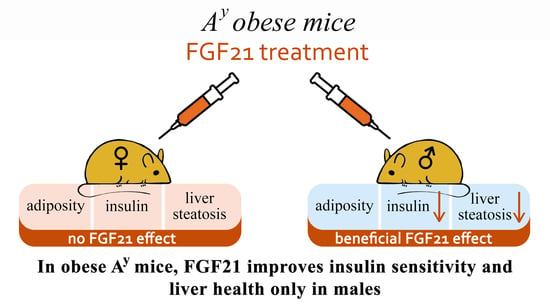
Fibroblast Growth Factor 21 (FGF21) Administration Sex-Specifically Affects Blood Insulin Levels and Liver Steatosis in Obese Ay Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
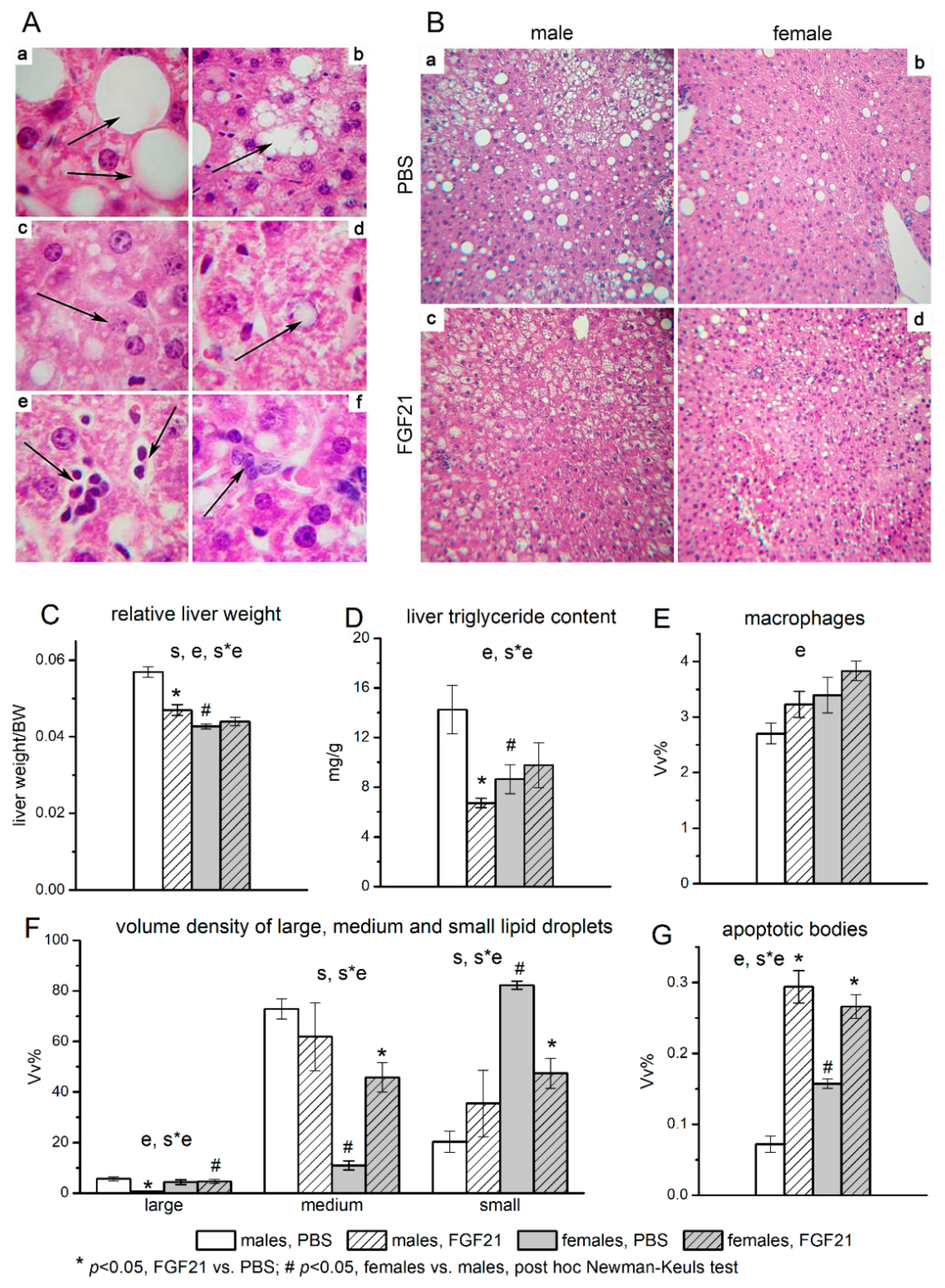
2.2. Histological Analysis of the Liver
2.3. Plasma Assays and Triglyceride and Glycogen Measurements
2.4. Expression and Purification of Mouse FGF21
2.5. Relative Quantitation Real-Time PCR
2.6. Statistical Analysis
3. Results
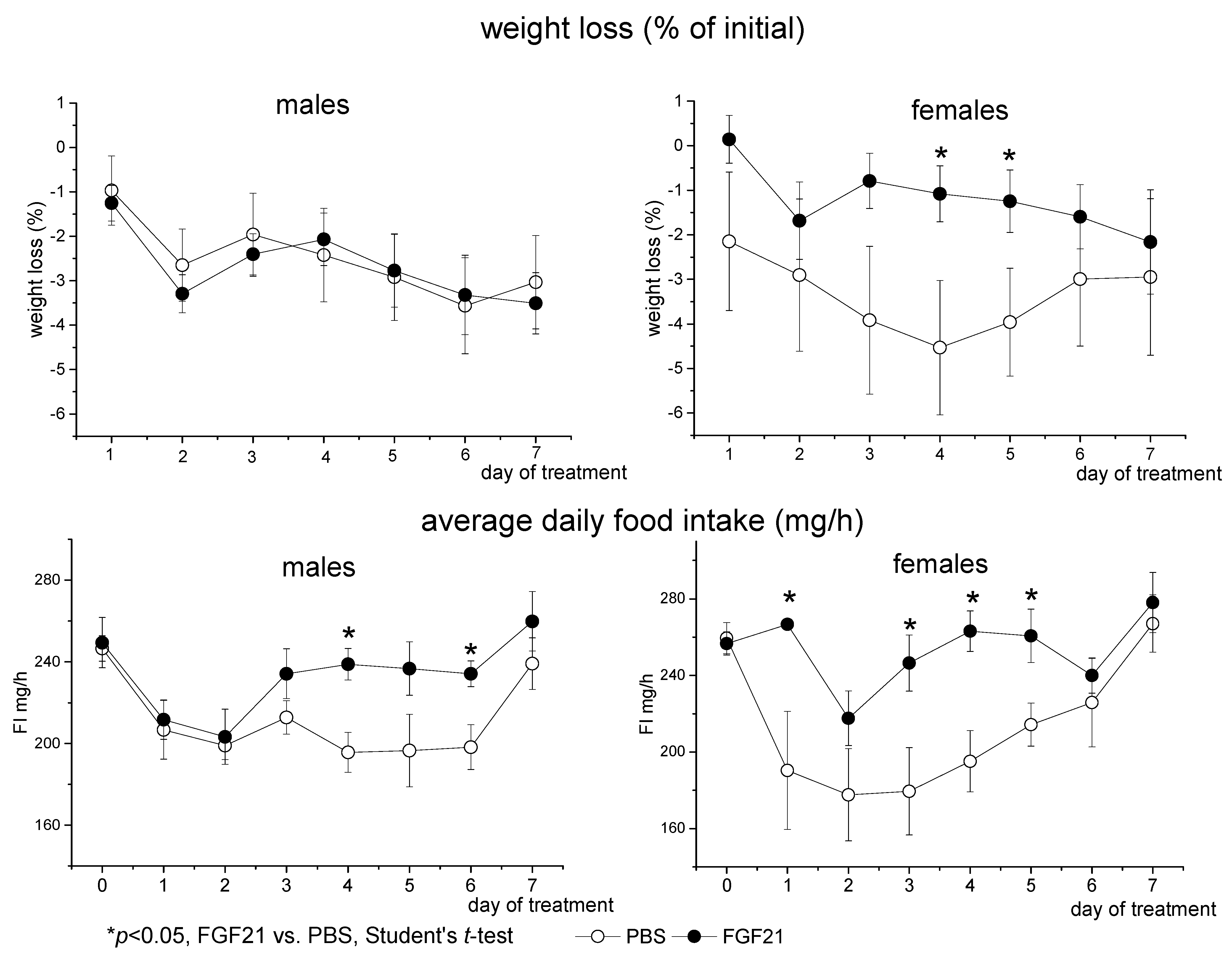
3.1. Effect of Exogenous FGF21 on Body Weight, Fat Mass, Food Intake, Locomotor Activity, and Hormonal and Metabolic Parameters in Obese Ay Male and Female Mice
3.2. Effect of Exogenous FGF21 on Liver Fat Content
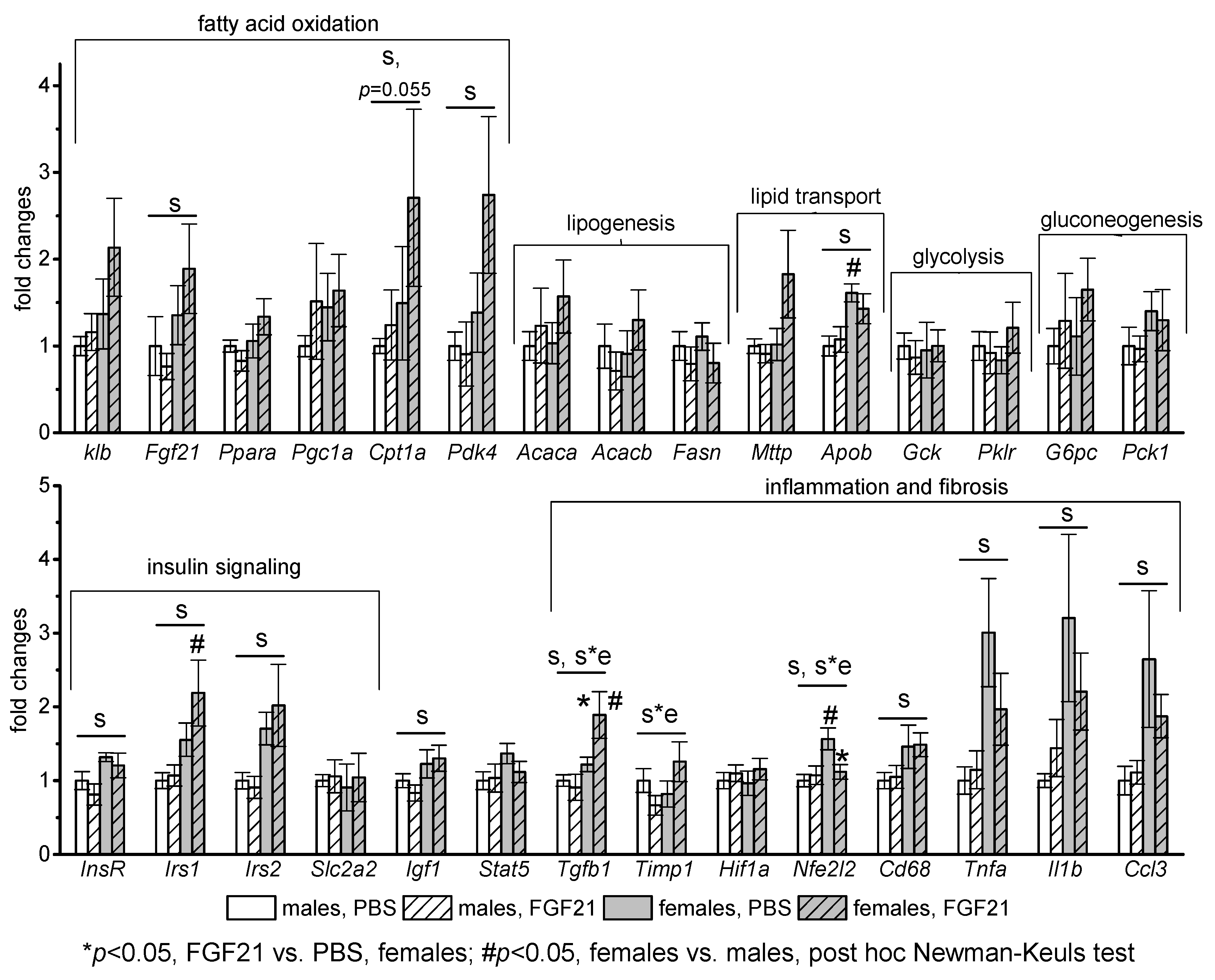
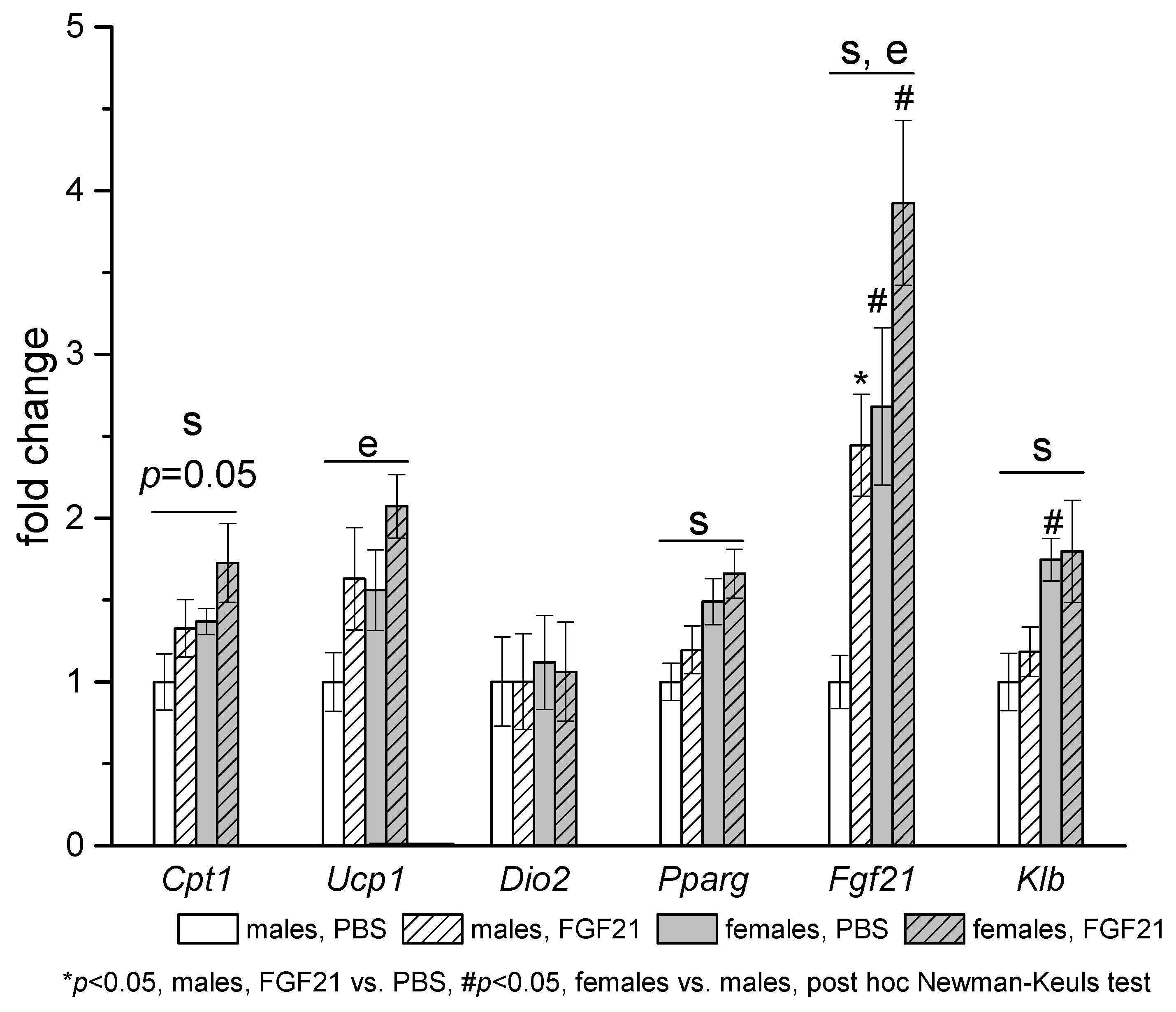
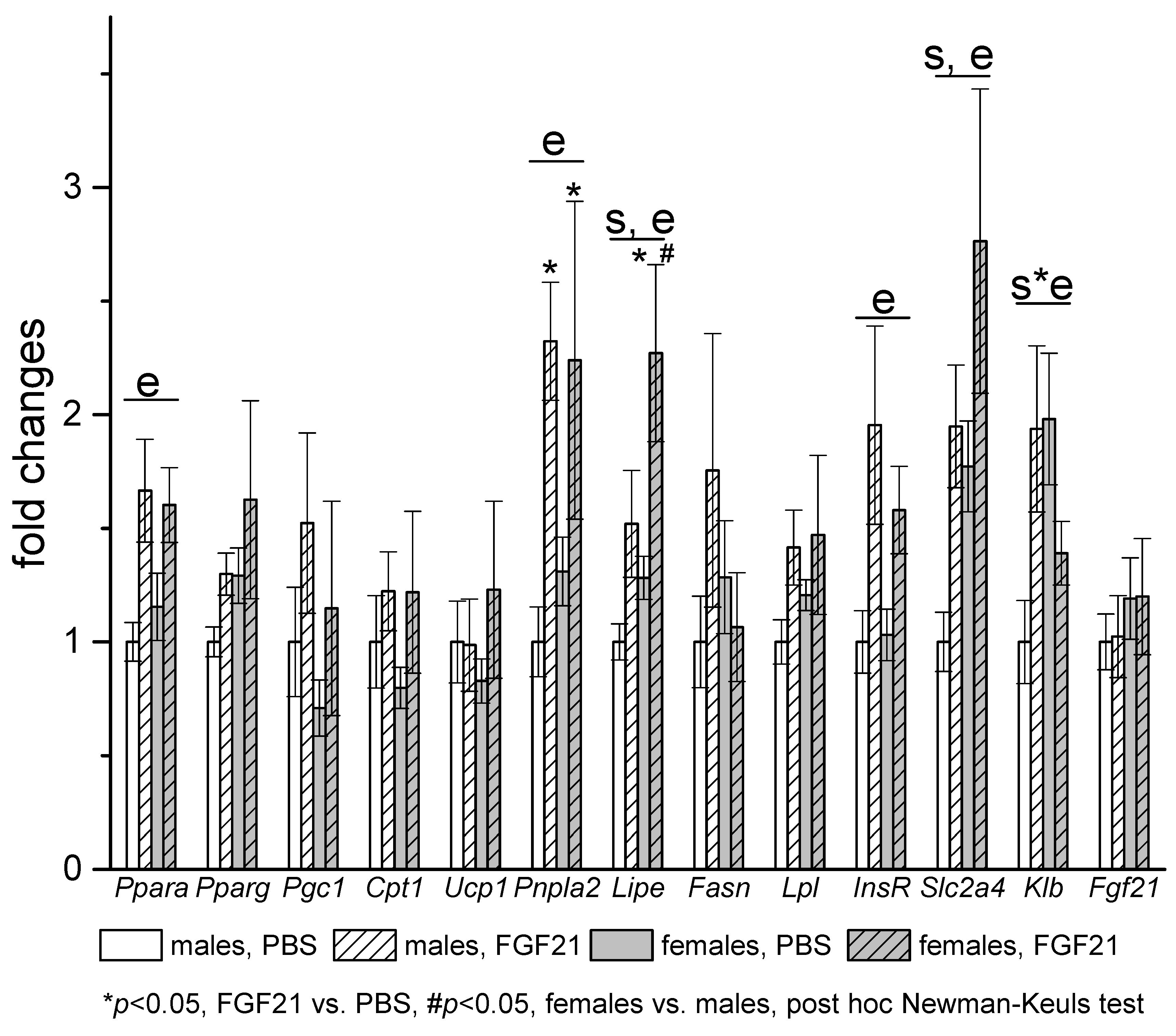
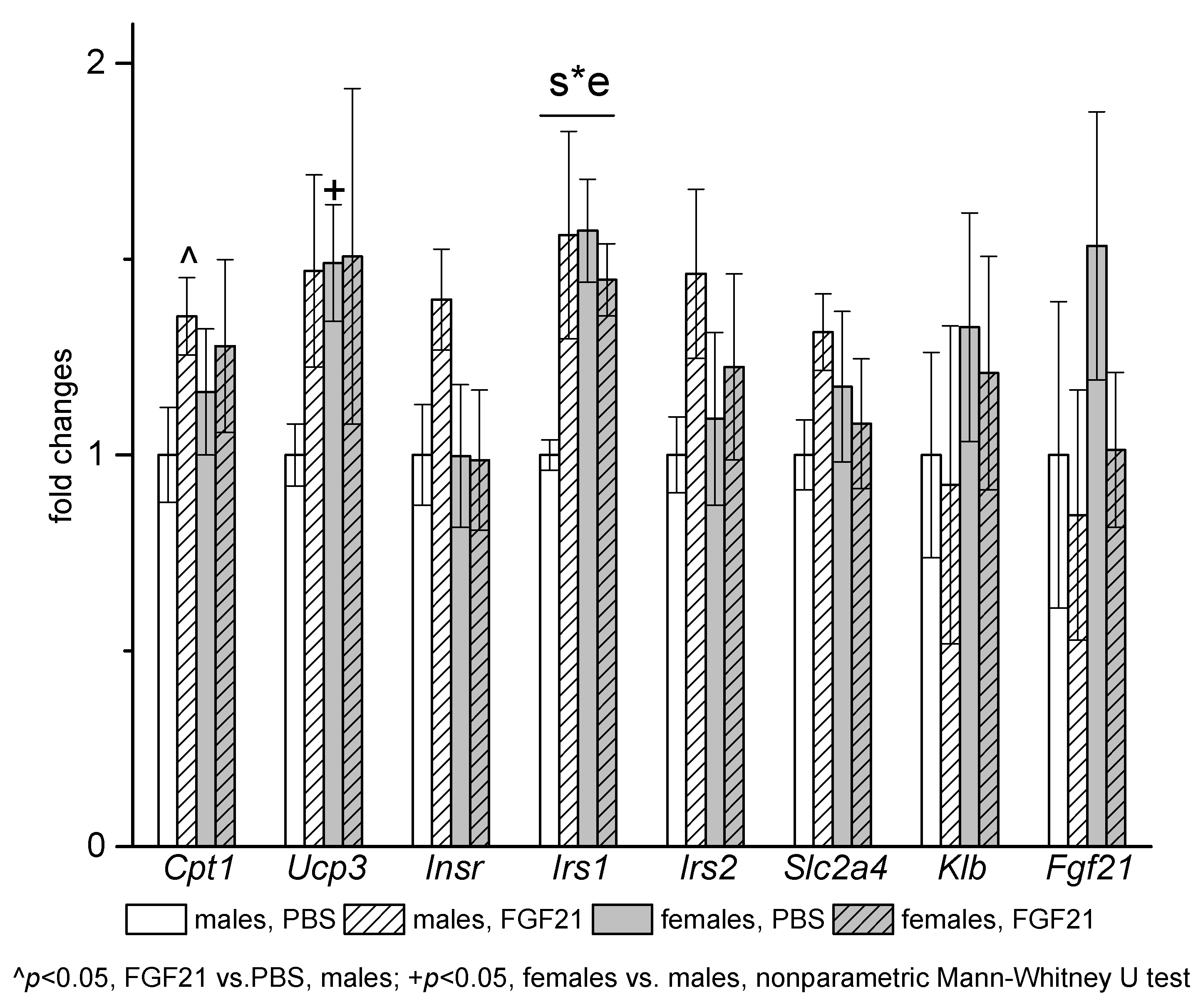
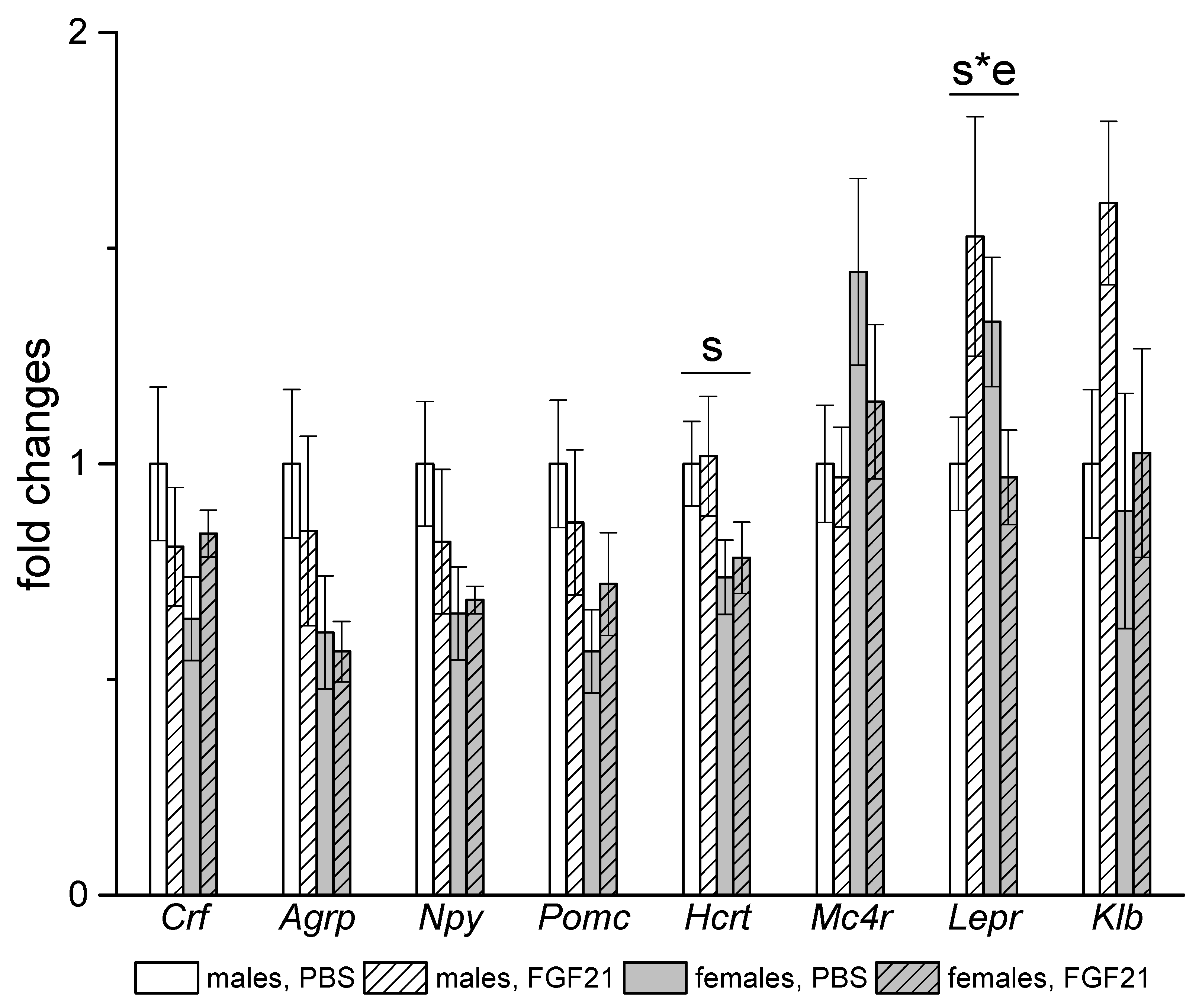
3.3. Effect of Exogenous FGF21 on Gene Expression in Liver, Muscle, White and Brown Adipose Tissues, and Hypothalamus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talukdar, S.; Kharitonenkov, A. FGF19 and FGF21: In NASH we trust. Mol. Metab. 2021, 46, 101152. [Google Scholar] [CrossRef]
- Flippo, K.H.; Potthoff, M.J. Metabolic Messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef]
- Martínez-Garza, Ú.; Torres-Oteros, D.; Yarritu-Gallego, A.; Marrero, P.F.; Haro, D.; Relat, J. Fibroblast Growth Factor 21 and the Adaptive Response to Nutritional Challenges. Int. J. Mol. Sci. 2019, 20, 4692. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.E.; Ebling, F.J.; Samms, R.J.; Tsintzas, K. Going Back to the Biology of FGF21: New Insights. Trends Endocrinol. Metab. 2019, 30, 491–504. [Google Scholar] [CrossRef]
- Zarei, M.; Pizarro-Delgado, J.; Barroso, E.; Palomer, X.; Vázquez-Carrera, M. Targeting FGF21 for the Treatment of Nonalcoholic Steatohepatitis. Trends Pharmacol. Sci. 2020, 41, 199–208. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast Growth Factor 21 Corrects Obesity in Mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef] [PubMed]
