Hyperglycaemia-Linked Diabetic Foot Complications and Their Management Using Conventional and Alternative Therapies
Abstract
:1. Introduction
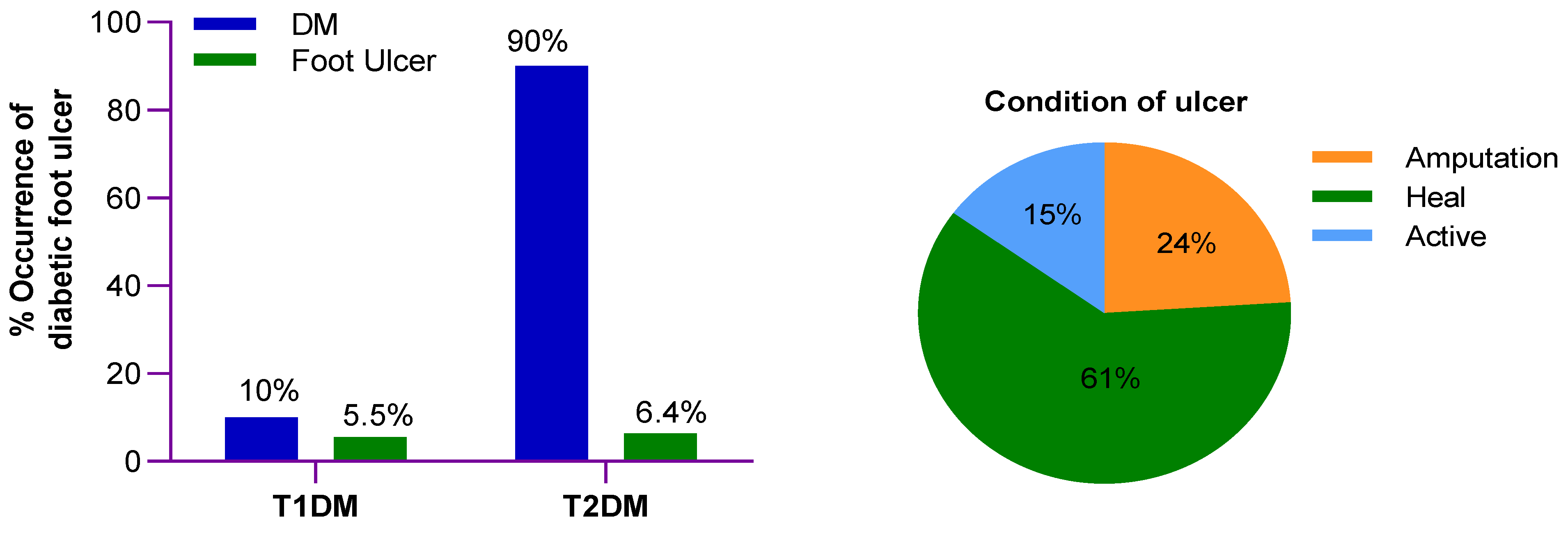
2. Epidemiology
3. Etiology
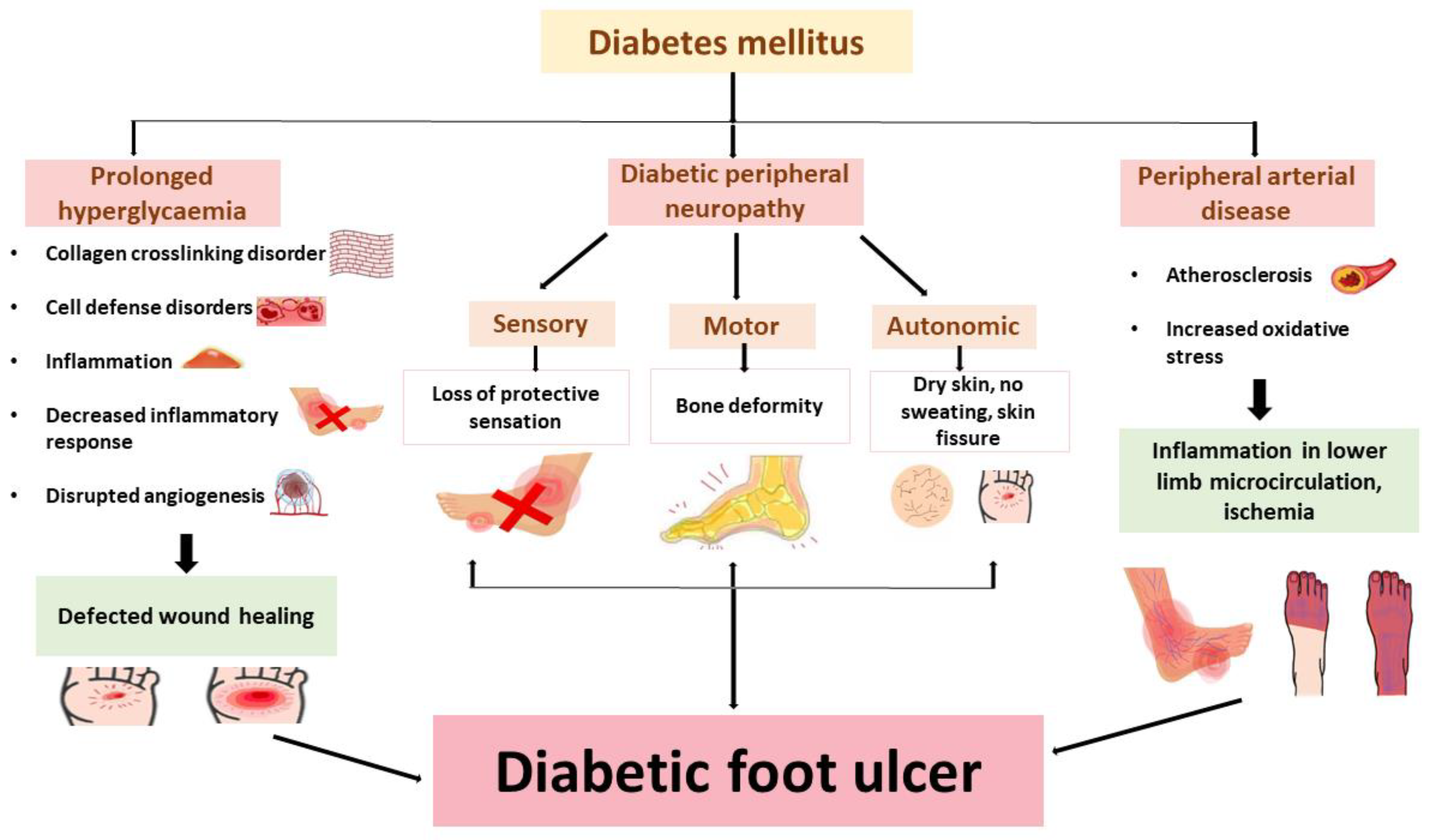
3.1. Diabetic Peripheral Neuropathy
3.2. Peripheral Arterial Disease
3.3. Other Contributive Factors
4. Types of Diabetic Foot Complications
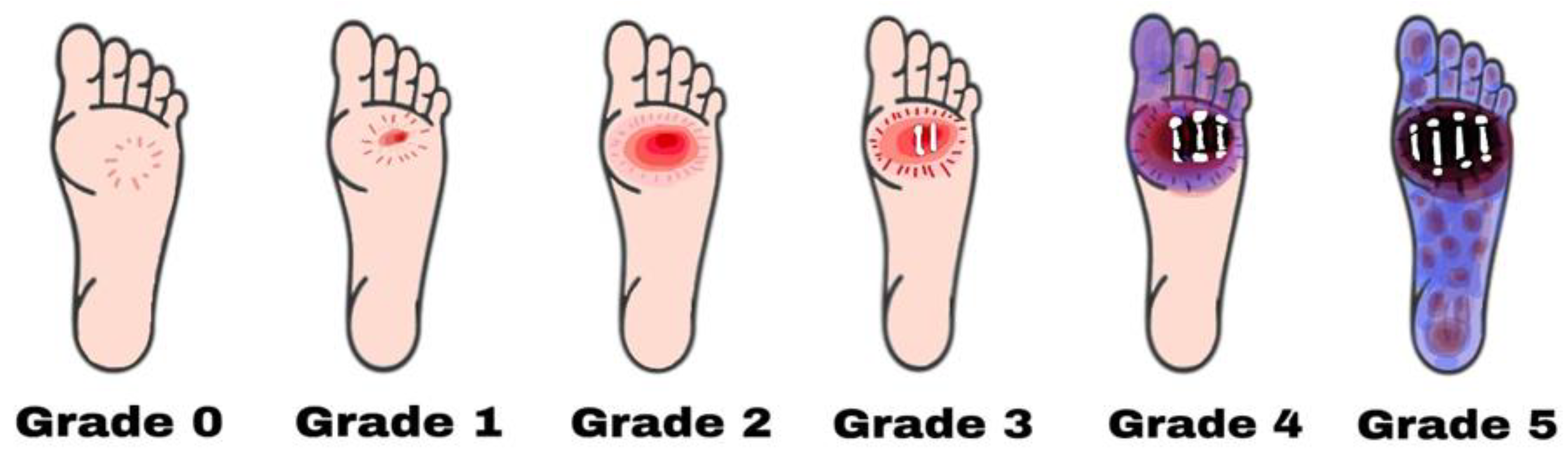
- Grade zero: No lesion on the skin, potential bone deformity or hyperkeratotic lesion, pain;
- Grade one: Superficial viable or necrotic ulcers; subcutaneous tissue loss; potential bone deformity; no penetration into the deeper layers of the skin;
- Grade two: Deeper penetrates including bones, tendons, ligaments, or deep fascia; bone deformity prominent in some aspects; absence of abscess or osteomyelitis;
- Grade Three: Presence of osteomyelitis, deep abscess, or tendinitis; severe infection symptoms (e.g., redness, heat, and swelling);
- Grade four: Gangrene (dry, wet, infected, or non-infected) in toe or forefoot; surgical ablation of the foot required with minimal blood supply for below-knee amputations;
- Grade 0: Intact skin; no sign of ulceration but the foot is at risk;
- Grade 1: Superficial ulcer; no sign of osteomyelitis or exposed bones; no deep ulceration;
- Grade 2: Deep ulceration; deeper penetration towards bones; bone deformity may be present to some extent;
- Grade 3: Presence of osteomyelitis or abscess; severe infection and redness; no gangrene exposure;
- Grade A: Not gangrenous; no ischemia;
- Grade B: Presence of ischemia but no gangrene;
- Grade C: Presence of ischemia and partial foot gangrene;
- Grade D: Presence of ischemia and complete foot gangrene [47].
