Evaluating the Feasibility and Acceptability of an Artificial-Intelligence-Enabled and Speech-Based Distress Screening Mobile App for Adolescents and Young Adults Diagnosed with Cancer: A Study Protocol
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Speech-Based Distress Assessment Tools
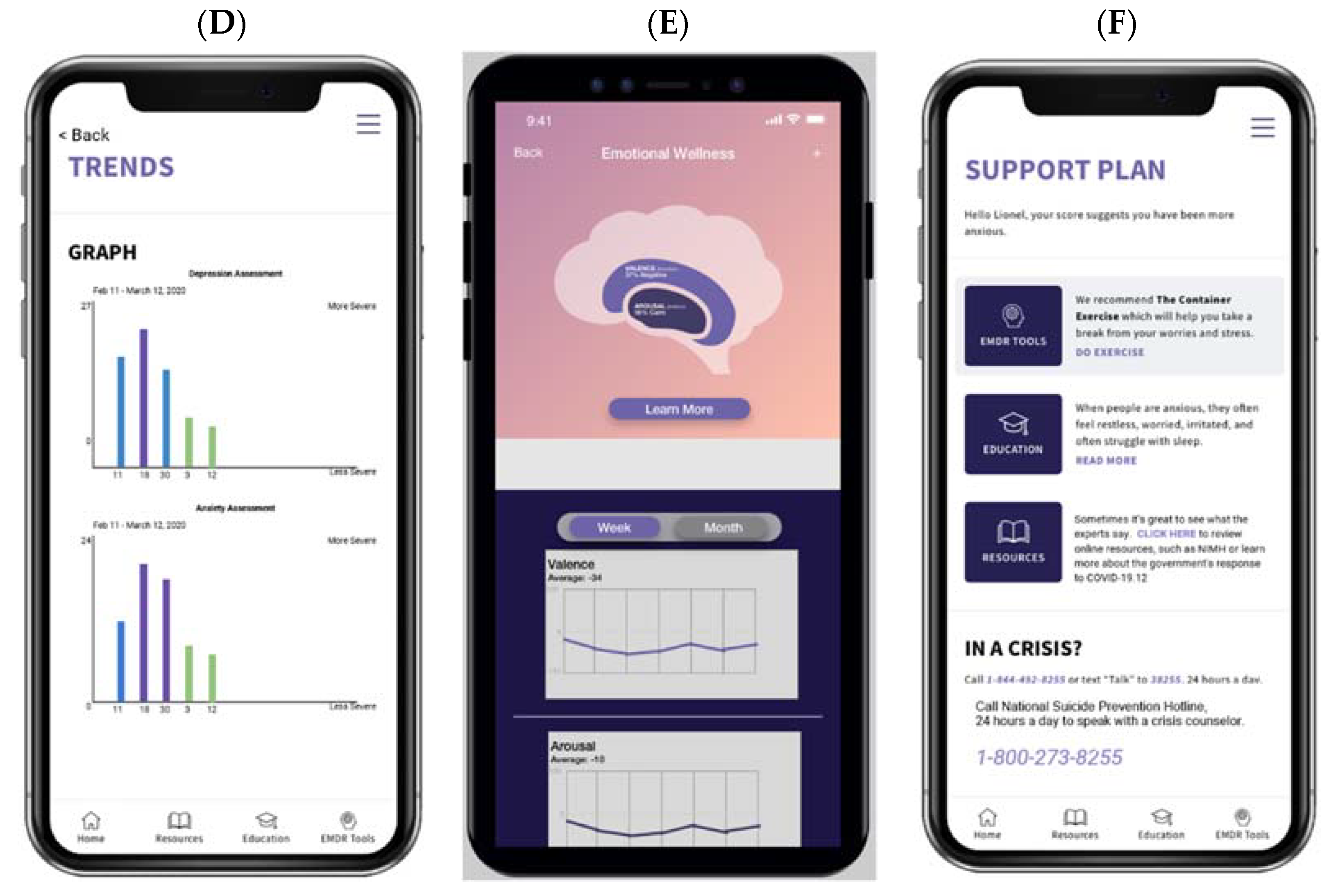
1.2. The Ellipsis Health Voice Tool (EH Voice Tool)
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Participant Recruitment and Study Procedures
2.4. Study Endpoints
2.5. Data Analysis
2.6. Power Calculation and Sample Size Considerations
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, Y.F.; Chen, M.X.; Yin, G.; Lin, R.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M. The Global, Regional, and National Burden of Cancer among Adolescents and Young Adults in 204 Countries and Territories, 1990–2019: A Population-Based Study. J. Hematol. Oncol. 2021, 14, 89. [Google Scholar] [CrossRef]
- Chao, C.; Bhatia, S.; Xu, L.; Cannavale, K.L.; Wong, F.L.; Huang, P.-Y.S.; Cooper, R.; Armenian, S.H. Chronic Comorbidities Among Survivors of Adolescent and Young Adult Cancer. J. Clin. Oncol. 2020, 38, 3161–3174. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer Statistics for Adolescents and Young Adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Frobisher, C.; Hawkins, M.M.; Nathan, P.C. Challenges and Opportunities in the Care of Survivors of Adolescent and Young Adult Cancers. Pediatric Blood Cancer 2019, 66, e27668. [Google Scholar] [CrossRef]
- Vetsch, J.; Wakefield, C.E.; McGill, B.C.; Cohn, R.J.; Ellis, S.J.; Stefanic, N.; Sawyer, S.M.; Zebrack, B.; Sansom-Daly, U.M. Educational and Vocational Goal Disruption in Adolescent and Young Adult Cancer Survivors. Psycho-Oncology 2018, 27, 532–538. [Google Scholar] [CrossRef]
- Kirchhoff, A.; Jones, S. Financial Toxicity in Adolescent and Young Adult Cancer Survivors: Proposed Directions for Future Research. JNCI J. Natl. Cancer Inst. 2021, 113, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Su, H.I.; Lee, Y.T.; Barr, R. Oncofertility: Meeting the Fertility Goals of Adolescents and Young Adults with Cancer. Cancer J. (Sudbury Mass.) 2018, 24, 328. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Wen, Y.; Wang, H.; Sun, H.; Liang, W.; Zhang, B.; Humphris, G. Fear of Cancer Recurrence in Adolescent and Young Adult Cancer Survivors: A Systematic Review of the Literature. Psycho-Oncology 2019, 28, 675–686. [Google Scholar] [CrossRef] [Green Version]
- Benedict, C.; Shuk, E.; Ford, J.S. Fertility Issues in Adolescent and Young Adult Cancer Survivors. J. Adolesc. Young Adult Oncol. 2016, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sender, A.; Friedrich, M.; Schmidt, R.; Geue, K. Cancer-Specific Distress, Supportive Care Needs and Satisfaction with Psychosocial Care in Young Adult Cancer Survivors. Eur. J. Oncol. Nurs. 2020, 44, 101708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Wang, K.; Zebrack, B.; Tan, C.Y.; Walling, E.; Chugh, R. Psychosocial, Behavioral, and Supportive Interventions for Pediatric, Adolescent, and Young Adult Cancer Survivors: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2021, 160, 103291. [Google Scholar] [CrossRef] [PubMed]