- Baruch, A.; Wong, C.; Chinn, L.W.; Vaze, A.; Sonoda, J.; Gelzleichter, T.; Chen, S.; Lewin-Koh, N.; Morrow, L.; Dheerendra, S.; et al. Antibody-mediated activation of the FGFR1/Klothoβ complex corrects metabolic dysfunction and alters food preference in obese humans. Proc. Natl. Acad. Sci. USA 2020, 117, 28992–29000. [Google Scholar] [CrossRef]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.-S.; Lindberg, R.A.; et al. Fibroblast Growth Factor 21 Reverses Hepatic Steatosis, Increases Energy Expenditure, and Improves Insulin Sensitivity in Diet-Induced Obese Mice. Diabetes 2008, 58, 250–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keinicke, H.; Sun, G.; Mentzel, C.; Fredholm, M.; John, L.M.; Andersen, B.; Raun, K.; Kjaergaard, M. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr. Connect. 2020, 9, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Lam, K.S.L.; Xu, A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat. Rev. Endocrinol. 2020, 16, 654–667. [Google Scholar] [CrossRef]
- Ritchie, M.; Hanouneh, I.A.; Noureddin, M.; Rolph, T.; Alkhouri, N. Fibroblast growth factor (FGF)-21 based therapies: A magic bullet for nonalcoholic fatty liver disease (NAFLD)? Expert Opin. Investig. Drugs 2020, 29, 197–204. [Google Scholar] [CrossRef]
- Li, X.; Ge, H.; Weiszmann, J.; Hecht, R.; Li, Y.-S.; Véniant, M.M.; Xu, J.; Wu, X.; Lindberg, R.; Li, Y. Inhibition of lipolysis may contribute to the acute regulation of plasma FFA and glucose by FGF21 in ob/ob mice. FEBS Lett. 2009, 583, 3230–3234. [Google Scholar] [CrossRef] [Green Version]
- Hale, C.; Chen, M.M.; Stanislaus, S.; Chinookoswong, N.; Hager, T.; Wang, M.; Véniant, M.M.; Xu, J. Lack of Overt FGF21 Resistance in Two Mouse Models of Obesity and Insulin Resistance. Endocrinology 2012, 153, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Weng, Y.; Chabot, J.R.; Bernardo, B.; Yan, Q.; Zhu, Y.; Brenner, M.B.; Vage, C.; Logan, A.; Calle, R.; Talukdar, S. Pharmacokinetics (PK), Pharmacodynamics (PD) and Integrated PK/PD Modeling of a Novel Long Acting FGF21 Clinical Candidate PF-05231023 in Diet-Induced Obese and Leptin-Deficient Obese Mice. PLoS ONE 2015, 10, e0119104. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, J.E.; Cha, J.J.; Hyun, Y.Y.; Kim, J.E.; Lee, M.H.; Song, H.K.; Nam, D.H.; Han, J.Y.; Han, S.Y.; et al. Fibroblast Growth Factor 21 Improves Insulin Resistance and Ameliorates Renal Injury in db/db Mice. Endocrinology 2013, 154, 3366–3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Qi, J.; Ren, G.; Xu, P.; Wu, Y.; Zhu, S.; Yu, D.; Li, S.; Wu, Q.; Muhi, R.L.; et al. Long-lasting anti-diabetic efficacy of PEGylated FGF-21 and liraglutide in treatment of type 2 diabetic mice. Endocrine 2015, 49, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Ye, X.; Wu, Q.; Li, S.; Yang, Y.; He, J.; Liu, Y.; Zhang, X.; Yuan, Q.; Liu, M.; et al. Insulin sensitizes FGF21 in glucose and lipid metabolisms via activating common AKT pathway. Endocrine 2016, 52, 527–540. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, Y.; Song, L.; Khoso, M.H.; Li, J.; Jiang, X.; He, J.; Li, J.; Ma, X.; Ren, G.; et al. Efficacy of a combination of high and low dosage of PEGylated FGF-21 in treatment of diabetes in db/db mice. Biomed. Pharmacother. 2016, 84, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Qi, J.; Yu, D.; Wu, Y.; Zhu, S.; Li, S.; Wu, Q.; Ren, G.; Li, D. Pharmacological efficacy of FGF21 analogue, liraglutide and insulin glargine in treatment of type 2 diabetes. J. Diabetes Its Complicat. 2017, 31, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cao, H.; Hou, Y.; Sun, G.; Li, D.; Wang, W. Liver Plays a Major Role in FGF-21 Mediated Glucose Homeostasis. Cell. Physiol. Biochem. 2018, 45, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Makarova, E.; Kazantseva, A.; Dubinina, A.; Jakovleva, T.; Balybina, N.; Baranov, K.; Bazhan, N. The Same Metabolic Response to FGF21 Administration in Male and Female Obese Mice Is Accompanied by Sex-Specific Changes in Adipose Tissue Gene Expression. Int. J. Mol. Sci. 2021, 22, 10561. [Google Scholar] [CrossRef] [PubMed]
- Kühnen, P.; Wiegand, S.; Biebermann, H. Pharmacological treatment strategies for patients with monogenic obesity. J. Pediatr. Endocrinol. Metab. 2020, 33, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; Keogh, J.M.; Yeo, G.S.H.; Lank, E.J.; Cheetham, T.; O’Rahilly, S. Clinical Spectrum of Obesity and Mutations in the Melanocortin 4 Receptor Gene. N. Engl. J. Med. 2003, 348, 1085–1095. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.W.; Faulkner, L.D. The Role of the Melanocortin System in Metabolic Disease: New Developments and Advances. Neuroendocrinology 2017, 104, 330–346. [Google Scholar] [CrossRef]
- Krashes, M.J.; Lowell, B.B.; Garfield, A.S. Melanocortin-4 receptor–regulated energy homeostasis. Nat. Neurosci. 2016, 19, 206–219. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.A.; do Carmo, J.M.; Hall, J.E. CNS Regulation of Glucose Homeostasis: Role of the Leptin-Melanocortin System. Curr. Diabetes Rep. 2020, 20, 29. [Google Scholar] [CrossRef]