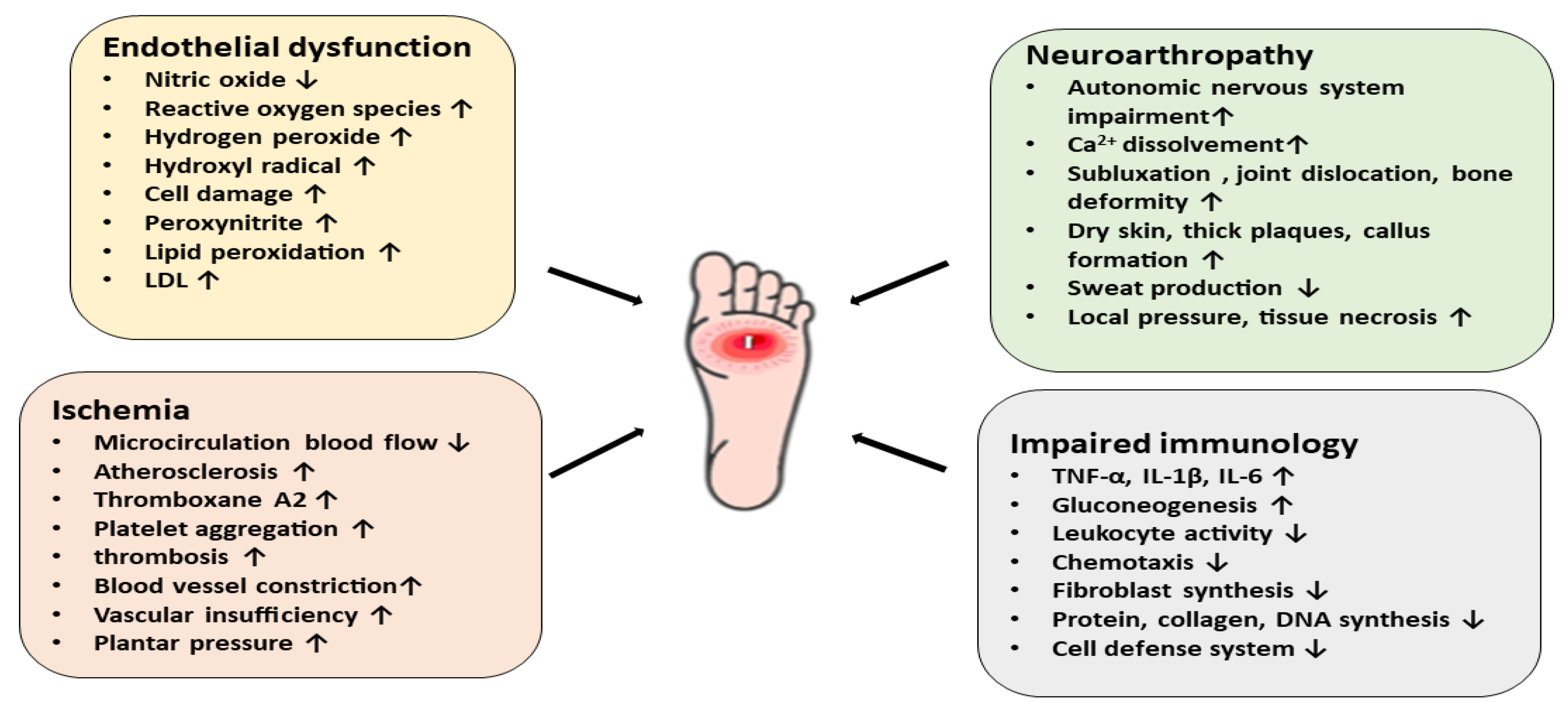
5. Pathophysiology
6. Current Therapy for Diabetic Foot Complications
7. Alternative Therapy for Diabetic Foot
8. Advantages of Alternative Therapies over Conventional Treatment Approaches for Diabetic Foot
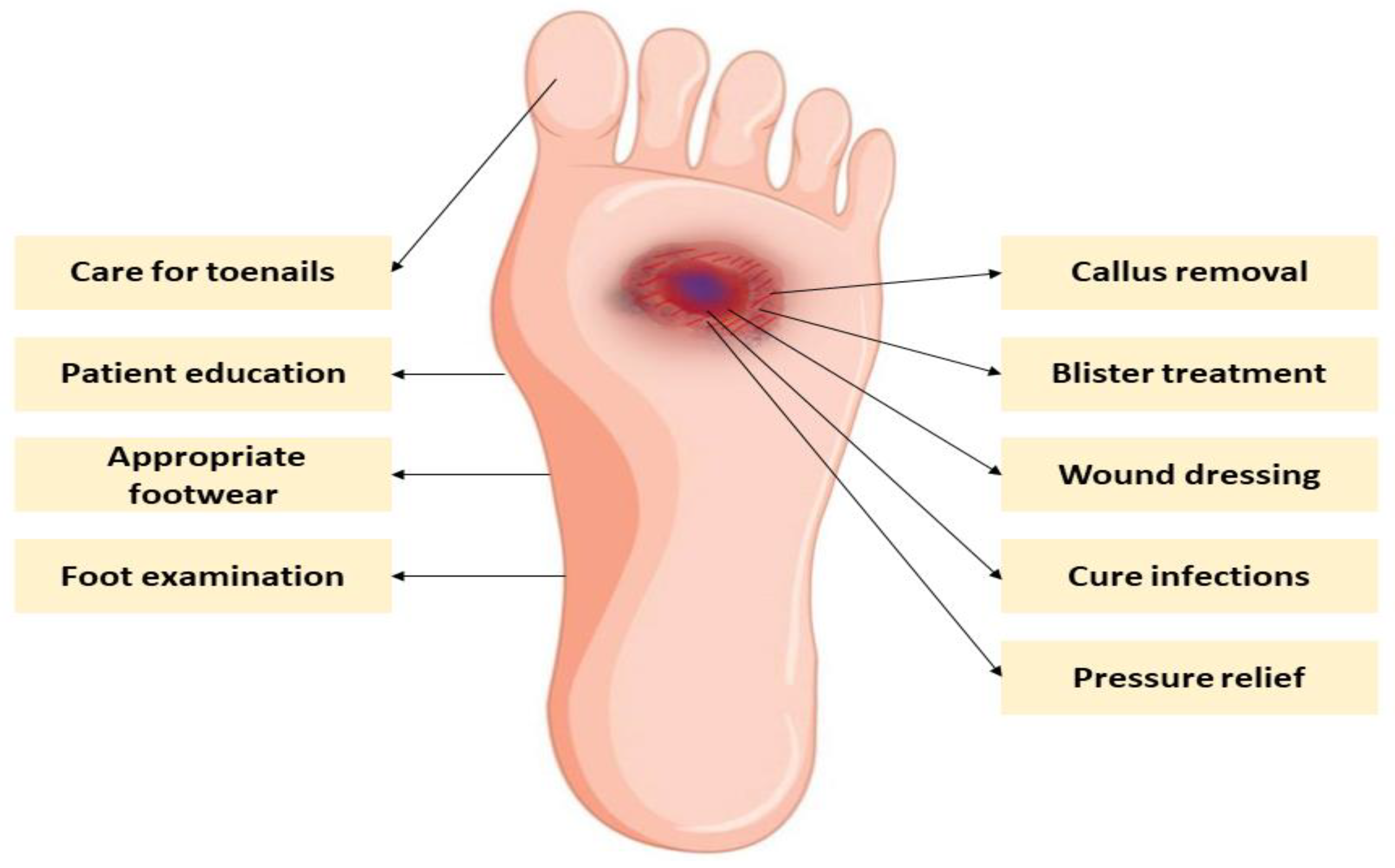
9. Prevention and Management of Diabetic Foot Complications
10. Future Strategies Used to Overcome the Burden of Diabetic Foot
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R.A. General aspects of diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 211–222. [Google Scholar]
- Mayfield, J.A. Diagnosis and classification of diabetes mellitus: New criteria. Am. Fam. Physician 1998, 58, 1355. [Google Scholar] [PubMed]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Prim. 2017, 3, 17016. [Google Scholar] [CrossRef]
- Ohlson, L.-O.; Larsson, B.; Björntorp, P.; Eriksson, H.; Svärdsudd, K.; Welin, L.; Tibblin, G.; Wilhelmsen, L. Risk factors for type 2 (non-insulin-dependent) diabetes mellitus. Thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia 1988, 31, 798–805. [Google Scholar] [CrossRef]
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular complications of type 2 diabetes mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Giurato, L.; Uccioli, L. The diabetic foot: Charcot joint and osteomyelitis. Nucl. Med. Commun. 2006, 27, 745–749. [Google Scholar] [CrossRef]
- Nathan, D.M. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993, 328, 1676–1685. [Google Scholar] [CrossRef]
- Volmer-Thole, M.; Lobmann, R. Neuropathy and diabetic foot syndrome. Int. J. Mol. Sci. 2016, 17, 917. [Google Scholar] [CrossRef] [PubMed]
- Brocco, E.; Ninkovic, S.; Marin, M.; Whisstock, C.; Bruseghin, M.; Boschetti, G.; Viti, R.; Forlini, W.; Volpe, A. Diabetic foot management: Multidisciplinary approach for advanced lesion rescue. J. Cardiovasc. Surg. 2018, 59, 670–684. [Google Scholar] [CrossRef]
- Markakis, K.; Bowling, F.; Boulton, A. The diabetic foot in 2015: An overview. Diabetes/Metab. Res. Rev. 2016, 32, 169–178. [Google Scholar] [CrossRef]
- Bandyk, D.F. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin. Vasc. Surg. 2018, 31, 43–48. [Google Scholar] [CrossRef]
- Bekele, F.; Chelkeba, L. Amputation rate of diabetic foot ulcer and associated factors in diabetes mellitus patients admitted to Nekemte referral hospital, western Ethiopia: Prospective observational study. J. Foot Ankle Res. 2020, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H.A. Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, R.; Bahramsoltani, R.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Medicinal Plants as Efficacious Agents for Diabetic Foot Ulcers: A Systematic Review of Clinical Studies. Wounds Compend. Clin. Res. Pract. 2021, 33, 207–218. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Medicinal plants and their effects on diabetic wound healing. Vet. World 2019, 12, 653. [Google Scholar] [CrossRef] [Green Version]
- Nagori, B.P.; Solanki, R. Role of medicinal plants in wound healing. Res. J. Med. Plant 2011, 5, 392–405. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal plants and their components for wound healing applications. Future J. Pharm. Sci. 2021, 7, 53. [Google Scholar] [CrossRef]
- El-Nashar, H.A.; Mostafa, N.M.; El-Shazly, M.; Eldahshan, O.A. The role of plant-derived compounds in managing diabetes mellitus: A review of literature from 2014 to 2019. Curr. Med. Chem. 2021, 28, 4694–4730. [Google Scholar] [CrossRef] [PubMed]
- Abdelghffar, E.A.; Mostafa, N.M.; El-Nashar, H.A.; Eldahshan, O.A.; Singab, A.N.B. Chilean pepper (Schinus polygamus) ameliorates the adverse effects of hyperglycaemia/dyslipidaemia in high fat diet/streptozotocin-induced type 2 diabetic rat model. Ind. Crops Prod. 2022, 183, 114953. [Google Scholar] [CrossRef]
- Talukdar, N.; Das, K.; Barman, I. A review on ethanobotanical survey of medicinal plants available in North-East India against microbes involved in diabetic foot ulcer. J. Diabetol. 2021, 12, 128. [Google Scholar] [CrossRef]
- Boulton, A.J.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef]
- Boulton, A.J. The diabetic foot: Grand overview, epidemiology and pathogenesis. Diabetes/Metab. Res. Rev. 2008, 24, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Chun, D.-I.; Kim, S.; Kim, J.; Yang, H.-J.; Kim, J.H.; Cho, J.-H.; Yi, Y.; Kim, W.J.; Won, S.H. Epidemiology and burden of diabetic foot ulcer and peripheral arterial disease in Korea. J. Clin. Med. 2019, 8, 748. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]
- Oliver, T.I.; Mutluoglu, M. Diabetic foot ulcer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Perez-Favila, A.; Martinez-Fierro, M.L.; Rodriguez-Lazalde, J.G.; Cid-Baez, M.A.; Zamudio-Osuna, M.D.J.; Martinez-Blanco, M.D.R.; Mollinedo-Montaño, F.E.; Rodriguez-Sanchez, I.P.; Castañeda-Miranda, R.; Garza-Veloz, I. Current therapeutic strategies in diabetic foot ulcers. Medicina 2019, 55, 714. [Google Scholar] [CrossRef] [Green Version]
- Boulton, A.J. The pathway to foot ulceration in diabetes. Med. Clin. 2013, 97, 775–790. [Google Scholar] [CrossRef]
- Alavi, A.; Sibbald, R.G.; Mayer, D.; Goodman, L.; Botros, M.; Armstrong, D.G.; Woo, K.; Boeni, T.; Ayello, E.A.; Kirsner, R.S. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J. Am. Acad. Dermatol. 2014, 70, e1. [Google Scholar] [CrossRef]
- Frykberg, R.G. Diabetic foot ulcers: Pathogenesis and management. Am. Fam. Physician 2002, 66, 1655. [Google Scholar] [PubMed]
- Rosyid, F.N. Etiology, pathophysiology, diagnosis and management of diabetics’ foot ulcer. Int. J. Res. Med. Sci. 2017, 5, 4206–4213. [Google Scholar] [CrossRef] [Green Version]
- Forsythe, R.; Brownrigg, J.; Hinchliffe, R. Peripheral arterial disease and revascularization of the diabetic foot. Diabetes Obes. Metab. 2015, 17, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Laing, P. The development and complications of diabetic foot ulcers. Am. J. Surg. 1998, 176, 11S–19S. [Google Scholar] [CrossRef]
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Shahbazian, H.; Yazdanpanah, L.; Latifi, S.M. Risk assessment of patients with diabetes for foot ulcers according to risk classification consensus of International Working Group on Diabetic Foot (IWGDF). Pak. J. Med. Sci. 2013, 29, 730. [Google Scholar] [CrossRef]
- Iraj, B.; Khorvash, F.; Ebneshahidi, A.; Askari, G. Prevention of diabetic foot ulcer. Int. J. Prev. Med. 2013, 4, 373. [Google Scholar]
- Waaijman, R.; De Haart, M.; Arts, M.L.; Wever, D.; Verlouw, A.J.; Nollet, F.; Bus, S.A. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care 2014, 37, 1697–1705. [Google Scholar] [CrossRef] [Green Version]
- Monteiro-Soares, M.; Boyko, E.; Ribeiro, J.; Ribeiro, I.; Dinis-Ribeiro, M. Predictive factors for diabetic foot ulceration: A systematic review. Diabetes/Metab. Res. Rev. 2012, 28, 574–600. [Google Scholar] [CrossRef]
- McEwen, L.N.; Ylitalo, K.R.; Herman, W.H.; Wrobel, J.S. Prevalence and risk factors for diabetes-related foot complications in Translating Research Into Action for Diabetes (TRIAD). J. Diabetes Its Complicat. 2013, 27, 588–592. [Google Scholar] [CrossRef] [Green Version]
- Tellechea, A.; Leal, E.; Veves, A.; Carvalho, E. Inflammatory and angiogenic abnormalities in diabetic wound healing: Role of neuropeptides and therapeutic perspectives. Open Circ. Vasc. J. 2010, 3, 43–55. [Google Scholar] [CrossRef]
- Schaper, N. Diabetic foot ulcer classification system for research purposes: A progress report on criteria for including patients in research studies. Diabetes/Metab. Res. Rev. 2004, 20, S90–S95. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoate, W.J.; Bus, S.A.; Game, F.L.; Hinchliffe, R.J.; Price, P.E.; Schaper, N.C. International Working Group on the Diabetic Foot and the European Wound Management Association. Reporting standards of studies and papers on the prevention and management of foot ulcers in diabetes: Required details and markers of good quality. Lancet Diabetes Endocrinol. 2016, 4, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Wagner, F.W., Jr. The dysvascular foot: A system for diagnosis and treatment. Foot Ankle 1981, 2, 64–122. [Google Scholar] [CrossRef] [PubMed]
- Abid, A.; Hosseinzadeh, S. Foot Ulcer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Robinson, A.; Pasapula, C.; Brodsky, J. Surgical aspects of the diabetic foot. J. Bone Jt. Surgery. Br. Vol. 2009, 91, 1–7. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Edmonds, M. The diabetic foot: Pathophysiology and treatment. Clin. Endocrinol. Metab. 1986, 15, 889–916. [Google Scholar] [CrossRef]
- Apelqvist, J.; Ragnarson-Tennvall, G.; Larsson, J.; Persson, U. Diabetic foot ulcers in a multidisciplinary setting an economic analysis of primary healing and healing with amputation. J. Intern. Med. 1994, 235, 463–471. [Google Scholar] [CrossRef]