- Lauer, A.L. Treatment of Anxiety and Depression in Adolescents and Young Adults with Cancer. J. Pediatr. Oncol. Nurs. 2015, 32, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Geue, K.; Brähler, E.; Faller, H.; Härter, M.; Schulz, H.; Weis, J.; Koch, U.; Wittchen, H.-U.; Mehnert, A. Prevalence of Mental Disorders and Psychosocial Distress in German Adolescent and Young Adult Cancer Patients (AYA). Psycho-Oncology 2018, 27, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ding, S.; He, S.; Duan, Y.; Yi, K.; Zhou, J. A Prevalence Study of Psychosocial Distress in Adolescents and Young Adults with Cancer. Cancer Nurs. 2017, 40, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Bitsko, M.J.; Cohen, D.; Dillon, R.; Harvey, J.; Krull, K.; Klosky, J.L. Psychosocial Late Effects in Pediatric Cancer Survivors: A Report From the Children’s Oncology Group. Pediatric Blood Cancer 2016, 63, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.; François, C.; Harju, E.; Dehler, S.; Roser, K. The Long-Term Impact of Cancer: Evaluating Psychological Distress in Adolescent and Young Adult Cancer Survivors in Switzerland. Psycho-Oncology 2019, 28, 577–585. [Google Scholar] [CrossRef]
- Wagner, L.I.; Spiegel, D.; Pearman, T. Using the Science of Psychosocial Care to Implement the New American College of Surgeons Commission on Cancer Distress Screening Standard. J. Natl. Compr. Cancer Netw. 2013, 11, 214–221. [Google Scholar] [CrossRef]
- Zebrack, B.; Kayser, K.; Bybee, D.; Padgett, L.; Sundstrom, L.; Jobin, C.; Oktay, J. A Practice-Based Evaluation of Distress Screening Protocol Adherence and Medical Service Utilization. J. Natl. Compr. Cancer Netw. 2017, 15, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Acquati, C.; Kayser, K. Addressing the Psychosocial Needs of Cancer Patients: A Retrospective Analysis of a Distress Screening and Management Protocol in Clinical Care. J. Psychosoc. Oncol. 2019, 37, 287–300. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Lord, K.; Slattery, J.; Grainger, L.; Symonds, P. How Feasible Is Implementation of Distress Screening by Cancer Clinicians in Routine Clinical Care? Cancer 2012, 118, 6260–6269. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Akechi, T.; Okamura, M.; Oba, A.; Fujimori, M.; Akizuki, N.; Uchitomi, Y. Usefulness of the Nurse-Assisted Screening and Psychiatric Referral Program. Cancer 2005, 103, 1949–1956. [Google Scholar] [CrossRef]
- Zebrack, B.J. Psychological, Social, and Behavioral Issues for Young Adults with Cancer. Cancer 2011, 117, 2289–2294. [Google Scholar] [CrossRef] [Green Version]
- Victorson, D.; Garcia, S.F.; Sanford, S.; Snyder, M.A.; Lampert, S.; Salsman, J.M. A Qualitative Focus Group Study to Illuminate the Lived Emotional and Social Impacts of Cancer and Its Treatment on Young Adults. J. Adolesc. Young Adult Oncol. 2019, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Jensen, L.; Larsen, H.B.; Johansen, C.; Frandsen, T.L.; Schmiegelow, K.; Wahlberg, A. Everyday Life Challenges among Adolescent and Young Adult Survivors of Childhood Acute Lymphoblastic Leukemia: An in-Depth Qualitative Study. Psycho-Oncology 2020, 29, 1630–1637. [Google Scholar] [CrossRef]
