. 2013, 134, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Ibrahim, D.; Omar, I.C. Enzymatic deinking of various types of waste paper: Efficiency and characteristics. Proces Biochem. 2013, 48, 299–305. [Google Scholar] [CrossRef]
- Elliston, A.; Collins, S.R.A.; Faulds, C.B.; Roberts, I.N.; Waldron, K.W. Biorefining of Waste Paper Biomass: Increasing the Concentration of Glucose by Optimising Enzymatic Hydrolysis. Appl. Biochem. Biotechnol. 2014, 172, 3621–3634. [Google Scholar] [CrossRef]
- Javed, M.; Buthe, A.; Rashid, M.; Wang, P. Cost-efficient entrapment of b-glucosidase in nanoscale latex and silicone polymeric thin films for use as stable biocatalysts. Food Chem. 2016, 190, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, K.; Megyeri, L.; Szakacs, G.; Kubicek, C.P.; Galbe, M.; Zachhi, G. Trichoderma atroviride mutants with enhanced production of cellulase and β-glucosidase on pretreated willow. Enzym. Microb. Technol. 2008, 43, 48–55. [Google Scholar] [CrossRef]
- Naz, S.; Ikram, N.; Rajoka, M.I.; Sadaf, S.; Akhtar, M.W. Enhanced Production and Characterization of a β-Glucosidase from Bacillus halodurans Expressed in Escherichia coli. Biochemistry 2010, 75, 513–518. [Google Scholar] [CrossRef]
- Suresh, P.; Yadav, K.; Shruthi, B.V.; Prasad, S.; Chandra, M.S. Enhanced Production of β-glucosidase by New Strain Aspergillus protuberus on Solid State Fermentation in Rice Husk. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 551–564. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, L.; Xia, L. High-level production of a fungal β-glucosidase with application potentials in the cost-effective production of Trichoderma reeseicellulase. Process Biochem. 2018, 70, 55–60. [Google Scholar] [CrossRef]
- Saha, B.C.; Bothast, R.J. Production, Purification, and Characterization of a Highly GlucoseTolerant Novel b-Glucosidase from Candida peltata. Appl. Environ. Microbiol. 1996, 62, 3165–3170. [Google Scholar]
- de Ovalle, S.; Brena, B.; Fariña, L.; González-Pombo, P. Novel beta-glucosidase from Issatchenkia orientalis: Characterization and assessment for hydrolysis of muscat wine glycosides. J. Biochem. Biotechnol. 2016, 4, 174–183. [Google Scholar]
- Yao, G.; Wu, R.; Kan, Q.; Gao, L.; Liu, M.; Yang, P.; Du, J.; Li, Z.; Qu, Y. Production of a high-efficiency cellulase complex via β-glucosidase engineering in Penicillium oxalicum. Biotechnol. Biofuels 2016, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Líter, J.A.; de Eugenio, L.I.; Prieto, A.L.; Martínez, M.J. The β-glucosidase secreted by Talaromyces amestolkiae under carbon starvation: A versatile catalyst for biofuel production from plant and algal biomass. Biotechnol. Biofuels 2018, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; He, R.; Zong, Z.; Zhan, D. A β-glucosidase Hyperproducing Strain, Pencillium Piceum: Novel Characterization of Lignocellulolytic Enzyme Systems and Its Application in Biomass Bioconversion. In Fungal Cellulolytic enzymesmicrobial Production and Application; Xu, F., Qu, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 81–106. [Google Scholar]