- Eslami, M.H.; Zayaruzny, M.; Fitzgerald, G.A. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. J. Vasc. Surg. 2007, 45, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Galkowska, H.; Wojewodzka, U.; Olszewski, W.L. Low recruitment of immune cells with increased expression of endothelial adhesion molecules in margins of the chronic diabetic foot ulcers. Wound Repair Regen. 2005, 13, 248–254. [Google Scholar] [CrossRef]
- Schaper, N.; Van Netten, J.; Apelqvist, J.; Lipsky, B.; Bakker, K.; Foot, I.W.G.o.t.D. Prevention and management of foot problems in diabetes: A Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes/Metab. Res. Rev. 2016, 32, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer—A review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Clayton, W., Jr.; Elasy, T.A. A review of the pathophysiology, classification, and treatment of foot ulcers in diabetic patients. Clin. Diabetes 2009, 27, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Wild, T.; Rahbarnia, A.; Kellner, M.; Sobotka, L.; Eberlein, T. Basics in nutrition and wound healing. Nutrition 2010, 26, 862–866. [Google Scholar] [CrossRef]
- Sharp, A.; Clark, J. Diabetes and its effects on wound healing. Nurs. Stand. 2011, 25, 41–47. [Google Scholar] [CrossRef]
- Singh, S.; Pai, D.R.; Yuhhui, C. Diabetic foot ulcer–diagnosis and management. Clin. Res. Foot Ankle 2013, 1, 120. [Google Scholar]
- Syafril, S. Pathophysiology diabetic foot ulcer. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Medan, Indonesia, 15–18 November 2017; IOP Publishing: Bristol, UK, 2018; p. 012161. [Google Scholar]
- Lipsky, B.A.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.; Kono, S.; Lavery, L.; Senneville, É.; Urbančič-Rovan, V.; Van Asten, S.; Peters, E.J. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes/Metab. Res. Rev. 2016, 32, 45–74. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Gupta, B. Choices and challenges of antibiotics therapy in diabetic foot infection. Indian J. Endocrinol. Metab. 2017, 21, 647. [Google Scholar] [CrossRef]
- Ramirez-Acuña, J.M.; Cardenas-Cadena, S.A.; Marquez-Salas, P.A.; Garza-Veloz, I.; Perez-Favila, A.; Cid-Baez, M.A.; Flores-Morales, V.; Martinez-Fierro, M.L. Diabetic foot ulcers: Current advances in antimicrobial therapies and emerging treatments. Antibiotics 2019, 8, 193. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.; Stapley, S. Debridement of diabetic foot ulcers. Cochrane Database Syst. Rev. 2010, 2010, CD003556. [Google Scholar] [CrossRef]
- Kavitha, K.V.; Tiwari, S.; Purandare, V.B.; Khedkar, S.; Bhosale, S.S.; Unnikrishnan, A.G. Choice of wound care in diabetic foot ulcer: A practical approach. World J. Diabetes 2014, 5, 546. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, R.; Andros, G.; Apelqvist, J.; Bakker, K.; Fiedrichs, S.; Lammer, J.; Lepantalo, M.; Mills, J.; Reekers, J.; Shearman, C. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes/Metab. Res. Rev. 2012, 28, 179–217. [Google Scholar] [CrossRef] [PubMed]
- Mulder, G.; Alfieri, D. The diabetic foot: Considerations for pressure reduction and off-loading. Prim. Intent. Aust. J. Wound Manag. 2007, 15, 58–65. [Google Scholar]
- Adams, C.A., Jr.; Deitch, E.A. Diabetic foot infections. In Surgical Treatment: Evidence-Based and Problem-Oriented; Holzheimer, R.G., Mannick, J.A., Eds.; Zuckschwerdt: Munich, Germany, 2001. [Google Scholar]
- Kerr, M.; Barron, E.; Chadwick, P.; Evans, T.; Kong, W.; Rayman, G.; Sutton-Smith, M.; Todd, G.; Young, B.; Jeffcoate, W. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet. Med. 2019, 36, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Stratman, S.; Schneider, C.; Kirsner, R.S. New Therapies for the Treatment of Diabetic Foot Ulcers: Updated Review of Clinical Trials. Surg. Technol. Int. 2020, 37, 37–47. [Google Scholar]
- Salehi, B.; Ata, A.V.; Anil Kumar, N.; Sharopov, F.; Ramirez-Alarcon, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulprachakarn, K.; Ounjaijean, S.; Wungrath, J.; Mani, R.; Rerkasem, K. Micronutrients and natural compounds status and their effects on wound healing in the diabetic foot ulcer. Int. J. Low. Extrem. Wounds 2017, 16, 244–250. [Google Scholar] [CrossRef]
- Li, S.; Zhao, J.; Liu, J.; Xiang, F.; Lu, D.; Liu, B.; Xu, J.; Zhang, H.; Zhang, Q.; Li, X. Prospective randomized controlled study of a Chinese herbal medicine compound Tangzu Yuyang Ointment for chronic diabetic foot ulcers: A preliminary report. J. Ethnopharmacol. 2011, 133, 543–550. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H.-J.; Wang, L.-Q.; Lu, C.-L.; Yan, Y.-Q.; Lu, H.; Zhang, K.; Zhang, H.-M.; Liu, J.-P. The effects of Chinese herbal medicines for treating diabetic foot ulcers: A systematic review of 49 randomized controlled trials. Complement. Ther. Med. 2019, 44, 32–43. [Google Scholar] [CrossRef]
- Debnath, P.; Prakash, A.; Banerjee, S.; Rao, P.N.; Tripathy, T.B.; Adhikari, A.; Shivakumar. Quality of life and treatment satisfaction observed among Indians with diabetes foot ulcers undergoing ayurvedic adjunct therapy. J. Evid.-Based Complement. Altern. Med. 2015, 20, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Alam, G.; Singh, M.P.; Singh, A. Wound healing potential of some medicinal plants. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 136–145. [Google Scholar]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound healing effects of curcumin: A short review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef]
- Pişkin, A.; Altunkaynak, B.Z.; Tümentemur, G.; Kaplan, S.; Yazıcı, Ö.B.; Hökelek, M. The beneficial effects of Momordica charantia (bitter gourd) on wound healing of rabbit skin. J. Dermatol. Treat. 2014, 25, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Koca, U.; Süntar, I.; Akkol, E.K.; Yılmazer, D.; Alper, M. Wound repair potential of Olea europaea L. leaf extracts revealed by in vivo experimental models and comparative evaluation of the extracts’ antioxidant activity. J. Med. Food 2011, 14, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Valecha, V. Diabetes and antidiabetic herbal formulations: An alternative to Allopathy. Eur. J. Med. 2014, 6, 226–240. [Google Scholar] [CrossRef]
- Papanas, N.; Maltezos, E. Polyherbal formulation as a therapeutic option to improve wound healing in the diabetic foot. Indian J. Med. Res. 2011, 134, 146. [Google Scholar]
- Ongarora, B.G. Recent technological advances in the management of chronic wounds: A literature review. Health Sci. Rep. 2022, 5, e641. [Google Scholar] [CrossRef]
- Mandrika, I.; Kumar, S.; Zandersone, B.; Eranezhath, S.S.; Petrovska, R.; Liduma, I.; Jezupovs, A.; Pirags, V.; Tracevska, T. Antibacterial and anti-inflammatory potential of polyherbal formulation used in chronic wound healing. Evid.-Based Complement. Altern. Med. 2021, 2021, 9991454. [Google Scholar] [CrossRef]
- Roozbeh, N.; Darvish, L. Acacia nilotica: New plant for help in pelvic organ prolapse. J. Menopausal Med. 2016, 22, 129–130. [Google Scholar] [CrossRef] [Green Version]
- Kankara, S.; Sani, D.; Ibrahim, M.; Mustafa, M.; Go, R. Acacia nilotica pods’ water extract enhances wound healing in Sprague-Dawley rats by alleviating oxidative stress and suppressing pro-inflammatory cytokines. Niger. J. Sci. Res. 2017, 16, 202–210. [Google Scholar]
- Islam, M.S.; Ara, H.; Ahmad, K.I.; Uddin, M.M. A review on medicinal uses of different plants of Euphorbiaceae family. UPRA3 2019, 4, 45–49. [Google Scholar] [CrossRef]
- Gutierrez, R.P. Evaluation of the wound healing properties of Acalypha langiana in diabetic rats. Fitoterapia 2006, 77, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Shalu, R.; Amanjot, G.S.P.; Kapil, K.; Sukhbir, K. Wound healing potential of medicinal plants with their screening models: A comprehensive review. J. Drug Deliv. Ther. 2016, 6, 56–66. [Google Scholar]
- He, X.; Fang, J.; Chen, X.; Zhao, Z.; Li, Y.; Meng, Y.; Huang, L. Actinidia chinensis Planch.: A review of chemistry and pharmacology. Front. Pharmacol. 2019, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, G.; Safaee, M.; Sanei, M.H. Effects of topical Kiwifruit on healing of neuropathic diabetic foot ulcer. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 520. [Google Scholar]
- Worasakwutiphong, S.; Termwattanaphakdee, T.; Kamolhan, T.; Phimnuan, P.; Sittichokechaiwut, A.; Viyoch, J. Evaluation of the safety and healing potential of a fibroin-aloe gel film for the treatment of diabetic foot ulcers. J. Wound Care 2021, 30, 1020–1028. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological update properties of Aloe vera and its major active constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [Green Version]