- Kwak, M.; Zebrack, B.J.; Meeske, K.A.; Embry, L.; Aguilar, C.; Block, R.; Hayes-Lattin, B.; Li, Y.; Butler, M.; Cole, S.; et al. Trajectories of Psychological Distress in Adolescent and Young Adult Patients With Cancer: A 1-Year Longitudinal Study. J. Clin. Oncol 2013, 31, 2160–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, A.R.; Bradford, M.C.; Bona, K.; Shaffer, M.L.; Wolfe, J.; Baker, K.S.; Lau, N.; Yi-Frazier, J. Hope, Distress, and Later Quality of Life among Adolescent and Young Adults with Cancer. J. Psychosoc. Oncol. 2018, 36, 137. [Google Scholar] [CrossRef]
- Bidstrup, P.E.; Christensen, J.; Mertz, B.G.; Rottmann, N.; Dalton, S.O.; Johansen, C. Trajectories of Distress, Anxiety, and Depression among Women with Breast Cancer: Looking beyond the Mean. Acta Oncol. 2015, 54, 789–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundt, J.C.; Snyder, P.J.; Cannizzaro, M.S.; Chappie, K.; Geralts, D.S. Voice Acoustic Measures of Depression Severity and Treatment Response Collected via Interactive Voice Response (IVR) Technology. J. Neurolinguistics 2007, 20, 50–64. [Google Scholar] [CrossRef] [Green Version]
- Galili, L.; Amir, O.; Gilboa-Schechtman, E. Acoustic Properties of Dominance and Request Utterances in Social Anxiety. J. Soc. Clin. Psychol. 2013, 32, 651–673. [Google Scholar] [CrossRef]
- Hashim, N.W.; Wilkes, M.; Salomon, R.; Meggs, J.; France, D.J. Evaluation of Voice Acoustics as Predictors of Clinical Depression Scores. J. Voice 2017, 31, 256.e1–256.e6. [Google Scholar] [CrossRef] [PubMed]
- Faurholt-Jepsen, M.; Busk, J.; Frost, M.; Vinberg, M.; Christensen, E.M.; Winther, O.; Bardram, J.E.; Kessing, L.V. Voice Analysis as an Objective State Marker in Bipolar Disorder. Transl. Psychiatry 2016, 6, e856. [Google Scholar] [CrossRef] [Green Version]
- Rogolo, F.; Magnavita, G.; Mota, N.B.; Ziebold, C.; Mabunda, D.; Pan, P.M.; Zugman, A.; Gadelha, A.; Corcoran, C.; Bressan, R.A. Lowering Costs for Large-Scale Screening in Psychosis: A Systematic Review and Meta-Analysis of Performance and Value of Information for Speech-Based Psychiatric Evaluation. Rev. Bras. Psiquiatr. 2020, 42, 673–686. [Google Scholar] [CrossRef] [Green Version]
- Trevino, A.C.; Quatieri, T.F.; Malyska, N. Phonologically-Based Biomarkers for Major Depressive Disorder. EURASIP J. Adv. Signal Process. 2011, 2011, 42. [Google Scholar] [CrossRef] [Green Version]
- Muaremi, A.; Gravenhorst, F.; Grünerbl, A.; Arnrich, B.; Tröster, G. Assessing Bipolar Episodes Using Speech Cues Derived from Phone Calls. Lect. Notes Inst. Comput. Sci. Soc.-Inform. Telecommun. Eng. LNICST 2014, 100, 103–114. [Google Scholar] [CrossRef]
- Cummins, N.; Scherer, S.; Krajewski, J.; Schnieder, S.; Epps, J.; Quatieri, T.F. A Review of Depression and Suicide Risk Assessment Using Speech Analysis. Speech Commun. 2015, 71, 10–49. [Google Scholar] [CrossRef]