- Nogueiras, R.; Wiedmer, P.; Perez-Tilve, D.; Veyrat-Durebex, C.; Keogh, J.M.; Sutton, G.M.; Pfluger, P.T.; Castaneda, T.R.; Neschen, S.; Hofmann, S.M.; et al. The central melanocortin system directly controls peripheral lipid metabolism. J. Clin. Investig. 2007, 117, 3475–3488. [Google Scholar] [CrossRef] [Green Version]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted Disruption of the Melanocortin-4 Receptor Results in Obesity in Mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Butler, A.; Cone, R. The melanocortin receptors: Lessons from knockout models. Neuropeptides 2002, 36, 77–84. [Google Scholar] [CrossRef]
- Litt, M.J.; Okoye, G.D.; Lark, D.; Cakir, I.; Moore, C.; Barber, M.; Atkinson, J.; Fessel, J.; Moslehi, J.; Cone, R.D. Loss of the melanocortin-4 receptor in mice causes dilated cardiomyopathy. eLife 2017, 6, e28118. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Suganami, T.; Nakagawa, N.; Tanaka, M.; Yamamoto, Y.; Kamei, Y.; Terai, S.; Sakaida, I.; Ogawa, Y. Melanocortin 4 Receptor–Deficient Mice as a Novel Mouse Model of Nonalcoholic Steatohepatitis. Am. J. Pathol. 2011, 179, 2454–2463. [Google Scholar] [CrossRef]
- Ramachandrappa, S.; Farooqi, I.S. Genetic approaches to understanding human obesity. J. Clin. Investig. 2011, 121, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.S.H.; Chao, D.H.M.; Siegert, A.M.; Koerperich, Z.M.; Ericson, M.D.; Simonds, S.E.; Larson, C.M.; Luquet, S.; Clarke, I.; Sharma, S.; et al. The melanocortin pathway and energy homeostasis: From discovery to obesity therapy. Mol. Metab. 2021, 48, 101206. [Google Scholar] [CrossRef]
- Lutz, T.A. An Overview of Rodent Models of Obesity and Type 2 Diabetes. In Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2128. [Google Scholar]
- Bultman, S.J.; Michaud, E.J.; Woychik, R. Molecular characterization of the mouse agouti locus. Cell 1992, 71, 1195–1204. [Google Scholar] [CrossRef]
- Mountjoy, K.G.; Willard, D.H.; Wilkison, W.O. Agouti Antagonism of Melanocortin-4 Receptor: Greater Effect with Desacetyl-α-Melanocyte-Stimulating Hormone (MSH) than with α-MSH. Endocrinology 1999, 140, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.L.; Roberts, D.W.; Mountjoy, K.G. Physiological consequences of ectopic agouti gene expression: The yellow obese mouse syndrome. Physiol. Genom. 1999, 1, 151–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derkach, K.; Zakharova, I.; Zorina, I.; Bakhtyukov, A.; Romanova, I.; Bayunova, L.; Shpakov, A. The evidence of metabolic-improving effect of metformin in Ay/a mice with genetically-induced melanocortin obesity and the contribution of hypothalamic mechanisms to this effect. PLoS ONE 2019, 14, e0213779. [Google Scholar] [CrossRef]
