Review Papers in (Re)programming Cells for Cardiac Repair
A special issue of Cells (ISSN 2073-4409). This special issue belongs to the section "Cells of the Cardiovascular System".
Deadline for manuscript submissions: closed (15 June 2024) | Viewed by 14864
Special Issue Editors
Interests: cardiac repair; stem cell therapy; cell reprogramming
Interests: 3D genomics; epigenetics; cardiac repair; stem cell therapy; exosomes
Special Issue Information
Dear Colleagues,
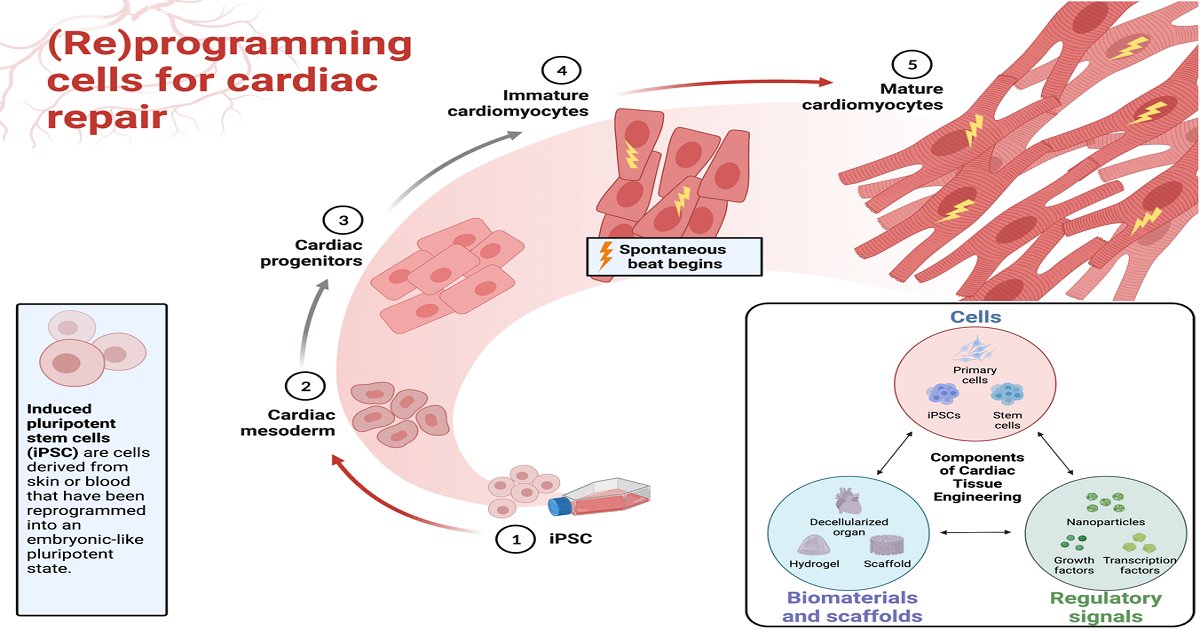
Cardiac disease is the leading cause of death worldwide due to the failure of the adult human heart to replenish the considerable loss of cardiomyocytes caused by various insults (e.g., ischemia). Regenerative medicine is an innovative approach to repair and replace damaged heart cells and is undergoing a major revolution due to the unprecedented need for therapeutics to treat cardiac patients around the world.
Induced pluripotent stem cells (iPSCs) have been widely studied in the field of cardiac regeneration and are considered one of the most promising candidate therapeutics. However, several issues remain to be resolved prior to clinical application, such as immaturity of iPSC-derived cardiomyocytes, poor survival of transplanted cells in the injured heart, and how we can best employ cardiac tissue engineering technologies to optimize repair. This Special Issue aims to publish high-quality review articles that provide novel insights and conceptual advancements in the field of cardiac (re)programming for cardiac repair, in particular in terms of the signaling pathways and transcriptional/epigenetic regulation for cardiac lineage commitment. We kindly encourage all research groups covering relevant areas within the issue’s scope to contribute up-to-date, full-length comprehensive reviews, highlighting the latest developments in their research field, or to invite relevant experts and colleagues to do so.
Prof. Dr. Yigang Wang
Dr. Yuliang Feng
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- cardiac programming
- cardiogenesis
- transcriptional regulation
- epigenetics
- cardiac tissue engineering
Benefits of Publishing in a Special Issue
- Ease of navigation: Grouping papers by topic helps scholars navigate broad scope journals more efficiently.
- Greater discoverability: Special Issues support the reach and impact of scientific research. Articles in Special Issues are more discoverable and cited more frequently.
- Expansion of research network: Special Issues facilitate connections among authors, fostering scientific collaborations.
- External promotion: Articles in Special Issues are often promoted through the journal's social media, increasing their visibility.
- e-Book format: Special Issues with more than 10 articles can be published as dedicated e-books, ensuring wide and rapid dissemination.
Further information on MDPI's Special Issue polices can be found here.