- Low, D.M.; Bentley, K.H.; Ghosh, S.S. Automated Assessment of Psychiatric Disorders Using Speech: A Systematic Review. Laryngoscope Investig. Otolaryngol. 2020, 5, 96–116. [Google Scholar] [CrossRef] [Green Version]
- Espinola, C.W.; Gomes, J.C.; Mônica, J.; Pereira, S.; Pinheiro, W.; Santos, D.; Espinola, C.W. Detection of Major Depressive Disorder Using Vocal Acoustic Analysis and Machine Learning. medRxiv 2020. [Google Scholar] [CrossRef]
- Rutowski, T.; Shriberg, E.; Harati, A.; Lu, Y.; Chlebek, P.; Oliveira, R. Depression and Anxiety Prediction Using Deep Language Models and Transfer Learning. In Proceedings of the 2020 7th IEEE International Conference on Behavioural and Social Computing, BESC 2020, Bournemouth, UK, 5–7 November 2020. [Google Scholar] [CrossRef]
- Harati, A.; Shriberg, E.; Rutowski, T.; Chlebek, P.; Lu, Y.; Oliveira, R. Speech-Based Depression Prediction Using Encoder-Weight-Only Transfer Learning and a Large Corpus. In Proceedings of the ICASSP, IEEE International Conference on Acoustics, Speech and Signal Processing—Proceedings 2021, Toronto, ON, Canada, 6–11 June 2021; pp. 7273–7277. [Google Scholar] [CrossRef]
- Rutowski, T.; Harati, A.; Lu, Y.; Shriberg, E. Optimizing Speech-Input Length for Speaker-Independent Depression Classification. Interspeech 2019, 3023–3027. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Harati, A.; Rutowski, T.; Oliveira, R.; Chlebek, P.; Shriberg, E. Robust Speech and Natural Language Processing Models for Depression Screening. In Proceedings of the 2020 IEEE Signal Processing in Medicine and Biology Symposium, SPMB 2020—Proceedings, Philadelphia, PA, USA, 5 December 2020. [Google Scholar] [CrossRef]
- Chlebek, P.; Shriberg, E.; Lu, Y.; Rutowski, T.; Harati, A.; Oliveira, R. Comparing Speech Recognition Services for HCI Applications in Behavioral Health. In Proceedings of the UbiComp/ISWC 2020 Adjunct—Proceedings of the 2020 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2020 ACM International Symposium on Wearable Computers, Virtual Event, 12–17 September 2020; pp. 483–487. [Google Scholar] [CrossRef]
- Rutowski, T.; Shriberg, E.; Harati, A.; Lu, Y.; Oliveira, R.; Chlebek, P. Cross-Demographic Portability of Deep NLP-Based Depression Models. In Proceedings of the 2021 IEEE Spoken Language Technology Workshop, SLT 2021—Proceedings, Shenzhen, China, 19–22 January 2021; pp. 1052–1057. [Google Scholar] [CrossRef]
- Zhao, Z.; Bao, Z.; Zhang, Z.; Deng, J.; Cummins, N.; Wang, H.; Tao, J.; Schuller, B. Automatic Assessment of Depression from Speech via a Hierarchical Attention Transfer Network and Attention Autoencoders. IEEE J. Sel. Top. Signal Process. 2020, 14, 423–434. [Google Scholar] [CrossRef]
- Furlong, A. Routledge Handbook of Youth and Young Adulthood, 2nd ed.; Taylor & Francis Group: London, UK, 2016; pp. 1–465. [Google Scholar] [CrossRef]