- Jalalpure, S.S.; Agrawal, N.; Patil, M.; Chimkode, R.; Tripathi, A. Antimicrobial and wound healing activities of leaves of Alternanthera sessilis Linn. Int. J. Green Pharm. (IJGP) 2008, 3, 141–144. [Google Scholar] [CrossRef]
- Muniandy, K.; Gothai, S.; Tan, W.S.; Kumar, S.S.; Mohd Esa, N.; Chandramohan, G.; Al-Numair, K.S.; Arulselvan, P. In vitro wound healing potential of stem extract of Alternanthera sessilis. Evid.-Based Complement. Altern. Med. 2018, 2018, 3142073. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Sasi, M.; Maheshwari, C.; Singh, S.; Dhumal, S. Custard apple (Annona squamosa L.) leaves: Nutritional composition, phytochemical profile, and health-promoting biological activities. Biomolecules 2021, 11, 614. [Google Scholar] [CrossRef]
- Ponrasu, T.; Suguna, L. Efficacy of Annona squamosa on wound healing in streptozotocin-induced diabetic rats. Int. Wound J. 2012, 9, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Evaluation of the antidiabetic and insulin releasing effects of A. squamosa, including isolation and characterization of active phytochemicals. Plants 2020, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Baghel, U.S.; Gautam, A.; Baghel, D.S.; Yadav, D.; Malik, J.; Yadav, R. The genus Anogeissus: A review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 194, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Motto, A.E.; Lawson-Evi, P.; Bakoma, B.; Eklu-Gadegbeku, K.; Aklikokou, K. Antihyperlipidemic and antioxidant properties of hydro-alcoholic extracts from Anogeissus leiocarpus (Combretaceae). Heliyon 2021, 7, e06648. [Google Scholar] [CrossRef]
- Alhassan, D.A.; Uba, A.I.; Muhammad, A.U.; Muhammad, Y.Y.U. Phytochemical screening and antimicrobial activity of crude stem bark extracts of Anogeissus leiocarpus. Eur. J. Med. Plants 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Hosseini, A.; Mirzaee, F.; Davoodi, A.; Jouybari, H.B.; Azadbakht, M. The traditional medicine aspects, biological activity and phytochemistry of Arnebia spp. Med. Glas. 2018, 15, 1–9. [Google Scholar]
- Nasiri, E.; Hosseinimehr, S.J.; Azadbakht, M.; Akbari, J.; Enayati-Fard, R.; Azizi, S.; Azadbakht, M. The healing effect of Arnebia euchroma ointment versus silver sulfadiazine on burn wounds in rat. World J. Plast. Surg. 2015, 4, 134. [Google Scholar]
- Gupta, S.K.; Sharma, O.P.; Raina, N.S.; Sehgal, S. Ethno-botanical study of medicinal plants of Paddar valley of Jammu and Kashmir, India. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Iabichella, M.L. The use of an extract of Hypericum perforatum and Azadirachta indica in advanced diabetic foot: An unexpected outcome. Case Rep. 2013, 2013, bcr2012007299. [Google Scholar] [CrossRef] [Green Version]
- Maan, P.; Yadav, K.S.; Yadav, N.P. Wound healing activity of Azadirachta indica A. juss stem bark in mice. Pharmacogn. Mag. 2017, 13, S316. [Google Scholar]
- Chothani, D.L.; Vaghasiya, H. A review on Balanites aegyptiaca Del (desert date): Phytochemical constituents, traditional uses, and pharmacological activity. Pharmacogn. Rev. 2011, 5, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annan, K.; Dickson, R. Evaluation of wound healing actions of Hoslundia opposita vahl, Anthocleista nobilis G. Don. and Balanites aegyptiaca L. J. Sci. Technol. 2008, 28, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Anti-hyperglycaemic and insulin-releasing effects of Camellia sinensis leaves and isolation and characterisation of active compounds. Br. J. Nutr. 2021, 126, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound healing and the use of medicinal plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hariono, M.; Julianus, J.; Djunarko, I.; Hidayat, I.; Adelya, L.; Indayani, F.; Auw, Z.; Namba, G.; Hariyono, P. The future of Carica papaya Leaf extract as an herbal medicine product. Molecules 2021, 26, 6922. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumar, S.; Mathan, S.V.; Tomar, M.S.; Singh, R.K.; Verma, P.K.; Kumar, A.; Kumar, S.; Singh, R.P.; Acharya, A. Therapeutic application of Carica papaya leaf extract in the management of human diseases. DARU J. Pharm. Sci. 2020, 28, 735–744. [Google Scholar] [CrossRef]
- Nayak, B.; Pinto Pereira, L.M. Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Complement. Altern. Med. 2006, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Lee, O.N.; Ak, G.; Zengin, G.; Cziáky, Z.; Jekő, J.; Rengasamy, K.R.; Park, H.Y.; Kim, D.H.; Sivanesan, I. Phytochemical composition, antioxidant capacity, and enzyme inhibitory activity in callus, somaclonal variant, and normal green shoot tissues of Catharanthus roseus (L) G. Don. Molecules 2020, 25, 4945. [Google Scholar] [CrossRef]
- Rivera-Mondragón, A.; Ortíz, O.O.; Bijttebier, S.; Vlietinck, A.; Apers, S.; Pieters, L.; Caballero-George, C. Selection of chemical markers for the quality control of medicinal plants of the genus Cecropia. Pharm. Biol. 2017, 55, 1500–1512. [Google Scholar] [CrossRef] [Green Version]
- Nayak, B.S. Cecropia peltata L. (Cecropiaceae) has wound-healing potential: A preclinical study in a Sprague Dawley rat model. Int. J. Low. Extrem. Wounds 2006, 5, 20–26. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A systematic review of the effect of Centella asiatica on wound healing. Int. J. Environ. Res. Public Health 2022, 19, 3266. [Google Scholar] [PubMed]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian J. Pharm. Sci. 2010, 72, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.; Ansari, M.; Alam, A.; Khan, T. Oral dose of citrus peel extracts promotes wound repair in diabetic rats. Pak. J. Biol. Sci. PJBS 2013, 16, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Lai, A.; Stange, R.R., Jr.; McCollum, T.G.; Schirra, M. Characterization of the wound-induced material in Citrus paradisi fruit peel by carbon-13 CP-MAS solid state NMR spectroscopy. Phytochemistry 2003, 63, 177–183. [Google Scholar] [PubMed]
- Uckoo, R.M.; Jayaprakasha, G.K.; Balasubramaniam, V.; Patil, B.S. Grapefruit (Citrus paradisi Macfad) phytochemicals composition is modulated by household processing techniques. J. Food Sci. 2012, 77, C921–C926. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.N.; Rani, R.; Kokane, A.D.; Ghosh, D.K.; Tomar, S.; Sharma, A.K. Characterization of a cytoplasmic 2-Cys peroxiredoxin from Citrus sinensis and its potential role in protection from oxidative damage and wound healing. Int. J. Biol. Macromol. 2022, 209, 1088–1099. [Google Scholar] [CrossRef]
- Favela-Hernández, J.M.J.; González-Santiago, O.; Ramírez-Cabrera, M.A.; Esquivel-Ferriño, P.C.; Camacho-Corona, M.D.R. Chemistry and Pharmacology of Citrus sinensis. Molecules 2016, 21, 247. [Google Scholar] [CrossRef] [Green Version]
- Rusak, A.; Rybak, Z. New directions of research related to chronic wound healing. Polim. Med. 2013, 43, 199–204. [Google Scholar]
- Balogun, F.O.; Ashafa, A.O.T. A review of plants used in South African traditional medicine for the management and treatment of hypertension. Planta Med. 2019, 85, 312–334. [Google Scholar] [CrossRef] [Green Version]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (Lemon) phenomenon—A review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Sami, D.G.; Abdellatif, A.; Azzazy, H.M. Turmeric/oregano formulations for treatment of diabetic ulcer wounds. Drug Dev. Ind. Pharm. 2020, 46, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Aggarwal, B.B. Turmeric, the golden spice. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, Florida, USA, 2011. [Google Scholar]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- Qabaha, K.; Ras, S.A.; Abbadi, J.; Al-Rimawi, F. Anti-inflammatory of both Eucalyptus spp. and Pistascia lentiscus were investigated along with their phenolic compounds analysis using HPLC with UV detection. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, R.; Zubair, M.; Khan, M.A.; Muzammil, S.; Siddique, M.H. Extracts of Eucalyptus alba Promote Diabetic Wound Healing by Inhibiting α-Glucosidase and Stimulating Cell Proliferation. Evid.-Based Complement. Altern. Med. 2022, 2022, 4953105. [Google Scholar] [CrossRef]
- Alam, P.; Shakeel, F.; Anwer, M.K.; Foudah, A.I.; Alqarni, M.H. Wound healing study of eucalyptus essential oil containing nanoemulsion in rat model. J. Oleo Sci. 2018, 67, ess18005. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Malhotra, R.; Kumar, D. Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacogn. Rev. 2010, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Tuhin, R.H.; Begum, M.; Rahman, M.; Karim, R.; Begum, T.; Ahmed, S.U.; Mostofa, R.; Hossain, A.; Abdel-Daim, M.; Begum, R. Wound healing effect of Euphorbia hirta linn.(Euphorbiaceae) in alloxan induced diabetic rats. BMC Complement. Altern. Med. 2017, 17, 423. [Google Scholar] [CrossRef]
- Bosisio, E.; Mascetti, D.; Verotta, L.; Zani, F.; Mazza, P.; Talbot, M. Guiera senegalensis JF Gmelin (Combretaceae): Biological activities and chemical investigation. Phytomedicine 1997, 3, 339–348. [Google Scholar] [CrossRef]