- Dillon, A.J.P.; Bettio, M.; Pozzan, F.G.; Andrighetti, T.; Camassola, M. A new Penicillium echinulatum strain with faster cellulase secretion obtained using hydrogen peroxide mutagenesis and screening with 2-deoxyglucose. J. Appl. Microbiol. 2011, 111, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, D.H.; Lee, S.H.; Kim, K.H. A novel β-glucosidase from Saccharophagus degradans 2-40T for the efficient hydrolysis of laminarin from brown macroalgae. Biotechnol. Biofuels 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-X.; Miao, L.-L.; Liu, Y.; Liu, H.-C.; Liu, Z.-P. Gene cloning and characterization of a cold-adapted β-glucosidase belonging to glycosyl hydrolase family 1 from a psychrotolerant bacterium Micrococcus antarcticus. Enzym. Microb. Technol. 2011, 49, 94–99. [Google Scholar] [CrossRef]
- Nishida, V.S.; de Oliveira, R.F.; Brugnari, T.; Correa, R.C.G.; Peralta, R.A.; Castoldi, R.; Peralta, R.M. Immobilization of Aspergillus awamori β-glucosidase on commercial gelatin: An inexpensive and efficient process. Int. J. Biol. Macromol. 2018, 111, 1206–1213. [Google Scholar] [CrossRef]
- Gottschalk, L.M.; de Sousa Paredes, R.; Teixeira, R.S.; da Silva, A.S.; da Silva Bon, E.P. Efficient production of lignocellulolytic enzymes xylanase, β-xylosidase, ferulic acid esterase and β-glucosidase by the mutant strain Aspergillus awamori 2B.361 U2/1. Braz. J. Microbiol. 2013, 44, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Santa-Rosa, P.S.; Souza, A.L.; Roque, R.A.; Andrade, E.V.; Astolfi-Filho, S.; Mota, A.J.; Nunes-Silva, C.G. Production of thermostable β-glucosidase and CMCase by Penicillium sp. LMI01 isolated from the Amazon region. Electr. J. Biotechnol. 2018, 31, 84–92. [Google Scholar] [CrossRef]
- Noor El-Deen, A.M.; Shata, H.M.A.H.; Farid, M.A.F. Improvement of β-glucosidase production by co-culture of Aspergillus niger and A. oryzae under solid state fermentation through feeding process. Ann. Microbiol. 2013, 64, 627–637. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Liu, L.; Chen, Y.; Li, S.; Jia, Y. Purification and characterization of a novel β-glucosidase from Aspergillus flavus and its application in saccharification of soybean meal. Prep. Biochem. Biotechnol. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bajar, S.; Bishnoi, N.R. Physico-chemical pretreatment and enzymatic hydrolysis of cotton stalk for ethanol production by Saccharomyces cerevisiae. Bioresour. Technol. 2017, 244, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.; Melo, M.; Schwan, R.; Silva, C. A new alternative use for coffee pulp from semi-dry process to β-glucosidase production by Bacillus subtilis. Lett. Appl. Microbiol. 2015, 61, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, J.; Jing, Y.; Teng, B.; Yang, P.; Chen, X.; Huang, H.; Zhao, T.; Che, T.; Zhang, C. A β-glucosidase-producing M-2 strain: Isolation from cow dung and fermentation parameter optimization for flaxseed cake. Anim. Nutr. 2018, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Gholamreza, S.J.; Mohammad, J. Advances in consolidated bioprocessing systems for bioethanol and butanol production from biomass: A comprehensive review. Biofuel Res. J. 2015, 5, 152–195. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Apps, M.J.; Jiang, H. Influence of nutrients disturbances and site conditions on carbon stocks along a boreal forest transect in central Canada. Plant Soil 2002, 242, 1–14. [Google Scholar] [CrossRef]
- de Souzaa, M.F.; da Silva Bona, E.P.; da Silva, A.S.A. On-site integrated production of cellulases and β-glucosidases by Trichoderma reesei Rut C30 using steam-pretreated sugarcane bagasse. Open Sci. 2018. [Google Scholar] [CrossRef]