- Makarova, E.N.; Yakovleva, T.V.; Balyibina, N.Y.; Baranov, K.O.; Denisova, E.I.; Dubinina, A.D.; Feofanova, N.A.; Bazhan, N.M. Pharmacological effects of fibroblast growth factor 21 are sex-specific in mice with the lethal yellow (Ay) mutation. Vavilov J. Genet. Breed. 2020, 24, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Hart-Unger, S.; Arao, Y.; Hamilton, K.J.; Lierz, S.; Malarkey, D.E.; Hewitt, S.; Freemark, M.; Korach, K.S. Hormone signaling and fatty liver in females: Analysis of estrogen receptor α mutant mice. Int. J. Obes. 2017, 41, 945–954. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zou, X.; Feng, Z.; Luo, C.; Liu, J.; Li, H.; Chang, L.; Wang, H.; Li, Y.; Long, J.; et al. Evidence for association of mitochondrial metabolism alteration with lipid accumulation in aging rats. Exp. Gerontol. 2014, 56, 3–12. [Google Scholar] [CrossRef]
- Roehrig, K.L.; Allred, J.B. Direct enzymatic procedure for the determination of liver glycogen. Anal. Biochem. 1974, 58, 414–421. [Google Scholar] [CrossRef]
- Marie, L.S.; Miura, G.I.; Marsh, D.J.; Yagaloff, K.; Palmiter, R.D. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 12339–12344. [Google Scholar] [CrossRef] [Green Version]
- Voss-Andreae, A.; Murphy, J.G.; Ellacott, K.L.J.; Stuart, R.C.; Nillni, E.A.; Cone, R.D.; Fan, W. Role of the Central Melanocortin Circuitry in Adaptive Thermogenesis of Brown Adipose Tissue. Endocrinology 2007, 148, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Albarado, D.C.; McClaine, J.; Stephens, J.M.; Mynatt, R.L.; Ye, J.; Bannon, A.W.; Richards, W.G.; Butler, A. Impaired Coordination of Nutrient Intake and Substrate Oxidation in Melanocortin-4 Receptor Knockout Mice. Endocrinology 2004, 145, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miltenberger, R.J.; Mynatt, R.L.; Wilkinson, J.E.; Woychik, R.P. The role of the agouti gene in the yellow obese syndrome. J. Nutr. 1997, 127, 1902S–1907S. [Google Scholar] [CrossRef] [PubMed]
- Laeger, T.; Baumeier, C.; Wilhelmi, I.; Würfel, J.; Kamitz, A.; Schürmann, A. FGF21 improves glucose homeostasis in an obese diabetes-prone mouse model independent of body fat changes. Diabetologia 2017, 60, 2274–2284. [Google Scholar] [CrossRef] [Green Version]