- Kroenke, K.; Strine, T.W.; Spitzer, R.L.; Williams, J.B.W.; Berry, J.T.; Mokdad, A.H. The PHQ-8 as a Measure of Current Depression in the General Population. J. Affect. Disord. 2009, 114, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Thombs, B.D.; Benedetti, A.; Kloda, L.A.; Levis, B.; Nicolau, I.; Cuijpers, P.; Gilbody, S.; Ioannidis, J.P.A.; McMillan, D.; Patten, S.B.; et al. The Diagnostic Accuracy of the Patient Health Questionnaire-2 (PHQ-2), Patient Health Questionnaire-8 (PHQ-8), and Patient Health Questionnaire-9 (PHQ-9) for Detecting Major Depression: Protocol for a Systematic Review and Individual Patient Data Meta-Analyses. Syst. Rev. 2014, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.S.; Kroenke, K.; Zack, M.M.; Strine, T.W.; Balluz, L.S. PHQ-8 Days: A Measurement Option for DSM-5 Major Depressive Disorder (MDD) Severity. Popul. Health Metr. 2011, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alpizar, D.; Laganá, L.; Plunkett, S.W.; French, B.F. Evaluating the Eight-Item Patient Health Questionnaire’s Psychometric Properties with Mexican and Central American Descent University Students. Psychol. Assess. 2018, 30, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, S.L.; Morrow, K.E.; Silk, J.S.; Kolko, D.J.; Pilkonis, P.A.; Lindhiem, O. National Norms and Correlates of the PHQ-8 and GAD-7 in Parents of School-Age Children. J. Child Fam. Stud. 2021, 30, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- López-Torres, S.; Pérez-Pedrogo, C.; Sánchez-Cardona, I.; Sánchez-Cesáreo, M. Psychometric Properties of the PHQ-A among a Sample of Children and Adolescents in Puerto Rico. Curr. Psychol. 2019, 41, 90–98. [Google Scholar] [CrossRef]
- Beard, C.; Björgvinsson, T. Beyond Generalized Anxiety Disorder: Psychometric Properties of the GAD-7 in a Heterogeneous Psychiatric Sample. J. Anxiety Disord. 2014, 28, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Osório, F.L.; Lima, M.P.; Chagas, M.H.N. Screening Tools for Psychiatry Disorders in Cancer Setting: Caution When Using. Psychiatry Res. 2015, 229, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, Y.E. The Psychometric Properties of the Generalized Anxiety Disorder Scale (GAD-7) among Korean University Students. Psychiatry Clin. Psychopharmacol. 2019, 29, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.U.; Ulvenes, P.G.; Øktedalen, T.; Hoffart, A. Psychometric Properties of the GAD-7 in a Heterogeneous Psychiatric Sample. Front. Psychol. 2019, 10, 1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutter, L.A.; Brown, T.A. Psychometric Properties of the Generalized Anxiety Disorder Scale-7 (GAD-7) in Outpatients with Anxiety and Mood Disorders. J. Psychopathol. Behav. Assess. 2016, 39, 140–146. [Google Scholar] [CrossRef]
- Patterson, P.; D’Agostino, N.M.; McDonald, F.E.J.; Church, T.D.; Costa, D.S.J.; Rae, C.S.; Siegel, S.E.; Hu, J.; Bibby, H.; Stark, D.P.; et al. Screening for Distress and Needs: Findings from a Multinational Validation of the Adolescent and Young Adult Psycho-Oncology Screening Tool with Newly Diagnosed Patients. Psycho-Oncology 2021, 30, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.; Klassen, A.F.; Tsangaris, E.; Breakey, V.; D’Agostino, N. Distress Screening in Adolescents and Young Adults with Cancer: Development of Cut-Points for the Cancer Distress Scales-Adolescent and Young Adults. J. Adolesc. Young-Adult Oncol. 2019, 8, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Sansom-Daly, U.M.; Wakefield, C.E. Distress and Adjustment among Adolescents and Young Adults with Cancer: An Empirical and Conceptual Review. Transl. Pediatrics 2013, 2, 167. [Google Scholar] [CrossRef]