- Rajan, S.S.; Yang, X.; Collart, F.; Yip, V.L.; Withers, S.G.; Varrot, A.; Thompson, J.; Davies, G.J.; Anderson, W.F. Novel catalytic mechanism of glycoside hydrolysis based on the structure of an NAD+/Mn2+-dependent phospho-α-glucosidase from Bacillus subtilis. Structure 2004, 12, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B.; Davies, G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Yang, S.; Zhu, M.; Wang, X. Technology Prospecting on Enzymes: Application, Marketing and Engineering. Comput. Struct. Biotech. J. 2012, 2, 1–11. [Google Scholar] [CrossRef]
- Vinod, K.; Punesh, S.; Dharmendra, S.; Prabhjot, K.G. Global Scenario of Industrial Enzyme Market from Industrial Enzymes: Trends, Scope and Relevance; Vikas, B., Anil, K.S., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 173–196. [Google Scholar]
- Available online: http://www.cazy.org/Glycoside-Hydrolases.html (accessed on 4 June 2019).
- Harris, P.V.; Welner, D.; McFarland, K.C.; Re, E.; Poulsen, J.N.; Brown, K.; Salbo, R.; Ding, H.; Vlasenko, E.; Merino, S.; et al. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: Structure and function of a large, enigmatic family. Biochemistry 2010, 49, 3305–3316. [Google Scholar] [CrossRef]
- Bohlin, C.; Praestgaard, E.; Baumann, M.J.; Borch, K.; Praestgaard, J.; Monrad, R.N.; Westh, P. A comparative study of hydrolysis and transglycosylation activities of fungal β-glucosidases. Appl. Microbiol. Biotechnol. 2013, 97, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Xu, X.; Qian, L.; Shi, P.; Bai, Y.; Luo, H.; Ma, R.; Yao, B. Engineering a highly active thermophilic β-glucosidase to enhance its pH stability and saccharification performance. Biotechnol. Biofuels 2016, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka-Woś, A.; Bartasun, P.; Cieśliński, H.; Kur, J. Cloning and characterization of a novel cold-active glycoside hydrolase family 1 enzyme with β-glucosidase, β-fucosidase and β-galactosidase activities. BMC Biotechnol. 2013, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Boudabbous, M.; Ben Hmad, I.; Saibi, W.; Mssawra, M.; Belghith, H.; Gargouri, A. Trans-glycosylation capacity of a highly glycosylated multi-specific β-glucosidase from Fusariumsolani. Bioprocess Biosyst. Eng. 2017, 40, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Mallek-Fakhfakh, H.; Belghith, H. Physicochemical properties of thermotolerant extracellular β-glucosidase from Talaromyces thermophilus and enzymatic synthesis of cello-oligosaccharides. Carbohydr. Res. 2016, 419, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Busk, P.K.; Grell, M.N.; Zhao, H.; Lange, L. Identification of a β-glucosidase from the Mucor circinelloides genome by peptide pattern recognition. Enzym. Microb. Technol. 2014, 67, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.S.; Sin, L.L.; Chan, K.; Shamsir, M.S.; Manan, F.A.; Sani, R.K.; Goh, K.M. Characterization of a glucose-tolerant β-glucosidase from Anoxybacillus sp. DT3-1. Biotechnol. Biofuels 2016, 9, 174. [Google Scholar] [CrossRef]
- Barrett, T.; Suresh, C.G.; Tolley, S.P.; Dodson, E.J.; Hughes, M.A. The crystal structure of a cyanogenic b-glucosidase from white clover, a family 1 glycosyl hydrolase. Structure 1995, 3, 951–960. [Google Scholar] [CrossRef]
- Varghese, J.N.; Hrmova, M.; Fincher, G.B. Three dimensional structure of a barley beta-D-glucan exohydrolase, a family 3 glycosyl hydrolase. Structure 1999, 7, 179–190. [Google Scholar] [CrossRef]