- Bazhan, N.; Yakovleva, T.; Kazantseva, A.; Makarova, E. Exaggerated anorexigenic response to restraint stress in Ay mice is associated with elevated CRFR2 mRNA expression in the hypothalamus. Physiol. Behav. 2013, 120, 19–25. [Google Scholar] [CrossRef]
- Owen, B.; Ding, X.; Morgan, D.A.; Coate, K.; Bookout, A.L.; Rahmouni, K.; Kliewer, S.A.; Mangelsdorf, D.J. FGF21 Acts Centrally to Induce Sympathetic Nerve Activity, Energy Expenditure, and Weight Loss. Cell Metab. 2014, 20, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Moure, R.; Cairó, M.; Morón-Ros, S.; Quesada-López, T.; Campderrós, L.; Cereijo, R.; Hernáez, A.; Villarroya, F.; Giralt, M. Levels of β-klotho determine the thermogenic responsiveness of adipose tissues: Involvement of the autocrine action of FGF21. Am. J. Physiol.-Endocrinol. Metab. 2021, 320, E822–E834. [Google Scholar] [CrossRef]
- Challa, T.D.; Dapito, D.H.; Kulenkampff, E.; Kiehlmann, E.; Moser, C.; Straub, L.; Sun, W.; Wolfrum, C. A Genetic Model to Study the Contribution of Brown and Brite Adipocytes to Metabolism. Cell Rep. 2020, 30, 3424–3433. [Google Scholar] [CrossRef] [Green Version]
- Berglund, E.D.; Liu, T.; Kong, X.; Sohn, J.-W.; Vong, L.; Deng, Z.; Lee, C.E.; Lee, S.; Williams, K.; Olson, D.; et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat. Neurosci. 2014, 17, 911–913. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Yu, M.; Arshad, M.; Wang, W.; Lu, Y.; Gong, J.; Gu, Y.; Li, P.; Xu, L. Coordination among Lipid Droplets, Peroxisomes, and Mitochondria Regulates Energy Expenditure through the CIDE-ATGL-PPARα Pathway in Adipocytes. Diabetes 2018, 67, 1935–1948. [Google Scholar] [CrossRef] [Green Version]
- BonDurant, L.D.; Ameka, M.; Naber, M.C.; Markan, K.; Idiga, S.O.; Acevedo, M.R.; Walsh, S.; Ornitz, D.; Potthoff, M.J. FGF21 Regulates Metabolism through Adipose-Dependent and -Independent Mechanisms. Cell Metab. 2017, 25, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.E.; Monnier, C.; Marshall, H.; Fowler, M.; Green, R.; Cooper, S.; Chiotellis, A.; Luckett, J.; Perkins, A.C.; Coskun, T.; et al. Whole-body and adipose tissue-specific mechanisms underlying the metabolic effects of fibroblast growth factor 21 in the Siberian hamster. Mol. Metab. 