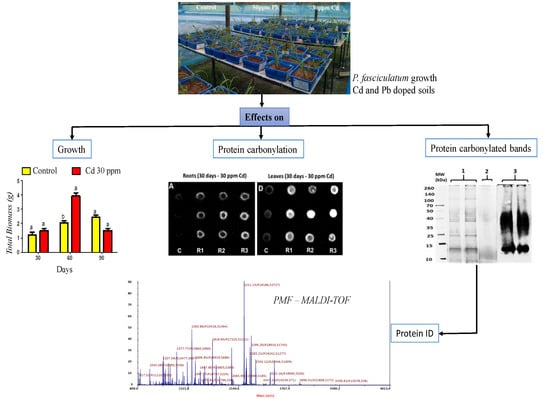
Protein Carbonylation As a Biomarker of Heavy Metal, Cd and Pb, Damage in Paspalum fasciculatum Willd. ex Flüggé
Abstract
1. Introduction
2. Results
2.1. Plant Growth
2.2. Cd and Pb Concentrations in Plants
2.3. Leaves and Roots Proteins Extract Obtained
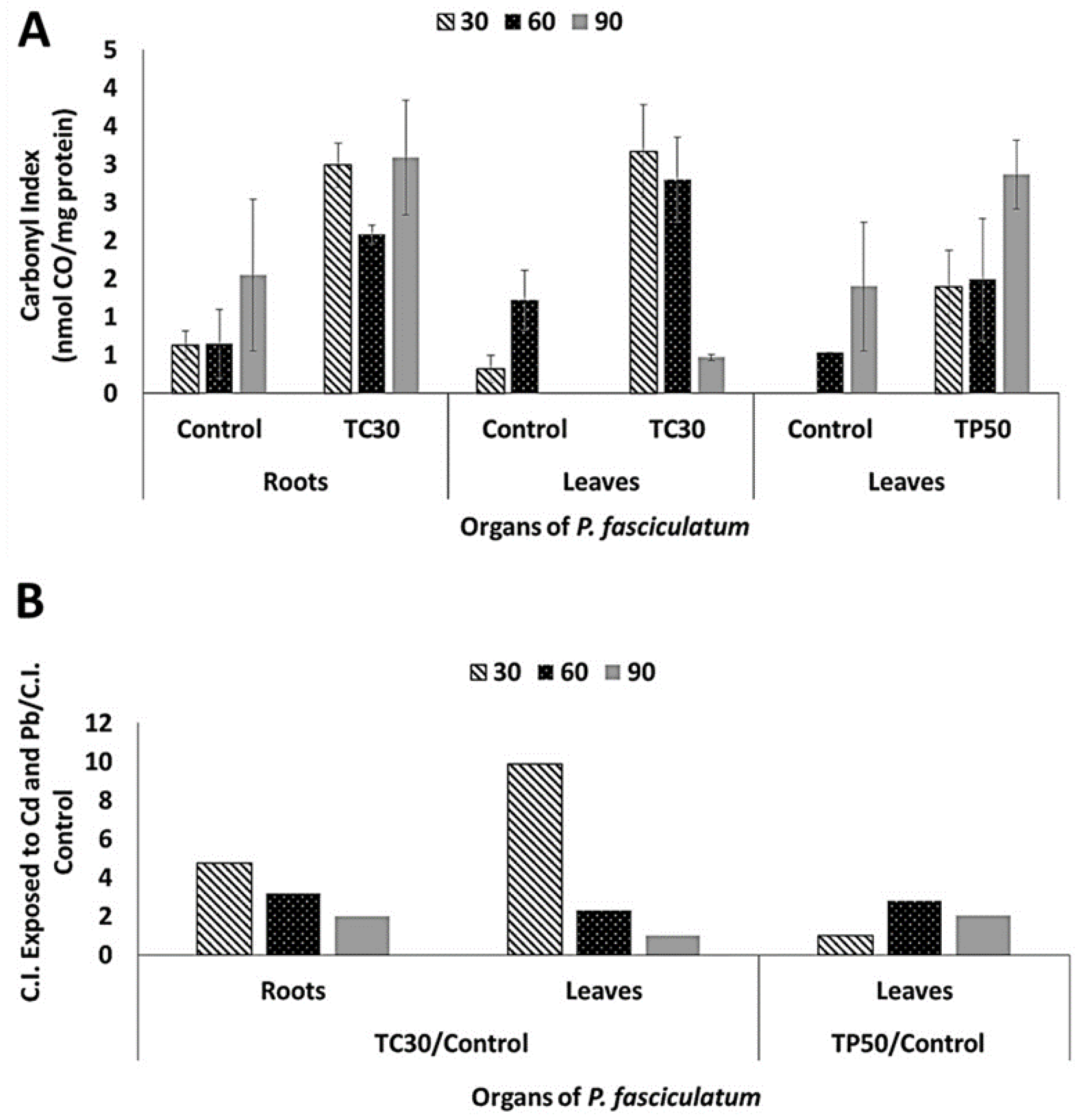
2.4. Oxidative Damage Induced by Cd and Pb in the Protein of P. fasciculatum
2.5. Carbonylation Patterns of Roots and Leaves Proteins of P. fasciculatum Exposed to Cd and Pb
2.6. Identification of Proteins in Carbonylated Bands
3. Discussion
4. Materials and Methods
4.1. Sampling and Preparation of Soils and Growing Conditions of the Plants
4.2. Analysis of Plants and Soil Samples
4.3. Protein Extraction of Roots and Leaves from P. fasciculatum
4.4. Measurement of Carbonyl Index
4.5. Identification of Proteins in Carbonylated Bands
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Belkadhi, A.; De Haro, A.; Soengas, P.; Obregon, S.; Cartea, M.E.; Djebali, W.; Chaïbi, W. Salicylic acid improves root antioxidant defense system and total antioxidant capacities of flax subjected to cadmium. OMICS J. Integr. Biol. 2013, 17, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 1–37. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Palma, J.M.; Gómez, M.; Del Río, L.A.; Sandalio, L.M. Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ. 2002, 25, 677–686. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, X.; Zhong, Y. Nitrogen as an important detoxification factor to cadmium stress in poplar plants. J. Plant Interact. 2014, 9, 249–258. [Google Scholar] [CrossRef]
- Bagheri, R.; Bashir, H.; Ahmad, J.; Baig, A.; Qureshi, M.I. Effects of Cadmium Stress on Plants. In Environmental Sustainability: Concepts, Principles, Evidences and Innovations; Kishangarh: New Delhi, India, 2014; pp. 271–277. [Google Scholar]
- Rogowska-Wrzesinska, A.; Wojdyla, K.; Nedić, O.; Baron, C.P.; Griffiths, H.R. Analysis of protein carbonylation—Pitfalls and promise in commonly used methods. Free Radic. Res. 2014, 48, 1145–1162. [Google Scholar] [CrossRef]
- Polge, C.; Jaquinod, M.; Holzer, F.; Bourguignon, J.; Walling, L.; Brouquisse, R. Evidence for the existence in Arabidopsis thaliana of the proteasome proteolytic pathway: Activation in response to cadmium. J. Biol. Chem. 2009, 284, 35412–35424. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, H.; Li, Z.; Zhuang, P.; Gao, B. Potential of four forage grasses in remediation of Cd and Zn contaminated soils. Bioresour. Technol. 2010, 101, 2063–2066. [Google Scholar] [CrossRef]