- Park, J.K.; Wang, L.X.; Patel, H.V.; Roseman, S. Molecular cloning and characterization of a unique b-glucosidase from Vibrio cholerae. J. Biol. Chem. 2002, 277, 29555–29560. [Google Scholar] [CrossRef]
- Qi, M.; Jun, H.S.; Forsberg, C.W. Cel9D, an atypical 1, 4-b-Dglucan glucohydrolase from Fibrobacter succinogenes: Characteristics, catalytic residues, and synergistic interactions with other cellulases. J. Bacteriol. 2008, 190, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Kempton, J.B.; Withers, S.G. Mechanism of Agrobacterium β-glucosidase: Kinetic studies. Biochemistry 1992, 31, 9961–9969. [Google Scholar] [CrossRef] [PubMed]
- Withers, S.G.; Rupitz, K.; Trimbur, D.; Warren, R.A.J. Mechanistic consequences of mutation of the active-site nucleophile Glu-358 in Agrobacterium β-glucosidase. Biochemistry 1992, 31, 9979–9985. [Google Scholar] [CrossRef] [PubMed]
- Street, I.P.; Kempton, J.B.; Withers, S.G. Inactivation of a bglucosidase through the accumulation of a stable 2-deoxy-2-fluoro-α-D-glucopyranosyl-enzyme intermediate: A detailed investigation. Biochemistry 1992, 31, 9970–9978. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Trimbur, D.; Graham, R.; Warren, R.A.J.; Withers, S.G. Identification of the acid/base catalyst in Agrobacterium faecalis β-glucosidase by kinetic analysis of mutants. Biochemistry 1995, 34, 14554–14562. [Google Scholar] [CrossRef]
- Li, Y.-K.; Chir, J.; Chen, F.-Y. Catalytic mechanism of a family 3 β-glucosidase and mutagenesis study on residue Asp-247. Biochem. J. 2001, 355, 835–884. [Google Scholar] [CrossRef]
- Davies, G.; Henrissat, B. Structures and mechanisms of glycosyl hydrolases. Structure 1995, 3, 853–859. [Google Scholar] [CrossRef]
- Litzinger, S.; Fischer, S.; Polzer, P.; Diederichs, K.; Welte, W.; Mayer, C. Structural and kinetic analysis of Bacillus subtilis N-acetylglucosaminidase reveals a unique Asp-His dyad mechanism. J. Biol. Chem. 2010, 285, 35675–35684. [Google Scholar] [CrossRef]
- Marana, S.R. Molecular basis of substrate specificity in family 1 glycoside hydrolases. IUBMB Life 2006, 58, 63–73. [Google Scholar] [CrossRef]
- Meleiro, L.P.; Salgado, J.C.S.; Maldonado, R.F.; Carli, S.; Moraes, L.A.B.; Ward, R.J.; Jorge, J.A.; Furriel, R.P.M. Engineering the GH1 β-glucosidase from Humicola insolens: Insights on the stimulation of activity by glucose and xylose. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Sawant, S.; Birhadea, S.; Anil, A.; Gilbert, H.; Lali, A. Two-way dynamics in β-glucosidase catalysis. J. Mol. Catal. B Enzym. 2016, 133, 161–166. [Google Scholar] [CrossRef]
- Dadheech, T.; Jakhesara, S.; Chauhan, P.S.; Pandit, R.; Hinsu, A.; Kunjadiya, A.; Joshi, C. Draft genome analysis of lignocellulolytic enzymes producing Aspergillus terreus with structural insight of β-glucosidases through molecular docking approach. Int. J. Biol. Macromol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bojarová, P.; Křen, V. Glycosidases: A key to tailored carbohydrates. Trends Biotechnol. 2009, 27, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Inhibition of cellulases by phenols. Enzym. Microb. Technol. 2010, 46, 170–176. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Gurram, R.N.; Datta, S.; Lin, Y.J.; Snyder, S.W.; Menkhaus, T.J. Removal of enzymatic and fermentation inhibitory compounds from biomass slurries for enhanced biorefinery process efficiencies. Bioresour. Technol. 2011, 102, 7850–7859. [Google Scholar] [CrossRef] [PubMed]