2020, 31, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wong, K.; Giles, A.; Jiang, J.; Lee, J.W.; Adams, A.C.; Kharitonenkov, A.; Yang, Q.; Gao, B.; Guarente, L.; et al. Hepatic SIRT1 Attenuates Hepatic Steatosis and Controls Energy Balance in Mice by Inducing Fibroblast Growth Factor 21. Gastroenterology 2014, 146, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Camporez, J.P.G.; Jornayvaz, F.; Petersen, M.C.; Pesta, D.; Guigni, B.; Serr, J.; Zhang, D.; Kahn, M.; Samuel, V.T.; Jurczak, M.; et al. Cellular Mechanisms by Which FGF21 Improves Insulin Sensitivity in Male Mice. Endocrinology 2013, 154, 3099–3109. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, J.T.; Turner, M.J.; Daves, M.; Vordermark, A.; Kleeberger, S.R. Genetic influence on daily wheel running activity level. Physiol. Genom. 2005, 19, 270–276. [Google Scholar] [CrossRef]
- Goforth, P.B.; Myers, M.G. Roles for Orexin/Hypocretin in the control of energy balance and metabolism. In Current Topics in Behavioral Neurosciences; Springer: Cham, Switzerland, 2017; Volume 33. [Google Scholar]
- Choudhury, J.; Sanyal, A.J. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin. Liver Dis. 2004, 8, 575–594. [Google Scholar] [CrossRef]
- Armandi, A.; Rosso, C.; Caviglia, G.; Bugianesi, E. Insulin Resistance across the Spectrum of Nonalcoholic Fatty Liver Disease. Metabolites 2021, 11, 155. [Google Scholar] [CrossRef]
- Toda, K.; Toda, A.; Ono, M.; Saibara, T. Lack of 17β-estradiol reduces sensitivity to insulin in the liver and muscle of male mice. Heliyon 2018, 4, e00772. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, physiological functions, role in diseases, and effects of nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- González-García, I.; Tena-Sempere, M.; López, M. Estradiol regulation of brown adipose tissue thermogenesis. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 1043. [Google Scholar]
- Monjo, M.; Rodríguez, A.M.; Palou, A.; Roca, P. Direct Effects of Testosterone, 17β-Estradiol, and Progesterone on Adrenergic Regulation in Cultured Brown Adipocytes: Potential Mechanism for Gender-Dependent Thermogenesis. Endocrinology 2003, 144, 4923–4930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Yang, W.; Zhou, F.; Li, X.; Pan, Q.; Shen, Z.; Han, G.; Newell-Fugate, A.; Tian, Y.; Majeti, R.; et al. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes 2019, 68, 291–304. [Google Scholar] [CrossRef] [Green Version]