- Alshafei, N.K.; Ahmed, S.; Abdelfattah, N. Preliminary observations on the uses of Guiera senegalensis as a traditional medicinal plants in Western Kurdofan, Sudan. J. Plant Biochem. Physiol. 2016, 2, 42–48. [Google Scholar]
- Miaffo, D.; Ntchapda, F.; Kamgue, O.G.; Mahamad, A.T.; Kamanyi, A. Glucose-lowering potential of Guiera senegalensis roots in a diabetic rat model. Avicenna J. Phytomedicine 2020, 10, 653. [Google Scholar]
- Ifijen, I.; Mamza, A.; Fasina, K.; Omoruyi, J.; Ikhuoria, E. Phytochemical analysis of Guiera senegalensis jF Gmel extract and its anti-plasmodial properties on wister albino mice via oral route. Int. J. Pharmacol. Phytochem. Ethnomed. 2019, 13, 35–44. [Google Scholar]
- Daisy, E.A.C.; Rajendran, N.K.; Houreld, N.N.; Marraiki, N.; Elgorban, A.M.; Rajan, M. Curcumin and Gymnema sylvestre extract loaded graphene oxide-polyhydroxybutyrate-sodium alginate composite for diabetic wound regeneration. React. Funct. Polym. 2020, 154, 104671. [Google Scholar] [CrossRef]
- Tiwari, P.; Mishra, B.; Sangwan, N.S. Phytochemical and pharmacological properties of Gymnema sylvestre: An important medicinal plant. BioMed Res. Int. 2014, 2014, 830285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskar, A.; Nithya, V. Evaluation of the wound-healing activity of Hibiscus rosa sinensis L. (Malvaceae) in Wistar albino rats. Indian J. Pharmacol. 2012, 44, 694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.-M.; Chen, C.; Jiang, J.-Y.; Zheng, Y.-L.; Cai, W.-F.; Wang, B.; Ling, Z.; Tang, L.; Wang, Y.-H.; Shi, G.-G. The N-butyl alcohol extract from Hibiscus rosa-sinensis L. flowers enhances healing potential on rat excisional wounds. J. Ethnopharmacol. 2017, 198, 291–301. [Google Scholar] [CrossRef]
- Ansari, P.; Azam, S.; Hannan, J.M.A.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Anti-hyperglycaemic activity of H. rosa-sinensis leaves is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of insulin secretion. J. Ethnopharmacol. 2020, 253, 112647. [Google Scholar] [CrossRef]
- Perez, G.R.; Vargas, S.R.; Ortiz, H.Y. Wound healing properties of Hylocereus undatus on diabetic rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2005, 19, 665–668. [Google Scholar]
- Joshi, M.; Prabhakar, B. Phytoconstituents and pharmaco-therapeutic benefits of pitaya: A wonder fruit. J. Food Biochem. 2020, 44, e13260. [Google Scholar] [CrossRef]
- Yadollah-Damavandi, S.; Chavoshi-Nejad, M.; Jangholi, E.; Nekouyian, N.; Hosseini, S.; Seifaee, A.; Rafiee, S.; Karimi, H.; Ashkani-Esfahani, S.; Parsa, Y. Topical Hypericum perforatum improves tissue regeneration in full-thickness excisional wounds in diabetic rat model. Evid.-Based Complement. Altern. Med. 2015, 2015, 245328. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.Z.; Al Mahmud, Z.; Ali, M.S.; Bachar, S.C. Phytochemical and pharmacological investigations of rhizome extracts of Kaempferia galanga. Int. J. Pharmacogn. 2014, 1, 185–192. [Google Scholar]
- Shanbhag, T.V.; Sharma, C.; Adiga, S.; Bairy, L.K.; Shenoy, S.; Shenoy, G. Wound healing activity of alcoholic extract of Kaempferia galanga in Wistar rats. Indian J. Physiol. Pharmacol. 2006, 50, 384–390. [Google Scholar]
- Wang, S.-Y.; Zhao, H.; Xu, H.-T.; Han, X.-D.; Wu, Y.-S.; Xu, F.-F.; Yang, X.-B.; Göransson, U.; Liu, B. Kaempferia galanga L.: Progresses in phytochemistry, pharmacology, toxicology and ethnomedicinal uses. Front. Pharmacol. 2021, 12, 675350. [Google Scholar] [CrossRef]
- Al-Snafi, A. A review on Lawsonia inermis: A potential medicinal plant. Int. J. Curr. Pharm. Res. 2019, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Franco, E.D.S.; de Aquino, C.M.F.; Medeiros, P.L.D.; Evêncio, L.B.; Góes, A.J.D.S.; Maia, M.B.D.S. Effect of a semisolid formulation of Linum usitatissimum L. (Linseed) oil on the repair of skin wounds. Evid.-Based Complement. Altern. Med. 2012, 2012, 270752. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, Z.; Hashemdokht, F.; Bahmani, F.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Clinical and metabolic response to flaxseed oil omega-3 fatty acids supplementation in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J. Diabetes Its Complicat. 2017, 31, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Hannon-Fletcher, M.P.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Effects of 22 traditional anti-diabetic medicinal plants on DPP-IV enzyme activity and glucose homeostasis in high-fat fed obese diabetic rats. Biosci. Rep. 2021, 41, BSR20203824. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.; Krishna, V.; Vidya, S.; Mankani, K.; Manohara, Y. Wound healing activity of Lycopodium serratum. Indian J. Pharm. Sci. 2007, 69, 283. [Google Scholar] [CrossRef] [Green Version]
- Lodhi, S.; Singhai, A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishna, K.; Vvs Nrk, B.M.; Kuppusamy, G. Potential use of herbal medicines in the treatment of diabetic foot ulcers. History 2014, 14, 34–42. [Google Scholar]
- Chorepsima, S.; Tentolouris, K.; Dimitroulis, D.; Tentolouris, N. Melilotus: Contribution to wound healing in the diabetic foot. J. Herb. Med. 2013, 3, 81–86. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical constituents and pharmacological effects of Melilotus Officinalis—A review. IOSR J. Pharm. 2020, 10, 26–36. [Google Scholar]
- Zippel, J.; Deters, A.; Hensel, A. Arabinogalactans from Mimosa tenuiflora (Willd.) Poiret bark as active principles for wound-healing properties: Specific enhancement of dermal fibroblast activity and minor influence on HaCaT keratinocytes. J. Ethnopharmacol. 2009, 124, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.S.O.D.; Albuquerque, U.P.D.; Monteiro, J.M.; Amorim, E.L.C.D. Jurema-Preta (Mimosa tenuiflora [Willd.] Poir.): A review of its traditional use, phytochemistry and pharmacology. Braz. Arch. Biol. Technol. 2008, 51, 937–947. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Cumming, E.; Manoharan, G.; Kalasz, H.; Adeghate, E. Suppl 2: Medicinal chemistry of the anti-diabetic effects of Momordica charantia: Active constituents and modes of actions. Open Med. Chem. J. 2011, 5, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ly, H.T.; Pham Nguyen, M.T.; Nguyen, T.K.O.; Bui, T.P.Q.; Ke, X.; Le, V.M. Phytochemical analysis and wound-healing activity of noni (Morinda citrifolia) leaf extract. J. Herbs Spices Med. Plants 2020, 26, 379–393. [Google Scholar] [CrossRef]
- Deng, S.; West, B.J.; Jensen, C.J. Phytochemical, antioxidant and toxicological investigation of Morinda citrifolia L. blossoms. Int. Sch. Res. Not. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Raeiszadeh, M.; Esmaeili-Tarzi, M.; Bahrampour-Juybari, K.; Nematollahi-Mahani, S.; Pardakhty, A.; Nematollahi, M.; Mehrabani, M. Evaluation the effect of Myrtus communis L. extract on several underlying mechanisms involved in wound healing: An in vitro study. S. Afr. J. Bot. 2018, 118, 144–150. [Google Scholar] [CrossRef]
- Sisay, M.; Gashaw, T. Ethnobotanical, ethnopharmacological, and phytochemical studies of Myrtus communis Linn: A popular herb in Unani system of medicine. J. Evid.-Based Complement. Altern. Med. 2017, 22, 1035–1043. [Google Scholar] [CrossRef] [Green Version]
- Sallehuddin, N.; Nordin, A.; Bt Hj Idrus, R.; Fauzi, M.B. Nigella sativa and its active compound, thymoquinone, accelerate wound healing in an in vivo animal model: A comprehensive review. Int. J. Environ. Res. Public Health 2020, 17, 4160. [Google Scholar] [CrossRef]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef]
- Abdullah, F.I.; Chua, L.S.; Mohd Bohari, S.P.; Sari, E. Rationale of Orthosiphon aristatus for healing diabetic foot ulcer. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Asraf, M.H.; Sani, N.S.; Williams, C.D.; Jemon, K.; Malek, N.A.N.N. In situ biosynthesized silver nanoparticle-incorporated synthesized zeolite A using Orthosiphon aristatus extract for in vitro antibacterial wound healing. Particuology 2022, 67, 27–34. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, S.; Gupta, A.; Jangra, M.; Pratap, R. Evaluation of Prosopis cineraria (Linn.) Druce leaves for wound healing activity in rats. Ann. Pharm. Res 2015, 3, 70–74. [Google Scholar]
- Yadav, E.; Singh, D.; Yadav, P.; Verma, A. Antioxidant and anti-inflammatory properties of Prosopis cineraria based phenolic rich ointment in wound healing. Biomed. Pharmacother. 2018, 108, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Manne, A.A.; Arigela, B.; Giduturi, A.K.; Komaravolu, R.K.; Mangamuri, U.; Poda, S. Pterocarpus marsupium Roxburgh heartwood extract/chitosan nanoparticles loaded hydrogel as an innovative wound healing agent in the diabetic rat model. Mater. Today Commun. 2021, 26, 101916. [Google Scholar] [CrossRef]