- Pareek, N.; Gillgren, T.; Jönsson, L.J. Adsorption of proteins involved in hydrolysis of lignocellulose on lignins and hemicelluloses. Bioresour. Technol. 2013, 148, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Rahikainen, R.; Martin Sampedro, H.; Heikkinen, S.; Rovio, K.; Marjamaa, T.; Tamminen, O.J.; Kruus, R.K. Inhibitory effect of lignin during cellulose bioconversion: The effect of lignin chemistry on non-productive enzyme adsorption. Bioresour. Technol. 2013, 133, 270–278. [Google Scholar] [CrossRef]
- Kumar, R.; Wyman, C.E. Strong cellulase inhibition by mannan polysaccharides in cellulose conversion to sugars. Biotechnol. Bioeng. 2014, 111, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Studer, M.H.; DeMartini, J.D.; Davis, M.F.; Sykes, R.W.; Davison, B.; Keller, M.; Tuskan, G.A.; Wyman, C.E. Lignin content in natural Populus variants affects sugar release. Proc. Natl. Acad. Sci. USA 2011, 108, 6300–6305. [Google Scholar] [CrossRef] [PubMed]
- Cavka, A.; Jönsson, L.J. Detoxification of lignocellulosic hydrolysates using sodium borohydride. Bioresour. Technol. 2013, 136, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Cannella, D.; Sveding, P.V.; Jørgensen, H. PEI detoxification of pretreated spruce for high solids ethanol fermentation. Appl. Energy 2014, 132, 394–403. [Google Scholar] [CrossRef]
- Duque, S.H.; Cardona, C.A.; Moncada, J. Techno-economic and environmental analysis of ethanol production from 10 agroindustrial residues in Colombia. Energy Fuel 2015, 29, 775–783. [Google Scholar] [CrossRef]
- Cao, G.; Ximenes, E.; Nichols, N.N.; Zhang, L.; Ladisch, M. Biological abatement of cellulase inhibitors. Bioresour. Technol. 2013, 146, 604–610. [Google Scholar] [CrossRef]
- Cao, G.; Ximenes, E.; Nichols, N.N.; Frazer, S.E.; Kim, D.; Cotta, M.A.; Ladisch, M. Bioabatement with hemicellulase supplementation to reduce enzymatic hydrolysis inhibitors. Bioresour. Technol. 2015, 190, 412–415. [Google Scholar] [CrossRef]
- Wimalasena, T.T.; Greetham, D.; Marvin, M.E.; Liti, G.; Chandelia, Y.; Hart, A.; Louis, E.J.; Phister, T.G.; Tucker, G.A.; Smart, K.A. Phenotypic characterization of Saccharomyces spp. yeast for tolerance to stresses encountered during fermentation of lignocellulosic residues to produce bioethanol. Microb. Cell Fact. 2014, 13, 47. [Google Scholar] [CrossRef]
- Smith, J.; van Rensburg, E.; Görgens, J.F. Simultaneously improving xylose fermentation and tolerance to lignocellulosic inhibitors through evolutionary engineering of recombinant Saccharomyces cerevisiae harbouring xylose isomerase. BMC Biotechnol. 2014, 14, 41. [Google Scholar] [CrossRef]
- Hasunuma, T.; Ismail, K.S.K.; Nambu, Y.; Kondo, A. Co-expression of TAL1 and ADH1 in recombinant xylose-fermenting Saccharomyces cerevisiae improves ethanol production from lignocellulosic hydrolysates in the presence of furfural. J. Biosci. Bioeng. 2014, 117, 165–169. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Pandey, A.; Ganansounou, E. Genetic modification: A tool for enhancing beta-glucosidase production for biofuel application. Bioresour. Technol. 2017, 245, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Rajasree, K.P.; Mathew, G.M.; Pandey, A.; Sukumaran, R.K. Highly glucose tolerant β-glucosidase from Aspergillus unguis: NII 08123 for enhanced hydrolysis of biomass. J. Ind. Microbiol. Biotechnol. 2013, 40, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.F.L.; Santos, F.R.S.; Gonçalves, F.A.; Paz, M.F.; Fonseca, G.G.; Simões, R.; Leite, R. Production of β-glucosidase on solid-state fermentation by Lichtheimia ramosa in agroindustrial residues: Characterization and catalytic properties of the enzymatic extract. Electr. J. Biotechnol. 2015, 18, 314–319. [Google Scholar] [CrossRef]
