Molecular and Cellular Mechanisms of Cardiovascular and Metabolic Diseases
A special issue of Cells (ISSN 2073-4409). This special issue belongs to the section "Cells of the Cardiovascular System".
Deadline for manuscript submissions: 31 January 2025 | Viewed by 3555
Special Issue Editor
2. Department of Pediatrics, Sanford School of Medicine, University of South Dakota, Sioux Falls, SD, USA
Interests: mitochondrial dysfunction; endoplasmic reticulum (ER) stress; oxidative/nitrosative stress; atherosclerosis; metabolomics; cell death
Special Issue Information
Dear Colleagues,
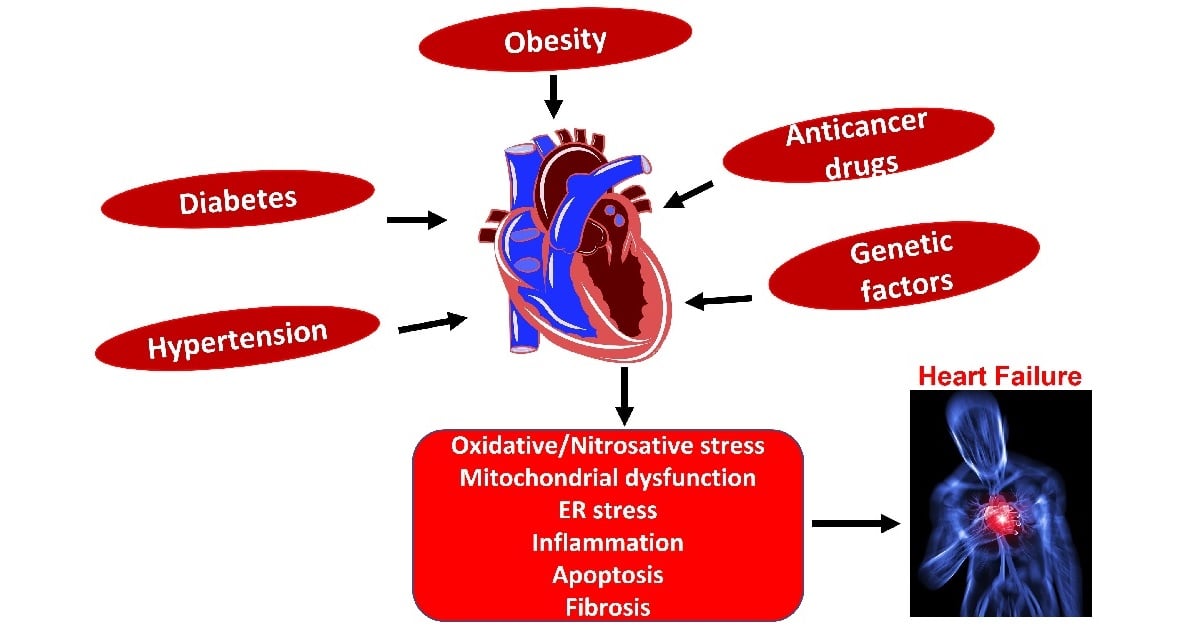
Globally, cardiovascular disease (CVD) is the leading cause of death, the primary contributor to disability, and causes about one third of all deaths. The prevalence of and disability due to CVD has doubled and is affecting more than 500 million people. CVD is influenced by both environmental and genetic triggers. Even though there is significant progress in identifying the multifactorial mechanisms associated with cardiometabolic complications; studies are still needed to unravel novel molecular mechanisms that may open new therapeutic horizons and will significantly reduce the prevalence and disability associated with this disease. This Special Issue focuses on the pathophysiology and novel molecular mechanism associated with cardiometabolic syndrome with a special emphasis on mitochondrial dysfunction, ER stress, oxidative stress, obesity, diabetes, anticancer drug-mediated cardiomyopathy, atherosclerosis, and nutrition. Identifying novel molecular mechanisms may help to implement cost effective, population-based early prevention.
Dr. Prathapan Ayyappan
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- mitochondrial dysfunction
- endoplasmic reticulum (ER) stress
- oxidative/nitrosative stress
- diabetes
- obesity
- diet
- atherosclerosis
- hypertension
- metabolomics
- cell death
- nutraceuticals/functional foods