- Giraldo-Cañas, D. CatáLogo de la familia poaceae en colombia. Darwiniana 2011, 49, 139–247. [Google Scholar]
- Giraldo-Cañas, D.A. Las Gramíneas en Colombia: Riqueza, Distribución, Endemismo, Invasión, Migración, Usos y Taxonomías Populares; Instituto de Ciencias Naturales, Universidad Nacional de Colombia: Bogotá DC, Colombia, 2013; p. 382. [Google Scholar]
- Salas-Moreno, M.; Marrugo-Negrete, J. Phytoremediation potential of Cd and Pb-contaminated soils by Paspalum fasciculatum Willd. ex Flugge. Int. J. Phytoremediation 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Li, T.; Zhang, X.; Yu, H.; Zhao, L. Rhizosphere characteristics of phytostabilizer Athyrium wardii (Hook.) involved in Cd and Pb accumulation. Ecotoxicol. Environ. Saf. 2018, 148, 892–900. [Google Scholar] [CrossRef]
- Deng, G.; Liu, L.; Wang, H.; Lao, C.; Wang, B.; Zhu, C.; Peng, D. Establishment and optimization of two-dimensional electrophoresis (2-DE) technology for proteomic analysis of ramie. Int. J. Agric. Biol. 2013, 15, 570–574. [Google Scholar]
- Bashir, N.Y.; Lockwood, P.; Chasteen, A.L.; Nadolny, D.; Noyes, I. The ironic impact of activists: Negative stereotypes reduce social change influence. Eur. J. Soc. Psychol. 2013, 43, 614–626. [Google Scholar] [CrossRef]
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Jaafar, H.Z.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 3–9. [Google Scholar] [CrossRef]
- Polatajko, A.; Feldmann, I.; Hayen, H.; Jakubowski, N. Combined application of a laser ablation-ICP-MS assay for screening and ESI-FTICR-MS for identification of a Cd-binding protein in Spinacia oleracea L. after exposure to Cd. Metallomics 2011, 3, 1001–1008. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Chakraborty, S.; Banerjee, D.K. Heavy metal uptake and its effect on macronutrients, chlorophyll, protein, and peroxidase activity of Paspalum distichum grown on sludge-dosed soils. Heavy metal uptake and its effect on P. distichum. Environ. Monit. Assess. 2010, 169, 15–26. [Google Scholar] [CrossRef]
- Shu, W.S.; Ye, Z.H.; Lan, C.Y.; Zhang, Z.Q.; Wong, M.H. Lead, zinc and copper accumulation and tolerance in populations of Paspalum distichum and Cynodon dactylon. Environ. Pollut. 2002, 120, 445–453. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Tan, Z.; Liu, J.; Zhuang, L.; Yang, Z.; Huang, B. Functional identification and characterization of genes cloned from halophyte seashore paspalum conferring salinity and cadmium tolerance. Front. Plant Sci. 2016, 7, 102. [Google Scholar] [CrossRef]