- Ng, I.-S.; Li, C.-W.; Chan, S.-P.; Chir, J.-L.; Chen, P.; Tong, C.-G.; Yu, S.-M.; Ho, T.-H. High-level production of a thermoacidophilic β-glucosidase from Penicillium citrinum YS40-5 by solid-state fermentation with rice bran. Bioresour. Technol. 2010, 101, 1310–1317. [Google Scholar] [CrossRef]
- De Oliveira, R.P.; dos Santos, B.V.; Costa, L.; Henrique, M.A.; Pasquini, D.; Baffi, M.A. Xylanase and β-glucosidase production by Aspergillus fumigatus using commercial and lignocellulosic substrates submitted to chemical pre-treatments. Ind. Crops Prod. 2017, 95, 453–459. [Google Scholar] [CrossRef]
- Almeida, J.M.; Lima, V.A.; Giloni-Lima, P.C.; Knob, A. Passion fruit peel as novel substrate for enhanced β-glucosidases production by Penicillium verruculosum: Potential of the crude extract for biomass hydrolysis. Biomass Bioenergy 2015, 72, 216–226. [Google Scholar] [CrossRef]
- Dias, L.; dos Santos, B.; Albuquerque, C.; Baeta, B.; Pasquini, D.; Baffi, M. Biomass sorghum as a novel substrate in solid-state fermentation for the production of hemicellulases and cellulases by Aspergillus niger and A. fumigatus. J. Appl. Microbiol. 2018, 124, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Helena, F.; Nicole, M.; Helena, P.; Aires, O.-T.; Isabel, B.; JoséManuel, S. Solid-State Fermentation of Ulva Rigida for Production of Cellulases, Xylanases and-Glucosidase. In Proceedings of the 4-CIAB—4th Iberoamerican Congress on Biorefineries, Jaén, Spain, 24–26 October 2018; Actas 48. pp. 455–460, ISBN 978-84-9159-144-3. [Google Scholar]
- Nabilah, I.; Ariff, M.; Bahrin, E.K.; Ramli, N.; Abd-Aziz, S. Direct Use of Spent Mushroom Substrate from Pleurotus pulmonarius as a Readily Delignified Feedstock for Cellulase Production. Waste Biomass Valoriz. 2019, 10, 839. [Google Scholar] [CrossRef]
- Morais, T.P.; de Barbosa, P.M.G.; Garcia, N.F.L.; Rosa-Garzon, N.G.; da Fonseca, G.G.; Paz, M.F.; da Leite, R.S.R. Catalytic and thermodynamic properties of β-glucosidases produced by Lichtheimia corymbifera and Byssochlamys spectabilis. Prep. Biochem. Biotechnol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Ruchi, A.; Alok, S.; Kumar, V.A. Utilization of Citrus sinensis waste for the production of β-glucosidase by solid-state fermentation using a Bacillus subtilis mutant. Environ. Eng. Manag. J. 2017, 16. [Google Scholar] [CrossRef]
- Regina da Silva Santos, F.; Nayara, F.L.G.; Marcelo Fossa da, P.; Gustavo, F.; Rodrigo, L. Production and characterization of -glucosidase from Gongronella butleri by solid-state fermentation. Afr. J. Biotechnol. 2016, 16, 633. [Google Scholar] [CrossRef]
- Liao, H.; Fan, X.T.; Mei, X.; Wei, Z.; Raza, W.; Shen, Q.; Xu, Y. Production and characterization of cellulolytic enzyme from Penicillium oxalicum GZ-2 and its application in lignocellulose saccharification. Biomass Bioenergy 2015, 74. [Google Scholar] [CrossRef]