- Nayak, S.B.; Rodrigues, V.; Maharaj, S.; Bhogadi, V.S. Wound healing activity of the fruit skin of Punica granatum. J. Med. Food 2013, 16, 857–861. [Google Scholar] [CrossRef]
- Arun, N.; Singh, D. Punica granatum: A review on pharmacological and therapeutic properties. Int. J. Pharm. Sci. Res. 2012, 3, 1240. [Google Scholar]
- Lau, T.; Lam, F.; Lau, K.; Chan, Y.; Lee, K.; Sahota, D.; Ho, Y.; Fung, K.; Leung, P.; Lau, C. Pharmacological investigation on the wound healing effects of Radix Rehmanniae in an animal model of diabetic foot ulcer. J. Ethnopharmacol. 2009, 123, 155–162. [Google Scholar] [CrossRef]
- Kim, S.-H.; Yook, T.-H.; Kim, J.-U. Rehmanniae radix, an effective treatment for patients with various inflammatory and metabolic diseases: Results from a review of Korean publications. J. Pharmacopunct. 2017, 20, 81. [Google Scholar]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [Green Version]
- de Macedo, L.M.; Santos, É.M.d.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., syn Salvia rosmarinus Spenn.) and its topical applications: A review. Plants 2020, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, R.R.; Sudheendra, A.T.; Nayak, P.G.; Paul, P.; Kutty, N.G.; Rao, C.M. Normal and delayed wound healing is improved by sesamol, an active constituent of Sesamum indicum (L.) in albino rats. J. Ethnopharmacol. 2011, 133, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Andargie, M.; Vinas, M.; Rathgeb, A.; Möller, E.; Karlovsky, P. Lignans of sesame (Sesamum indicum L.): A comprehensive review. Molecules 2021, 26, 883. [Google Scholar] [CrossRef]
- Singla, R.; Soni, S.; Patial, V.; Kulurkar, P.M.; Kumari, A.; Padwad, Y.S.; Yadav, S.K. Cytocompatible anti-microbial dressings of Syzygium cumini cellulose nanocrystals decorated with silver nanoparticles accelerate acute and diabetic wound healing. Sci. Rep. 2017, 7, 10457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramya, S.; Neethirajan, K.; Jayakumararaj, R. Profile of bioactive compounds in Syzygium cumini—A review. J. Pharm. Res. 2012, 5, 4548–4553. [Google Scholar]
- Singh, D.; Singh, D.; Choi, S.M.; Zo, S.M.; Painuli, R.M.; Kwon, S.W.; Han, S.S. Effect of extracts of Terminalia chebula on proliferation of keratinocytes and fibroblasts cells: An alternative approach for wound healing. Evid.-Based Complement. Altern. Med. 2014, 2014, 701656. [Google Scholar] [CrossRef]
- Sharma, P.; Dwivedee, B.P.; Bisht, D.; Dash, A.K.; Kumar, D. The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon 2019, 5, e02437. [Google Scholar] [CrossRef] [Green Version]
- Moghadam, F.H.; Vakili-Zarch, B.; Shafiee, M.; Mirjalili, A. Fenugreek seed extract treats peripheral neuropathy in pyridoxine induced neuropathic mice. Excli J. 2013, 12, 282. [Google Scholar]
- Taranalli, A.; Kuppast, I. Study of wound healing activity of seeds of Trigonella foenum graecum in rats. Indian J. Pharm. Sci. 1996, 58, 117. [Google Scholar]
- Mohamed, A.H.B.; Osman, A.A.F. Antibacterial and wound healing potential of ethanolic extract of Zingiber Officinale in albino rats. J. Dis. Med. Plants 2017, 3, 1–6. [Google Scholar]
- Kumar Gupta, S.; Sharma, A. Medicinal properties of Zingiber officinale Roscoe-A review. J. Pharm. Biol. Sci 2014, 9, 124–129. [Google Scholar]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Physician 2016, 8, 1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.Z.M.; Ng, N.S.L.; Thomas, C. Prevention and treatment of diabetic foot ulcers. J. R. Soc. Med. 2017, 110, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A.; Board, I.E. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36, e3266. [Google Scholar]
- Braun, L.; Kim, P.J.; Margolis, D.; Peters, E.J.; Lavery, L.A. What’s new in the literature: An update of new research since the original WHS diabetic foot ulcer guidelines in 2006. Wound Repair Regen. 2014, 22, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Van Netten, J.J.; Woodburn, J.; Bus, S.A. The future for diabetic foot ulcer prevention: A paradigm shift from stratified healthcare towards personalized medicine. Diabetes/Metab. Res. Rev. 2020, 36, e3234. [Google Scholar] [CrossRef]
- Eldor, R.; Raz, I.; Ben Yehuda, A.; Boulton, A. New and experimental approaches to treatment of diabetic foot ulcers: A comprehensive review of emerging treatment strategies. Diabet. Med. 2004, 21, 1161–1173. [Google Scholar] [CrossRef]
- Seyed, M.A.; Ayesha, S. Modern Phytomedicine in Treating Diabetic Foot Ulcer: Progress and Opportunities. In Diabetic Foot Ulcer; Zubair, M., Ahmad, J., Malik, A., Talluri, M.R., Eds.; Springer: Singapore, 2021; pp. 281–313. [Google Scholar]

| Conventional Therapies for Diabetic Foot Management | Examples | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Tapentadol, pregabalin, tramadol, duloxetine, acetaminophen, oxycodone | Alleviates diabetic peripheral neuropathy-induced pain | Constipation, nausea, drowsiness, confusion, drug abuse (opioids) | [29] |
| Nafcillin, ceftazidime, cefazolin, clindamycin, dalbavancin, sulfamethoxazole/trimethoprim | The most common treatment for diabetic foot infections | Antibiotic resistance, high cost, unavailability | [29,61] |
| Surgical, autolytic, mechanical, enzymatic, maggot debridement, polysaccharide beads/paste | Aids in a complete assessment of the wound, enhances breakdown of necrotic tissue, speeds up ulcer healing | High cost, time-consuming, patient reluctance, surgical debridement may increase wound size | [62,63,64] |
| Hydrogels, hydrocolloids | Prevent infections, easy to use, effective, provides thermal insulation and mechanical protection | Costly, unavailability | [29,62,64] |
| - | Heals diabetic foot ulcers | Costly | [65] |
| Half-shoes, rigid-soled post-operative shoes, total contact casts, accommodative dressings | Heals diabetic foot ulcers, significantly reduces pressure, high acceptability | Costly, patient compliance, ineffective against inflammation | [29,66] |
| Low-level laser therapy | Effective in healing wounds, reduces inflammatory phase | High cost, discontinuous in efficacy | [29] |
| - | Heals amputation or non-healing ulcers, one of the most effective methods to treat foot ulcers | Costly, lack of skilled surgeons | [67] |
| Medicinal Plants | Parts Used | Isolated Phytochemicals | Traditional Uses | Extract Form | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| Leaves, roots, bark, seeds | Gallic acid, ellagic acid, isoquercitrin, leucocianidolum, kaempferol | Diabetes, wounds, burns, stomachache, cough, malaria, pneumonia | Aqueous extract | Decreases oxidative stress; suppresses TNF-α and IL-1β activity; enhances cellular proliferation, re-epithelization of wounds, and dermal tissue regeneration | [83,84] |
| Leaves | Acalyphine, triacetoneamine | Diabetes, wounds, diarrhea, urinary infection, asthma, tuberculosis | Aqueous extract | Minimizes wound area, faster tissue regeneration in granulation section, improves congestion and edema | [18,85,86,87] |
| Fruit, stem, roots | Ursolic acid, oleanolic acid, epicatechin, quercetin, emodin | Diabetes, wound healing, fever, diarrhea, hypertension, hyperlipidemia | Pure normal concentrated kiwifruit extract | Enhances angiogenesis and vascularization, increases collagen and granulation tissues, minimizes ulcer size, initiates wound closure, inhibits bacterial infection | [88,89] |
| Leaves | Emodin, aloin, aloesin, acemannan | Diabetes, wound healing, dermatitis, constipation, infection, worm infestation | Aqueous extract | Enhances collagen cross-linking, increases DNA and glycosaminoglycans, reduces wound area, and accelerates healing rate of ulcer | [90,91] |
| Stem, leaves | Sebacic acid, bis(2-ethylhexyl) ester, neophytadiene, hexadecanoic acid | Diabetes, ulcer, wounds, lesion, fever, skin rash, burn, indigestion | Ethanol extract | Initiates wound closure, improves dermal fibroblast and keratinocyte proliferation | [92,93] |
| Leaves | Squamosin, squamostatin, rutin, linalool, β-caryophyllene | Diabetes, ulcer, hypertension, wounds, hemorrhage, epilepsy | Ethanol extract | Increases DNA synthesis, protein and collagen contents; decreases lipid peroxidation and wound size, restores epidermis; enhances tensile strength, proliferation, epithelialization, and migration of keratinocytes | [94,95,96] |
| Bark, leaves | Ellagic acid, gallic acid, anogeissinin, rutin, quercetin | Diabetes, infection, wounds, diabetes, tuberculosis, hyperlipidemia | Ethanol extract | Reduces oxidative stress, lipid peroxidation, and wound area; enhances vascularity, wound contraction, and rate of epithelialization; prevents onset of cell necrosis and microbial infection | [97,98,99] |