- Gutsch, A.; Keunen, E.; Guerriero, G.; Renaut, J.; Cuypers, A.; Hausman, J.F.; Sergeant, K. Long-term cadmium exposure influences the abundance of proteins that impact the cell wall structure in Medicago sativa stems. Plant Boil. (Stuttg) 2018, 20, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Pochodylo, A.L.A.L.; Aristilde, L. Molecular dynamics of stability and structures in phytochelatin complexes with Zn, Cu, Fe, Mg, and Ca: Implications for metal detoxification. Environ. Chem. Lett. 2017, 15, 495–500. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Yang, Y.; Yang, S.; Sun, X.; Yang, Y. Physiological and proteomics analyses reveal the mechanism of Eichhornia crassipes tolerance to high-concentration cadmium stress compared with Pistia stratiotes. PLoS ONE 2015, 10, e0124304. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Aarts, M.G. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. 2012, 69, 3187–3206. [Google Scholar] [CrossRef]
- Prasad, A.; Kumar, A.; Suzuki, M.; Kikuchi, H.; Sugai, T.; Kobayashi, M.; Pospíšil, P.; Tada, M.; Kasai, S. Detection of hydrogen peroxide in Photosystem II (PSII) using catalytic amperometric biosensor. Front. Plant Sci. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Khan, K.Y. Effect of humic acid amendment on cadmium bioavailability and accumulation by pak choi (Brassica rapa ssp. chinensis L.) to alleviate dietary toxicity risk. Arch. Agron. Soil Sci. 2017, 63, 1431–1442. [Google Scholar] [CrossRef]
- Stefanic, P.P.; Cvjetko, P.; Biba, R.; Domijan, A.M.; Letofsky-Papst, I.; Tkalec, M.; Šikić, S.; Cindrić, M.; Balen, B. Physiological, ultrastructural and proteomic responses of tobacco seedlings exposed to silver nanoparticles and silver nitrate. Chemosphere 2018, 209, 640–653. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Hajduch, M.; Rakwal, R.; Agrawal, G.K.; Yonekura, M.; Pretova, A. High-resolution two-dimensional electrophoresis separation of proteins from metal-stressed rice (Oryza sativa L.) leaves: Drastic reductions/fragmentation of ribulose-1,5-bisphosphate carboxylase/oxygenase and induction of stress-related proteins. Electrophoresis 2001, 22, 2824–2831. [Google Scholar] [CrossRef]
- Wientjes, E.; Philippi, J.; Borst, J.W.; van Amerongen, H. Imaging the Photosystem I/Photosystem II chlorophyll ratio inside the leaf. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 259–265. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Bashir, H.; Qureshi, M.I.; Ibrahim, M.M.; Iqbal, M. Chloroplast and photosystems: Impact of cadmium and iron deficiency. Photosynthetica 2015, 53, 321–335. [Google Scholar] [CrossRef]