- Nascimento, C.V.; Souza, F.H.M.; Masui, D.C.; Leone, F.A.; Peralta, R.M.; Jorge, J.A.; Furriel, R.P.M. Purification and biochemical properties of a glucose-stimulated β-d-glucosidase produced by Humicola grisea var. thermoidea grown on sugarcane bagasse. J. Microbiol. 2010, 48, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Annette, S.; Julie JABirgitte, K.A.; Philip, J.T.; Mette, L. Screening of carbon sources for beta-glucosidase production by Aspergillus saccharolyticus. Int. Biodeterior. Biodegrad. 2014, 93, 78–83. [Google Scholar] [CrossRef]
- Amsal, A.G.; Anisah, J.; Dang, A.R.; Nur Yuhasliza, A.R.; Musaalbakri, M. Effect of nitrogen supplementation on the production of β-glucosidase in solid state fermentation of broken rice and rice bran by Aspergillus oryzae. Int. J. Pharm. Bio Sci. 2019, 9, 173–178. [Google Scholar]
- Pryor, S.W.; Nahar, N. β-glucosidase supplementation during biomass hydrolysis: How low can we go? Biomass Bioenergy 2015, 80, 298–302. [Google Scholar] [CrossRef]
- Illanes, A. Enzyme Biocatalysis: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Mól, P.C.G.; Veríssimo, L.A.A.; Minim, L.A.; Boscolo, M.; Gomes, E.; da Silva, R. Production and capture of β-glucosidase from Thermoascus aurantiacus using a tailor made anionic cryogel. Process Biochem. 2019, in press. [Google Scholar] [CrossRef]
- Chen, T.; Yang, W.; Guo, Y.; Yuan, R.; Xu, L.; Yan, Y. Enhancing catalytic performance of β-glucosidase via immobilization on metal ions chelated magnetic nanoparticles. Enzym. Microb. Technol. 2014, 63, 50–57. [Google Scholar] [CrossRef]
- Abraham, R.E.; Verma, M.L.; Barrow, C.J.; Puri, M. Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol. Biofuels 2014, 7, 90. [Google Scholar] [CrossRef]
- Goffé, J.; Ferrasse, J.-H. Stoichiometry impact on the optimum efficiency of biomass conversion to biofuels. Energy 2019, 170, 438–458. [Google Scholar] [CrossRef]
- Ahmed, W.; Sarkar, B. Impact of carbon emissions in a sustainable supply chain management for a second generation biofuel. J. Clean. Prod. 2018, 186, 807–820. [Google Scholar] [CrossRef]
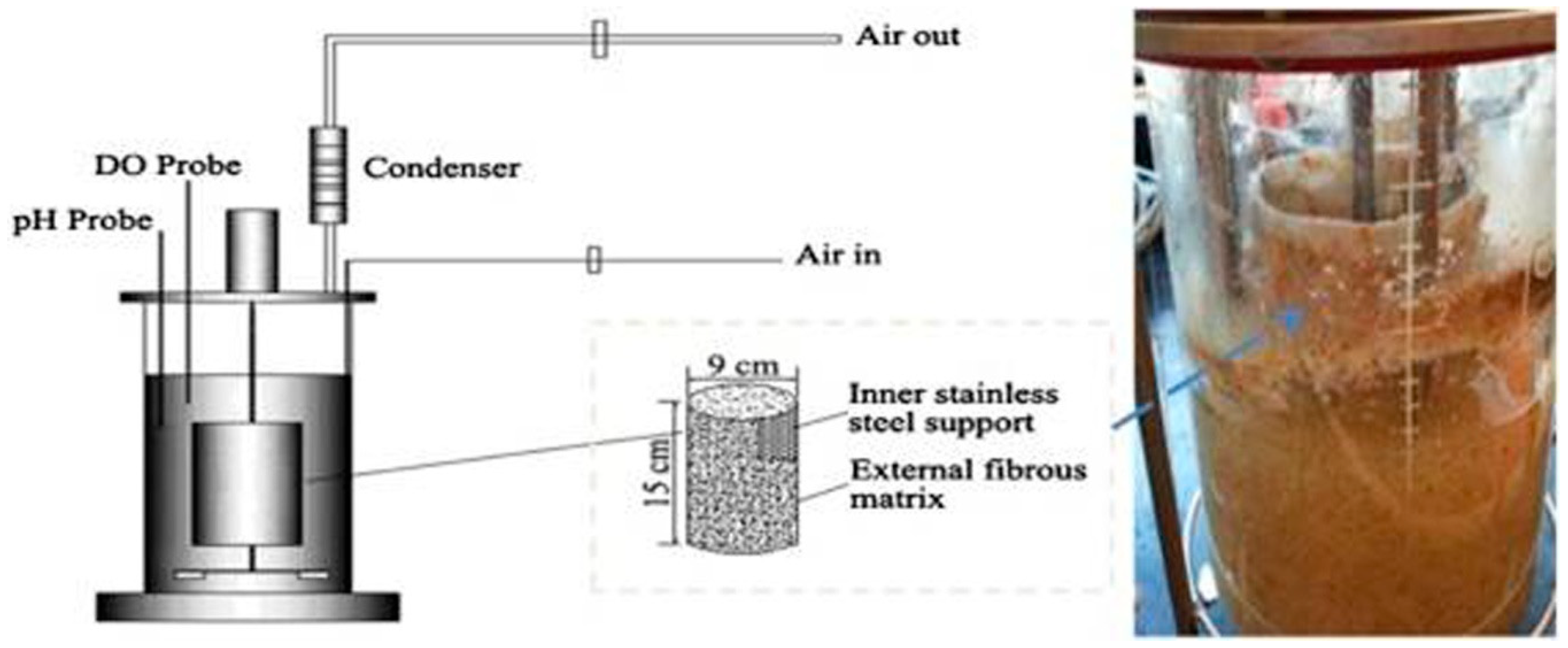
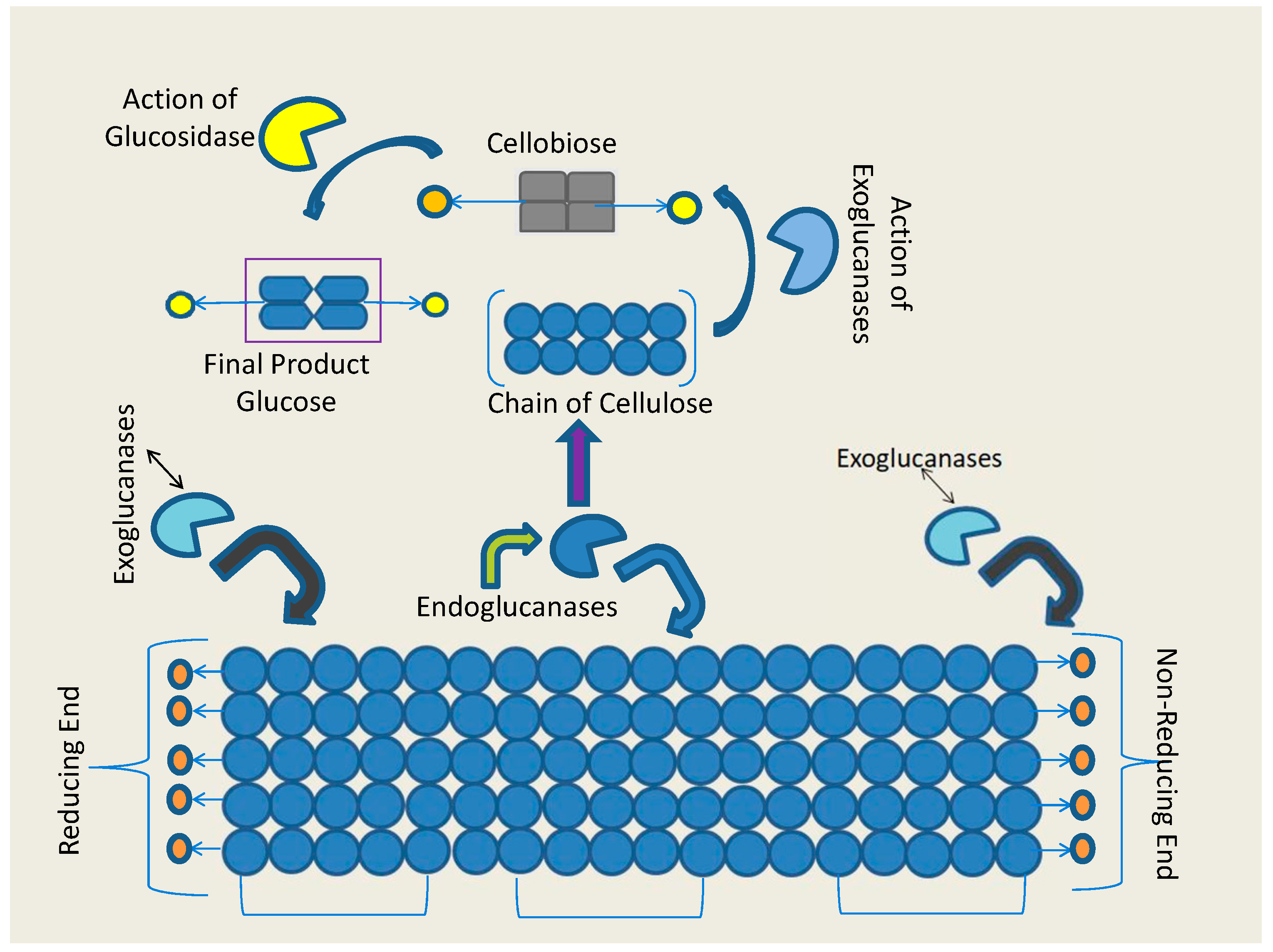
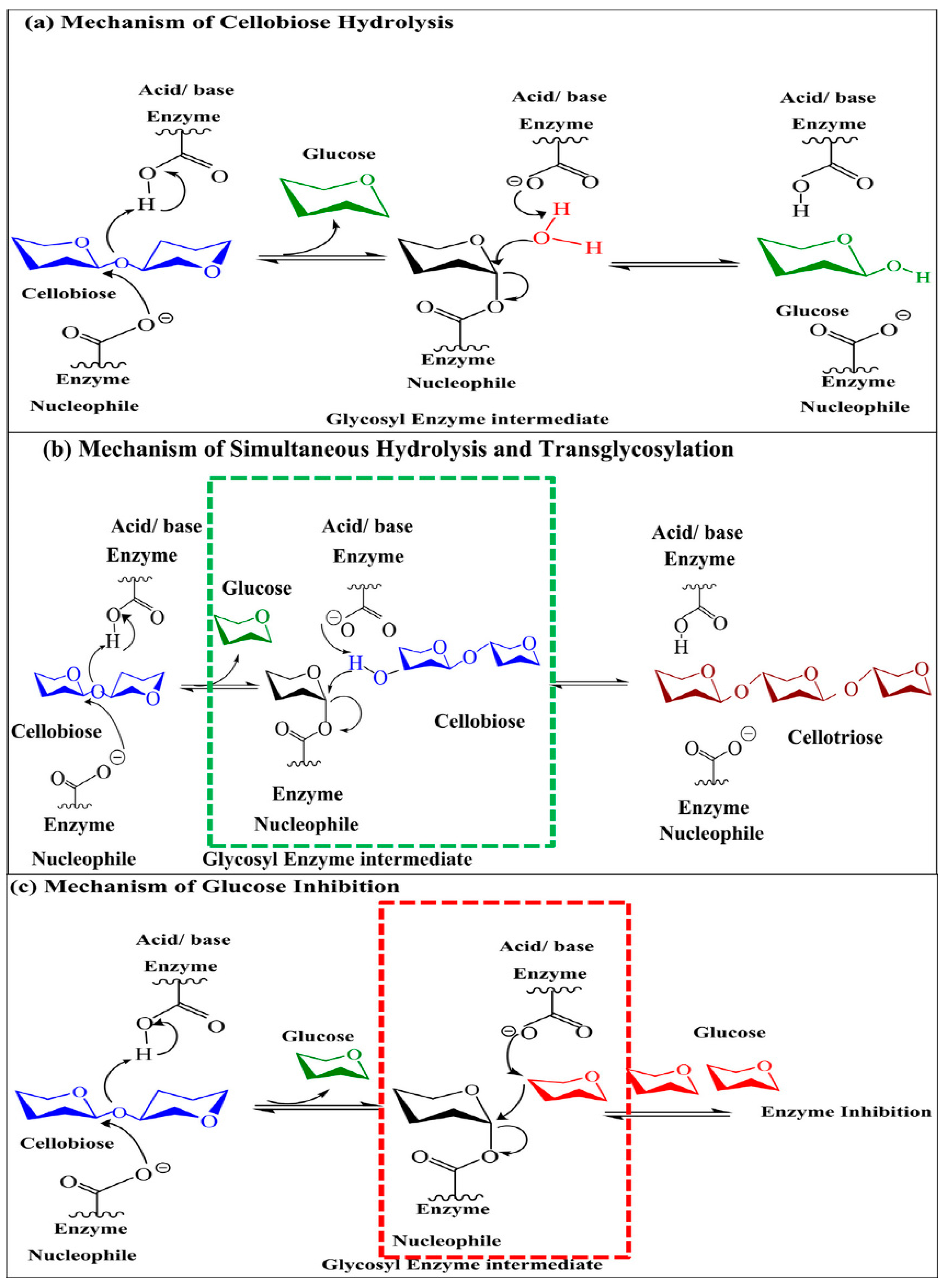
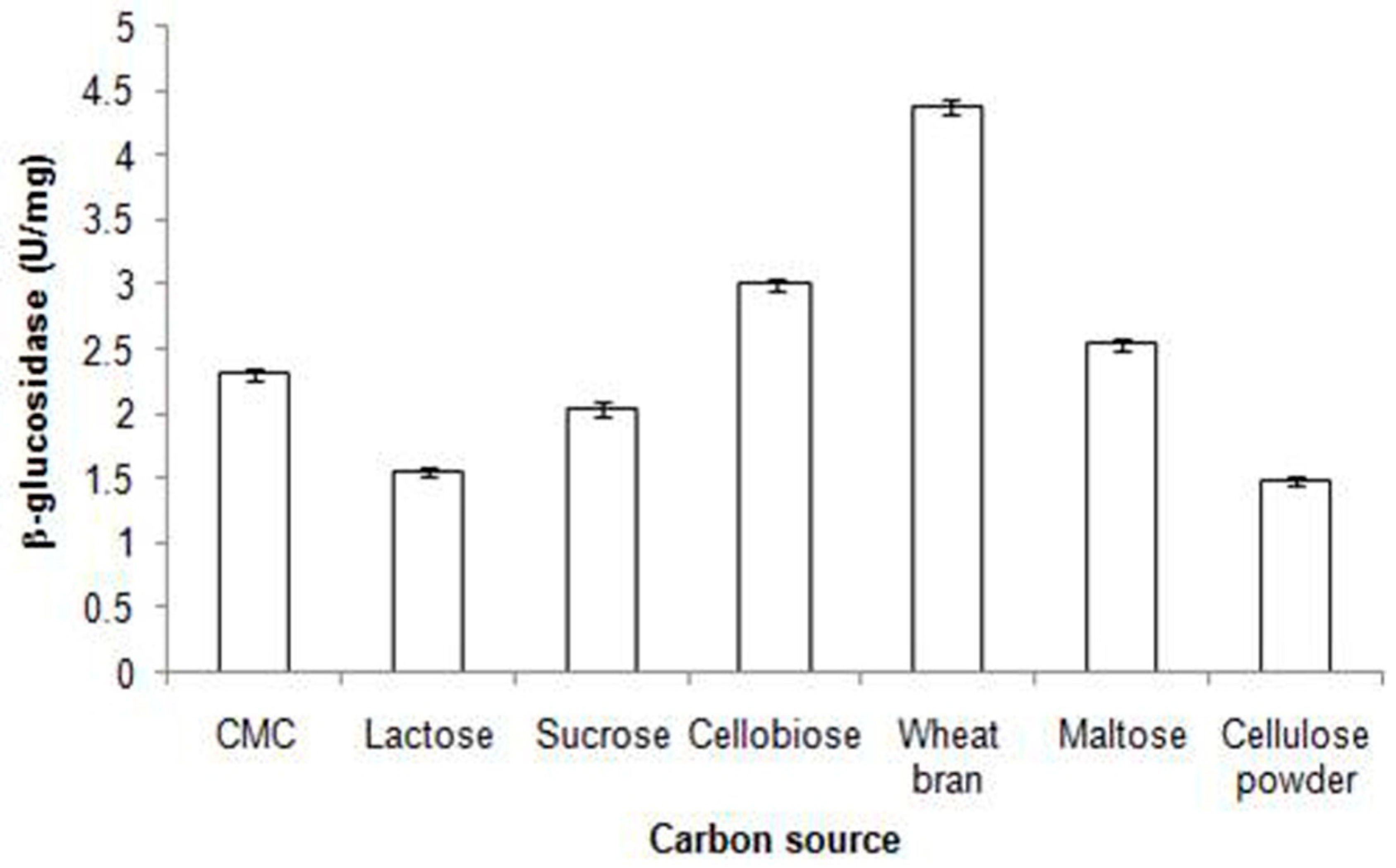
| S.No | Microorganism | Physiological conditions(Temperature, pH, Mode of fermentation and substrate) | BGL Activity | Ref. | |||
|---|---|---|---|---|---|---|---|
| Temp | pH | Mode of Fermentation | Substrate | (IU/mL) | |||
| 1. | Trichodermaatroviridae TUB F-1505 TUB F-1724 TUB F- 1753 | 30 °C 30 °C 30 °C | 6.2 6.2 6.2 | Submerged Submerged Submerged | Steam pretreated willow | 5.30 11.70 10.28 | [102] |
| 2. | Bacillus halodurans C-125 | 45 °C | 8.0 | Submerged | Lactose induced Luria broth LB media | 95 | [103] |
| 3. | Aspergillusprotuberus | 30 °C | 3.0 | Solid state | Rice husk | 26.06 IU/g | [104] |
| 4. | Pichiapastoris Bgl gene from Aspergillus niger | 30 °C | 5.0 | Fed batch | Glycerol+ methanol(1:5 ratio) | 129 | [105] |
| 5. | Candida peltata NRRL Y-6888 | 50 °C | 5.0 | Submerged | Glucose+xylose+sucrose+ maltose+arabinose | 1.5 | [106] |
| 6. | Issatchenkiaorientalis | 50 °C | 5.0 | Submerged | Esculine | 6 × 10−3 | [107] |
| 7. | Bacillus licheniformis | 60 °C | 7.0 | Submerged | Glucose+ sucrose | 45.44 | [108] |
| 8. | Penicillium oxalicum | 30 °C | - | Submerged | Microcrystalline cellulose | 150 | [109] |
| 9. | Talaromycesamestolkial | 70 °C | 4.0 | Submerged | Glucose | 1.8 | [110] |
| 10. | Penicillium piceum | 55 °C | 5.0 | Submerged | Avicel | 53.12 | [111] |