| Roots | Naphthoquinone, shikonin, teracrylshikonin β-hydroxyisovalerylalkannin | Diabetes, eye infection, cuts, wound, toothache, earache | Aqueous, ethanol extract | Enhances granulation tissue, wound contraction, re-epithelization; restores epidermis; minimizes wound size; accelerates wound healing | [100,101,102] |
| Leaves | Rutin, nimbin, deacetylnimbin, quercetin, azadiradione | Diabetes, ulcer, lesion, wounds, piles, intestinal worms, dermatitis, asthma, cough | Ethanol extract | Increases the rate of wound contraction, improves DNA content and nitric oxide level, enhances tissue regeneration, increases hydroxyproline and protein content | [16,87,103,104] |
| Bark, fruit, seed, leaves | Furanocoumarin, quercetin, diosgenin, linoleic acid, palmitic acid | Diabetes, malaria, wound healing, diarrhea, constipation, asthma | Aqueous extract | Prevents microbial infection, suppresses lipid peroxidation, enhances wound contraction, and accelerates its healing | [16,105,106] |
| Leaves | (-) Epigallocatechin-3-gallate, quercitrin, rutin | Diabetes, wound healing, anxiety, flatulence, cardiovascular diseases | Hot water extract | Lowers blood sugar levels, increases insulin secretion, stimulates proliferation and differentiation of keratinocytes and fibroblasts, increases collagen synthesis and angiogenesis | [16,107,108] |
| Leaves, fruits | Apigenin, hesperitin, kaempferol, rutin, naringenin | Diabetes, dengue, malaria, ulcer, hypertension, wound, psoriasis | Aqueous, ethanol, methanol extract | Improves granulation tissue weight, collagen and hydroxyproline content, wound size, and contraction; epithelizes faster and prevents microbial infection | [18,109,110] |
| Flower, roots, leaves | Catharanthine, vindolinine, isorhamnetin, quercetin, kaempferol, O-feruloylquinic acids | Diabetes, sepsis, wound healing, blood dysentery, blood purification | Ethanol extract | Enhances wound contraction, tensile strength, angiogenesis, epithelialization, vascularization, granulation tissue weight, and hydroxyproline content and protects against microbial infection | [111,112] |
| Leaves | Orientin, vitexin, rutin, chlorogenic acid, catechin | Diabetes, respiratory tract infection, wounds, kidney diseases, hypertension, sleep disorder | Aqueous, ethanol extract | Minimizes wound area; enhances hydroxyproline, protein, and hexosamine contents; accelerates wound healing | [113,114] |
| Leaves | Kaempferol, hydrochotine, linoleic, oleic, stearic, palmitic acid | Diabetes, ulcer, wounds, eczema, urinary tract infection, amenorrhea, diarrhea | Aqueous, ethanol extract | Improves angiogenesis, collagen content, fibroblast growth factor, and vascular endothelial growth factor; inhibits TNF-α, IL-6, IL-1β, COX-2, prostaglandin E2, and lipoxygenase; initiates re-epithelialization | [115,116] |
| Peel, fruit | Narirutin, hesperidin, naringin, neohesperidin, limoline | Diabetes, hypertension, gastrointestinal problems, wound healing, infection, cold, cough | Aqueous peel extract | Reduces wound size; increases collagen synthesis, hydroxyproline and protein contents, and regeneration of tissue; initiates wound closure and healing | [117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190] |
| Peel, fruit | Kaempferol, naringin, hesperetin, limocitrin, naringenin | Diabetes, bronchitis, cough, obesity, hypertension, tuberculosis | Aqueous extract | Protects from oxidative damage, inflammatory cytokines, and hydrogen-peroxide-induced cellular damage; enhances cell proliferation, blood flow in tissues, angiogenesis, keratinocyte and fibroblast migration, wound healing | [120,121,122] |
| Peel, fruit, leaves | D-limonene, naringin, hesperetin, hesperidin, apigenin, quercetin, orientin | Diabetes, cough, hypertension, sore throat, scurvy, kidney stones | Aqueous, acetone, ethanol extract | Inhibits gluconeogenesis; enhances collagen synthesis, protein and hydroxyproline contents; increases tissue growth rate; reduces wound area and healing time | [123,124] |
| Rhizome | Curcumin, curcuminoids, turmerin, vanillic acid, β-sitosterol, zingiberene | Diabetes, liver disorders, cough, inflammation, ulcer, wounds, pain, infections | Ethannol extract | Improves ulcers, inflammation, wound contraction, proliferation, fibroblast formation, collagen synthesis, granulation tissue and epithelialization; helps in tissue remodeling and wound size minimization; suppresses inflammatory cytokines | [16,76,125,126,127] |
| Leaves | Chlorogenic acid, gallic acid, caffeic acid, vanillic acid, ferulic acid | Diabetes, sinusitis, bronchitis, cold, cough, wounds, sores, | Ethanol, methanol, acetone extract | Inhibits TNF-α, IL-6, and α-glucosidase enzyme; reduces oxidative stress; decreases oxidative free radicals; increases cell proliferation, collagen synthesis, and blood flow to the wound; prevents microbial attack | [128,129,130] |
| Leaves, stem, flower | Heptacosane, camphol, leucocyanidin, kaempferol, gallic acid | Diabetes, cold, asthma, dysentery, wound healing, dermatitis | Ethanol extract | Initiates wound closure and healing; restores epidermis, collagen tissue, and extensive fibrosis; improves granulation tissue weight, inflammatory cells, cellular damage, and re-epithelialization; decreases lipid peroxidation | [16,87,131,132] |
| Roots, leaves | Epigallocatechin, epicatechin, gallic acid, quercetin | Diabetes, wound healing, gastrointestinal disorders, cough, malaria, enteritis | Aqueous, methanol extract | Exhibits antimicrobial action, inhibits lipid peroxidation and inflammatory cytokines, minimizes wound size, improves healing time | [133,134,135,136] |
| Leaves | Gymnemic acid, gurmarin, lupeol, stigmasterol, kaempferol | Diabetes, anemia, constipation, indigestion, infections, cardiopathy | Hot water, ethanol, methanol extract | Decreases blood glucose levels, bacterial growth, and inflammation; decreases formation of reactive oxidants; improves wound contraction; increases fibroblasts and enhances tissue regeneration | [137,138] |
| Flower, leaves, roots | Quercetin, stigmasterol, β-sitosterol, cyaniding, taraxeryl acetate | Diabetes, wounds, stomach ulcer, hypertension, hypercholesterolemia | Ethanol extract, n-butyl alcohol extract | Increases DNA, collagen, and protein contents of granulation tissues; improves rates of epithelialization and wound contraction, closure rate, granuloma weight, tensile strength | [139,140,141] |
| Peel, fruit | Gallic acid, betacyanin, lycopene, ascorbic acid | Diabetes, asthma, intestinal disease, wounds, ulcer, allergy, hypertension | Aqueous extract | Increases total protein, collagen, hydroxyproline, and DNA contents; enhances rate of epithelialization, wound size, tensile strength, and wound closure | [142,143] |
| Leaves, flowers | Amentoflavone, hypericin, hyperforin | Diabetes, depression, wounds healing, mycobacterium and viral infections, GIT disorders, eczema | Aqueous, ethanol extract | Improves glycemic levels, decreases inflammation, inhibits extracellular matrix degradation by matrix metalloproteinase-2 and urokinase, increases epithelial regeneration and revascularization | [103,144] |
| Rhizome | Ethyl p-methoxy cinnamate, 4-methoxy cinnamic acid, kaempferol, kaempferide, luteolin | Diabetes, hypertension, asthma, urticaria, rheumatism, wound healing, ulcers | Ethanol extract | Lowers blood glucose levels, exhibits anti-inflammatory and antioxidant effects, enhances wound contraction, increases rate of epithelialization | [19,145,146,147] |
| Leaves | Kaempferol, quercetin, luteolin, | Diabetes, wound healing, bacterial and fungal infections, jaundice, skin problems | Aqueous, ethanol extract | Exhibits antibacterial activity, improves wound healing in fissures and cracks in diabetic foot, prevents decubitus ulcers, improves fibroblasts and collagen synthesis, increases granulation tissue | [19,148] |
| Seeds | α- linolenic acid, omega-3-fatty acids, p-coumaric acid | Diabetes, diarrhea, skin diseases, wound healing, gastrointestinal infections | Ethanol extract | Improves insulin sensitivity; stimulates fibrin proliferation and collagen synthesis; increases granulation tissue around wounds; promotes vascular contraction, chemotaxis, adhesion, transmigration, and cell activation for tissue repair | [16,149,150,151] |
| Leaves | Lycoposerramine A, serratezomines A, B, C, α- pyridine, | Diabetes, wounds, sores, cuts, and burns, to improve learning and memory efficiency, Alzheimer’s disease, otitis media | Ethanol extract | Increases rates of epithelialization and wound contraction, increases content of hydroxyproline at the wound site, and exerts antioxidant and antibacterial effects | [19,152] |
| Leaves, flowers | p-hydroxy benzoic acid, chlorogenic acid, luteolin | Diabetes, epilepsy, sore throat, wounds, inflammation | Ethanol extract | Inhibits oxidative stress, exerts antibacterial action, improves angiogenesis of fibroblasts, increases collagen synthesis and hyroxyproline content, enhances epithelialization, increases wound contraction rate | [153,154] |