- Perreault, F.; Dionne, J.; Didur, O.; Juneau, P.; Popovic, R. Effect of cadmium on photosystem II activity in Chlamydomonas reinhardtii: Alteration of O-J-I-P fluorescence transients indicating the change of apparent activation energies within photosystem II. Photosynth. Res. 2011, 107, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Pribil, M.; Labs, M.; Leister, D. Structure and dynamics of thylakoids in land plants. J. Exp. Bot. 2014, 65, 1955–1972. [Google Scholar] [CrossRef] [PubMed]
- Sigfridsson, K.G.V.; Bernát, G.; Mamedov, F.; Styring, S. Molecular interference of Cd2+ with Photosystem II. Biochim. Biophys. Acta Bioenerg. 2004, 1659, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Seelert, H.; Dencher, N.A. ATP synthase superassemblies in animals and plants: Two or more are better. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Matsuoka, Y.; Hara, S.; Konno, H.; Hisabori, T. Distinct redox behaviors of chloroplast thiol enzymes and their relationships with photosynthetic electron transport in arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 1415–1425. [Google Scholar] [CrossRef]
- Buchert, F.; Forreiter, C. Singlet oxygen inhibits ATPase and proton translocation activity of the thylakoid ATP synthase CF1CFo. FEBS Lett. 2010, 584, 147–152. [Google Scholar] [CrossRef]
- Mahler, H.; Wuennenberg, P.; Linder, M.; Przybyla, D.; Zoerb, C.; Landgraf, F.; Forreiter, C. Singlet oxygen affects the activity of the thylakoid ATP synthase and has a strong impact on its γ subunit. Planta 2007, 225, 1073–1083. [Google Scholar] [CrossRef]
- Shah, K.; Dubey, R.S. A18 kDa cadmium inducible protein Complex: Its isolation and characterisation from rice (Oryza sativa L.) seedlings. J. Plant Physiol. 1998, 152, 448–454. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Díez, S. Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ. Res. 2017, 154, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Kabata-pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA; London, UK, 2001; p. 403. [Google Scholar]
- ECDGE. Heavy Metals and Organic Compounds from Wastes Used as Organic Fertilisers; ECDGE: Perchtoldsdorf, Austria, 2004. [Google Scholar]
- Jedrzejczak, R. Determination of total mercury in foods of plant origin in Poland by cold vapour atomic absorption spectrometry. Food Addit. Contam. 2002, 19, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Method 3051A (SW-846): Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils; United States Environmental Protection Agency: Washington, DC, USA, 2007.