| 11. | Penicilliume chinulatum | 50 °C | 4.8 | Submerged | Microcrystalline cellulose+glucose+ soy bran | 1.5 | [111] |
| 12. | Saccharophagus degradans, 2-40T | 30 °C | 6.0 | Submerged | Laminarin | - | [112] |
| 13. | Micrococcus antarcticus | 25 °C | 6.5 | Submerged | Cellobiose | 289 | [113] |
| 14. | Aspergillus awamori | 28 °C | 4.5 | Solid state | Pineapple crown leaves + wheat bran | 820 ± 30 IU/g | [114] |
| 15. | Aspergillus awamori2B.361 U2/1 | 30 °C | 8.0 | Submerged | Wheat bran | 104.7 | [115] |
| 16. | Penicilliumsp. LMI01 | 60 °C | 6.0 | Submerged | Carboxymethyl cellulose | 0.058 ± 0.004 | [116] |
| 17. | Aspergillus niger and Aspergillus oryzae | 28–30 °C | - | Solid state | Sugarcane bagasse | 814 IU/g | [117] |
| 18. | Aspergillus flavus | 37 °C | - | Submerged | Wheat bran | 0.64 | [118] |
| 19. | Aspergillus flavus ITCC 7680 | 30 ± 2 °C | 4.8 | Solid state | Pretreated cotton stalk | 96 ± 2.9 IU/g | [119] |
| 20. | Bacillus subtilisCCMA 0087. | 36.6 °C | 3.64 | Submerged | Coffee pulp | 22.59 | [120] |
| 21. | Lichtheimia ramosa | 32 °C | - | Submerged | Flaxseed | 3.54 | [121] |
| S. No | Microorganism | Carbon Substrate | Activity of β-glucosidase (IU/g) | Reference |
|---|---|---|---|---|
| 1. | Aspergillus fumigatus | Microcrystalline cellulose (Avicel) Kraft pulp | 27.5 5.68 | [175] |
| 2. | Penicillium verruculosum | Alkali pretreated passion fruit peel | 8.54 IU/ml | [176] |
| 3. | Lichtheimia ramose | Wheat bran Soy bran Sugarcane bagasse | 162.2 ± 4.2 11.5 ± 0.7 11.1 ± 0.25 | [173] |
| 4. | Aspergillus nigerSCBM1 | Biomass sorghum +0.5% peptone | 54.90 | [177] |
| 5. | Aspergillus ibericus | Washed seaweed | 6.94 ± 0.21 | [178] |
| 6. | Pleurotus pulmonarius | Spent mushroom | 6.83 | [179] |
| 7. | Byssochlamys spectabilis Lichtheimia corymbifera | Wheat bran Wheat bran | 51.0 ± 0.75 11.6 ± 0.8 | [180] |
| 8. | Bacillus subtilis PS-CM5-UM3 | Citrus sinensis bagasse +1% peptone | 264.0 | [181] |
| 9. | Gongronella butleri | Wheat bran | 215.4 | [182] |
| 10. | Penicillium oxalicumGZ-2 | Rice straw | 2.7 IU/mL | [183] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, N.; Rathour, R.; Jha, S.; Pandey, K.; Srivastava, M.; Thakur, V.K.; Sengar, R.S.; Gupta, V.K.; Mazumder, P.B.; Khan, A.F.; et al. Microbial Beta Glucosidase Enzymes: Recent Advances in Biomass Conversation for Biofuels Application. Biomolecules 2019, 9, 220. https://doi.org/10.3390/biom9060220
Srivastava N, Rathour R, Jha S, Pandey K, Srivastava M, Thakur VK, Sengar RS, Gupta VK, Mazumder PB, Khan AF, et al. Microbial Beta Glucosidase Enzymes: Recent Advances in Biomass Conversation for Biofuels Application. Biomolecules. 2019; 9(6):220. https://doi.org/10.3390/biom9060220
Chicago/Turabian StyleSrivastava, Neha, Rishabh Rathour, Sonam Jha, Karan Pandey, Manish Srivastava, Vijay Kumar Thakur, Rakesh Singh Sengar, Vijai K. Gupta, Pranab Behari Mazumder, Ahamad Faiz Khan, and et al. 2019. "Microbial Beta Glucosidase Enzymes: Recent Advances in Biomass Conversation for Biofuels Application" Biomolecules 9, no. 6: 220. https://doi.org/10.3390/biom9060220
APA StyleSrivastava, N., Rathour, R., Jha, S., Pandey, K., Srivastava, M., Thakur, V. K., Sengar, R. S., Gupta, V. K., Mazumder, P. B., Khan, A. F., & Mishra, P. K. (2019). Microbial Beta Glucosidase Enzymes: Recent Advances in Biomass Conversation for Biofuels Application. Biomolecules, 9(6), 220. https://doi.org/10.3390/biom9060220