- Zhang, A.; Hu, R.; Wang, K.; Antalis, E.P. Age Moderates the Association between Psychological Distress and Engagement in Mindfulness among Cancer Patients and Survivors: A Population-Based Study. J. Psychosoc. Oncol. 2020, 38, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; Zebrack, B.J. Perceived Impact of Cancer among Adolescents and Young Adults: Relationship with Health-Related Quality of Life and Distress. Psycho-Oncology 2017, 26, 1307–1315. [Google Scholar] [CrossRef] [PubMed]

| Recruitment Clusters | ND | CT | ET | PT | Total |
|---|---|---|---|---|---|
| Male Adolescent (15–17 years) | 4 | 4 | 4 | 4 | 16 |
| Female Adolescent (15–17 years) | 4 | 4 | 4 | 4 | 16 |
| Male emerging adult (18–26 years) | 4 | 4 | 3 | 3 | 14 |
| Female emerging adult (18–26 years) | 4 | 4 | 3 | 3 | 14 |
| Total | 16 | 16 | 14 | 14 | 60 |
| Measure | Measure Administration Time Points and Personnel | |||
|---|---|---|---|---|
| T0 | T1–T6 | T7 | Admin | |
| MINI Interview | X | RS | ||
| PHQ-8 | X | RS | ||
| GAD-7 | X | RS | ||
| EH Voice Tool Admin. Data | Ongoing all participants | Platform | ||
| AIM Measure | X | RS | ||
| Qualitative Interview | X | RS | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Kamat, A.; Acquati, C.; Aratow, M.; Kim, J.S.; DuVall, A.S.; Walling, E. Evaluating the Feasibility and Acceptability of an Artificial-Intelligence-Enabled and Speech-Based Distress Screening Mobile App for Adolescents and Young Adults Diagnosed with Cancer: A Study Protocol. Cancers 2022, 14, 914. https://doi.org/10.3390/cancers14040914
Zhang A, Kamat A, Acquati C, Aratow M, Kim JS, DuVall AS, Walling E. Evaluating the Feasibility and Acceptability of an Artificial-Intelligence-Enabled and Speech-Based Distress Screening Mobile App for Adolescents and Young Adults Diagnosed with Cancer: A Study Protocol. Cancers. 2022; 14(4):914. https://doi.org/10.3390/cancers14040914
Chicago/Turabian StyleZhang, Anao, Aarti Kamat, Chiara Acquati, Michael Aratow, Johnny S. Kim, Adam S. DuVall, and Emily Walling. 2022. "Evaluating the Feasibility and Acceptability of an Artificial-Intelligence-Enabled and Speech-Based Distress Screening Mobile App for Adolescents and Young Adults Diagnosed with Cancer: A Study Protocol" Cancers 14, no. 4: 914. https://doi.org/10.3390/cancers14040914
APA StyleZhang, A., Kamat, A., Acquati, C., Aratow, M., Kim, J. S., DuVall, A. S., & Walling, E. (2022). Evaluating the Feasibility and Acceptability of an Artificial-Intelligence-Enabled and Speech-Based Distress Screening Mobile App for Adolescents and Young Adults Diagnosed with Cancer: A Study Protocol. Cancers, 14(4), 914. https://doi.org/10.3390/cancers14040914