- Coquery, M.; Welbourn, P.M. The relationship between metal concentration and organic matter in sediments and metal concentration in the aquatic macrophyte Eriocaulon septangulare. Water Res. 1995, 29, 2094–2102. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wehr, N.B.; Levine, R.L. Quantitation of protein carbonylation by dot blot. Anal. Biochem. 2012, 423, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Puentes, N.; Rodriguez-Cavallo, E.; Mendez-Cuadro, D. Membrane protein carbonylation of Plasmodium falciparum infected erythrocytes under conditions of sickle cell trait and G6PD deficiency. Mol. Biochem. Parasitol. 2019, 227, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Mendez, D.; Linares, M.; Diez, A.; Puyet, A.; Bautista, J.M. Stress response and cytoskeletal proteins involved in erythrocyte membrane remodeling upon Plasmodium falciparum invasion are differentially carbonylated in G6PD A-deficiency. Free Radic. Biol. Med. 2011, 50, 1305–1313. [Google Scholar] [CrossRef]
- Sechi, S.; Chait, B.T. Modification of cysteine residues by alkylation. A tool in peptide mapping and protein identification. Anal. Chem. 1998, 70, 5150–5158. [Google Scholar] [CrossRef]





| Treatments | Plant Tissue | Cd30 (mg kg−1) | Pb50 (mg kg−1) | ||||
|---|---|---|---|---|---|---|---|
| 30 Days | 60 Days | 90 Days | 30 Days | 60 Days | 90 Days | ||
| Roots | 190.5 ± 8 a | 107.1 ± 22.7 a | 130.8 ± 22.7 a | 36.7 ± 6.9 a | 20.8 ± 2.2 a | 45.7 ± 1.9 a | |
| Stems | 23.2 ± 3.8 a | 12.7 ± 3.67 a | 7.6 ± 0.7 a | 5.4 ± 0.6 a | 1.1 ± 0.02 a | 3.5 ± 0.1 a | |
| Leaves | 27.6 ± 5.6 a | 16.4 ± 7.7 a | 16.1 ± 0.74 a | 4.8 ± 2.6 a | 1.3 ± 0.71 a | 2.9 ± 0.3 a | |
| Control | Roots | 2.7 ± 0.5 b | 3.0 ± 0.4 b | 2.1 ± 0.1 b | 0.6 ± 0.4 b | 1.2 ± 0.05 b | 0.9 ± 0.7 b |
| Stems | 0.6 ± 0.5 b | 0.5 ± 0.02 b | 0.9 ± 0.2 b | ND b | ND a | ND b | |
| Leaves | 1.4 ± 1.1 b | 1.2 ± 0.2 b | 1.3 ± 0.2 b | ND b | ND a | ND b | |
| Days | Carbonyl Index of Protein Exposed to Cd30 | Carbonyl Index of Protein Exposed to Pb50 | ||||
|---|---|---|---|---|---|---|
| Roots | Leaves | Leaves | ||||
| Control | Cd30 | Control | Cd30 | Control | Pb50 | |
| 30 | 0.6 ± 0.2 | 2.9 ± 0.3 | 0.3 ± 0.2 | 3.2 ± 0.6 | ND | 1.4 ± 0.5 |
| 60 | 0.7 ± 0.5 | 2.08 ± 0.1 | 1.2 ± 0.4 | 2.8 ± 0.6 | 0.5 ± 0.01 | 1.5 ± 0.8 |
| 90 | 1.6 ± 0.9 | 3.1 ± 0.8 | ND | 0.5 ± 0.04 | 1.4 ± 0.8 | 2.9 ± 0.5 |
| Band | Submitted Name | Score | Accession | Biological Process | Encoded on | Condition of Exposure | |
|---|---|---|---|---|---|---|---|
| 1 | Ribulose bisphosphate carboxylase large chain [Hordeum vulgare] | 52.0 | 104.0 | RBL_HORVU | Catalyzes: CO2 fixation, oxidative fragmentation of the pentose substrate in the photorespiration process. | Plastic, Chloroplast | Cd30 |
| 2 | ATP synthase subunit alpha [Oryza nivara] | 55.6 | 157.0 | ATPA_ORYNI | Translocase, ATP synthesis, Hydrogen ion transport, Ion transport | Plastic, chloroplastic | Cd30 |