| Flowers, flowering stems | Quercetin, kaempferol, lupeol, oleanolic acid, coumarin compounds | Diabetes, intestinal worms, gastric ulcers, wound treatment, vein problems, hemorrhoids | Methanol, ethanol, hexane, chloroform extract | Stimulates the proliferation of endothelial cells and vascular neoangiogenesis, improves blood circulation, increases wound contraction and collagen synthesis, exerts anti-inflammatory and antioxidant effects | [155,156] |
| Bark | Lupeol, mimonoside A,B,C, arabinogalactans, campesterol-3-O-β-D-glucopyranosyl, stigmasterol-3-O-β-D-glucopyranosyl | Diabetes, skin burns, ulcers, wounds, psoriasis, inflammation | Aqueous, ethanol extract | Enhances proliferation of dermal fibroblasts, stimulates production of keratinocytes, exerts antimicrobial activity to improve wound healing | [157,158] |
| Leaves, fruit | Momocharin, momordicin | Diabetes, wound healing, microbial infections, hypertension, inflammation, skin disease | Aqueous, ethanol, ether extract | Lowers blood glucose levels, increases insulin secretion, rectifies structural abnormalities in peripheral nerve, improves wound contraction, increases granulation tissue and neovascularization | [77,151,159] |
| Leaves, fruits | Rutin, quercetin, kaempferol, coumarin compounds | Diabetes, wounds, pain and inflammation, hypertension, constipation, gastric ulcers | Aqueous, ethanol extract | Reduces blood glucose levels, decreases wound size, decreases period of epithelialization, inhibits bacterial growth, reduces inflammation, exerts antioxidant action | [75,160,161] |
| Leaves, fruits | Limonene, α,β-pinene, myrtucommulone, gallic acid, ellagic acid, linalool | Diabetes, skin diseases, hemorrhage, peptic ulcers, wounds, conjunctivitis | Aqueous, ethanol extract | Lowers blood glucose levels, stimulates angiogenesis, promotes proliferation and migration of cells to wound sites, reduces inflammation, exerts free radical scavenging activity, prevents microbial infection at the wound site | [162,163] |
| Seeds | Thymoquinone, thymohydroquinone, thymol, oleic acid, linoleic acid | Diabetes, wound healing, microbial infections, rheumatism, dysentery, respiratory tract diseases | Aqueous, ethanol extract | Lowers blood glucose levels; induces angiogenesis, fibroblast proliferation, and collagen synthesis; minimizes bacterial infection; exhibits anti-inflammatory action; decreases neuronal degeneration | [16,151,164,165] |
| Leaves | Oleuropein, oleanolic acid, hydroxytyrosol, luteolin | Diabetes, hypertension, constipation, UTI, skin ulcers, inflammatory wounds | Ethanol extract | Decreases blood sugar levels; exhibits antioxidant activity; improves blood circulation in healing tissues; inhibits bacterial growth; enhances epithelialization, collagen synthesis, and development of fibroblasts | [16,78] |
| Leaves | Sinensetin, caffeic acid, rosmarinic acid, eupatorin, eugenol, linalool | Diabetes, nephritis, kidney and gallstones, hypertension, arteriosclerosis epilepsy, rheumatism, | Aqueous, ethanol extract | Reduces blood glucose levels; increases insulin secretion and actio;, exerts antioxidant and anti-inflammatory action; enhances epithelialization rate; increases wound contraction and collagen synthesis in fibroblasts | [166,167] |
| Leaves, stem bark | Pautelin, linolenic acid, luteolin, gallic acid, rutin | Diabetes, rheumatism, asthma, bronchitis, eye infections, wound healing | Ethanol extract | Increases rates of wound healing and tissue repair, enhances wound contraction and epithelialization, increases cell proliferation, exerts antioxidant and antimicrobial actions, increases collagen synthesis, regulates inflammatory markers and oxidative stress | [168,169] |
| Bark | Marsupin, marsupol, carsupin, epicatechin, pterostilbene | Diabetes, wound healing, skin diseases, ulcer, cough, diarrhea, dysentery | Ethanol extract | Lowers blood glucose levels, improves glucose tolerance, promotes re-epithelialization, exhibits free radical scavenging activity, decrease TNF-α levels, increases expression of angiogenesis-related proteins, reduces bacterial infection in wounds | [16,170] |
| Fruit, flowers | Ellagic acid, gallic acid, luteolin, catechin, rutin, quercetin | Diabetes, inflammation, rheumatism, sore throat, leprosy, burns | Aqueous, methanol, ethanol, ethyl acetate extract | Lowers blood sugar levels, increases wound healing rate, enhances cell proliferation and collagen synthesis, reduces inflammation | [171,172] |
| Roots | Catalpol | Diabetes, ulcers, hypertension, liver and kidney diseases, depression | Aqueous extract | Reduces blood sugar levels, heals diabetic foot ulcers, promotes tissue regeneration and angiogenesis, inhibits TNF-α production, increases IL-2 and IFN-γ production | [173,174] |
| Leaves, stems, flowers | Rosmarinic acid, carnosic acid, chlorogenic acid, apigenin, luteolin | Diabetes, anxiety, inflammation, muscle, and joint pain, wound, colds | Ethanol, methanol, chloroform extract | Exhibits free radical scavenging activity, inhibits lipid peroxidation, decreases growth of microorganisms, exerts anti-inflammatory and neuroprotective actions, promotes angiogenesis, improves granulation tissue for faster wound healing | [175,176] |
| Seeds | Sesamol, sesamin, sesamolin, sesaminol | Diabetes, healing burns and wounds, hypertension, constipation | Ethanol extract | Lowers blood glucose levels, accelerates wound healing, increases wound contraction, decreases wound inflammation, exerts neuroprotection | [19,151,177,178] |
| Seeds, bark | Quercetin, ellagic acid, gallic acid, kaempferol, iso-coumarin | Diabetes, sore throat, constipation, wounds, skin ulcers, gastritis, constipation | Aqueous extract | Decreases blood sugar levels, enhances insulin secretion and action and re-epithelialization, increases collagen deposition, promotes neovascularization | [16,179,180] |
| Fruit, leaves | Ellagic acid, gallic acid, tannic acid, chebulagic acid, corilagin | Diabetes, constipation, dementia, wound healing, astringent | Aqueous, methanol, ethyl acetate extract | Lowers blood glucose levels, improves rate of wound contraction, increases proliferation of keratinocytes and fibroblasts, improves free radical scavenging activity | [16,181] |
| Leaves, bark, roots | Tinosporaside, tinosporine, berberine, jatrorrhizine, β-sitosterol, arabinogalactan | Diabetes, asthma, dysentery, diarrhea, jaundice, anemia, allergy, wound healing | Aqueous, methanol, ethanol extract | Exerts immunomodulatory effects, enhances wound contraction, promotes collagen synthesis, exhibits antioxidant and free radical scavenging activity levels, inhibits microbial growth at wound site | [16,182] |
| Seeds | Galactomannan, trigonelline, 4-hydroxy isoleuscine | Diabetes, wound healing, indigestion, pneumonia, bronchitis, constipation | Aqueous, ethanol extract | Decreases blood glucose levels, increases insulin secretion, restores function of nerve fibers, improves collagen synthesis and maturation, increases granuloma tissue | [19,151,183,184] |
| Roots, rhizome | Gingerol, paradol, shogaol | Diabetes, wound healing, microbial infections, cough, asthma | Ethanol extract | Decreases blood sugar levels, increases insulin secretion, improves collagen synthesis and maturation, increases wound contraction and epithelialization, inhibits bacterial colonization | [185,186] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, P.; Akther, S.; Khan, J.T.; Islam, S.S.; Masud, M.S.R.; Rahman, A.; Seidel, V.; Abdel-Wahab, Y.H.A. Hyperglycaemia-Linked Diabetic Foot Complications and Their Management Using Conventional and Alternative Therapies. Appl. Sci. 2022, 12, 11777. https://doi.org/10.3390/app122211777
Ansari P, Akther S, Khan JT, Islam SS, Masud MSR, Rahman A, Seidel V, Abdel-Wahab YHA. Hyperglycaemia-Linked Diabetic Foot Complications and Their Management Using Conventional and Alternative Therapies. Applied Sciences. 2022; 12(22):11777. https://doi.org/10.3390/app122211777
Chicago/Turabian StyleAnsari, Prawej, Samia Akther, Joyeeta T. Khan, Sara S. Islam, Md. Samim R. Masud, Anisur Rahman, Veronique Seidel, and Yasser H. A. Abdel-Wahab. 2022. "Hyperglycaemia-Linked Diabetic Foot Complications and Their Management Using Conventional and Alternative Therapies" Applied Sciences 12, no. 22: 11777. https://doi.org/10.3390/app122211777
APA StyleAnsari, P., Akther, S., Khan, J. T., Islam, S. S., Masud, M. S. R., Rahman, A., Seidel, V., & Abdel-Wahab, Y. H. A. (2022). Hyperglycaemia-Linked Diabetic Foot Complications and Their Management Using Conventional and Alternative Therapies. Applied Sciences, 12(22), 11777. https://doi.org/10.3390/app122211777