| Ribulose bisphosphate carboxylase large chain [Cuscuta sandwichiana] | 53.4 | 99.4 | RBL_CUSSA | Catalyzes: carbon dioxide fixation, oxidative fragmentation of the pentose substrate in the photorespiration process | Plastic, chloroplastic | Cd30 | |
| 3 | Ribulose bisphosphate carboxylase large chain [Avena sativa] | 52.9 | 150.0 | RBL_AVESA | Catalyzes: carbon dioxide fixation, oxidative fragmentation of the pentose substrate in the photorespiration process | Plastic, chloroplastic | Cd30 |
| 4 | Ribulose bisphosphate carboxylase large chain [Avena sativa] | 52.9 | 150.0 | RBL_AVESA | Catalyzes: carbon dioxide fixation, oxidative fragmentation of the pentose substrate in the photorespiration process | Plastic, chloroplastic | Cd30 |
| 5 | Fructose-bisphosphate aldolase [Oryza sativa subsp. Japonica] | 42.0 | 145.0 | ALFP_ORYSJ | Allosteric enzyme, kinase, transferase, photosynthesis, Glycolysis; Plays a key role in glycolysis and gluconeogenesis | Cytoplasm | Cd30 and Pb50 |
| ATP synthase subunit gamma [Zea mays] | 39.8 | 67.5 | ATPG_MAIZE | ATP synthesis, Hydrogen ion transport, Ion transport, Transport, proton-transporting ATP synthase activity, rotational mechanism; | Chloroplast; chloroplast thylakoid membrane, Peripheral membrane protein | Cd30 and Pb50 | |
| Chlorophyll a-b binding protein CP26 [Arabidopsis thaliana] | 30.1 | 79.1 | CB5_ARATH | light-harvesting in photosystem I, The light-harvesting complex (LHC) functions as a light receptor | Chloroplast, chloroplast thylakoid membrane | Cd30 |
| Soil Properties | |||||
|---|---|---|---|---|---|
| Properties of Bioavailability | Texture | Metals | |||
| pH | 3.67 ± 0.03 | Sand (%) | 27.5 ± 0.03 | Cd (mg kg−1) | 7.27 ± 0.1 |
| OM (%) | 1.54 ± 0.09 | Clay (%) | 4.4 ± 0.09 | Pb (mg kg−1) | 2.72 ± 0.4 |
| CEC | 13.1 ± 0.01 | Silt (%) | 68.1 ± 0.06 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Moreno, M.; Contreras-Puentes, N.; Rodríguez-Cavallo, E.; Jorrín-Novo, J.; Marrugo-Negrete, J.; Méndez-Cuadro, D. Protein Carbonylation As a Biomarker of Heavy Metal, Cd and Pb, Damage in Paspalum fasciculatum Willd. ex Flüggé. Plants 2019, 8, 513. https://doi.org/10.3390/plants8110513
Salas-Moreno M, Contreras-Puentes N, Rodríguez-Cavallo E, Jorrín-Novo J, Marrugo-Negrete J, Méndez-Cuadro D. Protein Carbonylation As a Biomarker of Heavy Metal, Cd and Pb, Damage in Paspalum fasciculatum Willd. ex Flüggé. Plants. 2019; 8(11):513. https://doi.org/10.3390/plants8110513
Chicago/Turabian StyleSalas-Moreno, Manuel, Neyder Contreras-Puentes, Erika Rodríguez-Cavallo, Jesús Jorrín-Novo, José Marrugo-Negrete, and Darío Méndez-Cuadro. 2019. "Protein Carbonylation As a Biomarker of Heavy Metal, Cd and Pb, Damage in Paspalum fasciculatum Willd. ex Flüggé" Plants 8, no. 11: 513. https://doi.org/10.3390/plants8110513
APA StyleSalas-Moreno, M., Contreras-Puentes, N., Rodríguez-Cavallo, E., Jorrín-Novo, J., Marrugo-Negrete, J., & Méndez-Cuadro, D. (2019). Protein Carbonylation As a Biomarker of Heavy Metal, Cd and Pb, Damage in Paspalum fasciculatum Willd. ex Flüggé. Plants, 8(11), 513. https://doi.org/10.3390/plants8110513