- López, M.; Tena-Sempere, M. Estradiol effects on hypothalamic AMPK and BAT thermogenesis: A gateway for obesity treatment? Pharmacol. Ther. 2017, 178, 109–122. [Google Scholar] [CrossRef]
- Lieu, L.; Chau, D.; Afrin, S.; Dong, Y.; Alhadeff, A.L.; Betley, J.N.; Williams, K.W. Effects of metabolic state on the regulation of melanocortin circuits. Physiol. Behav. 2020, 224, 113039. [Google Scholar] [CrossRef]
- Masaki, T.; Chiba, S.; Yasuda, T.; Tsubone, T.; Kakuma, T.; Shimomura, I.; Funahashi, T.; Matsuzawa, Y.; Yoshimatsu, H. Peripheral, but Not Central, Administration of Adiponectin Reduces Visceral Adiposity and Upregulates the Expression of Uncoupling Protein in Agouti Yellow (Ay/a) Obese Mice. Diabetes 2003, 52, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [Green Version]
- Camporez, J.P.G.; Jornayvaz, F.; Lee, H.-Y.; Kanda, S.; Guigni, B.; Kahn, M.; Samuel, V.T.; Carvalho, C.; Petersen, K.F.; Jurczak, M.; et al. Cellular Mechanism by Which Estradiol Protects Female Ovariectomized Mice From High-Fat Diet-Induced Hepatic and Muscle Insulin Resistance. Endocrinology 2013, 154, 1021–1028. [Google Scholar] [CrossRef]
- D’Souza, A.M.; Neumann, U.H.; Glavas, M.M.; Kieffer, T.J. The glucoregulatory actions of leptin. Mol. Metab. 2017, 6, 1052–1065. [Google Scholar] [CrossRef]
- Boston, B.A.; Blaydon, K.M.; Varnerin, J.; Cone, R.D. Independent and Additive Effects of Central POMC and Leptin Pathways on Murine Obesity. Science 1997, 278, 1641–1644. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.A.; Lyon, C.J.; Hsueh, W.A. Nuclear Factor (Erythroid-Derived 2)-Like-2 Factor (Nrf2), a Key Regulator of the Antioxidant Response to Protect Against Atherosclerosis and Nonalcoholic Steatohepatitis. Curr. Diabetes Rep. 2013, 13, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Matsuoka, S.; Yamazaki, M.; Shibata, T.; Nirei, K.; Takahashi, H.; Kaneko, T.; Fujisawa, M.; Higuchi, T.; Nakamura, H.; et al. Apoptosis and non-alcoholic fatty liver diseases. World J. Gastroenterol. 2018, 24, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Caceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Dijke, P.T.; IT-LIVER Consortium. TGF-β signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [Green Version]
- Thiele, N.D.; Wirth, J.W.; Steins, D.; Koop, A.C.; Ittrich, H.; Lohse, A.W.; Kluwe, J. TIMP-1 is upregulated, but not essential in hepatic fibrogenesis and carcinogenesis in mice. Sci. Rep. 2017, 7, 714. [Google Scholar] [CrossRef]
- Tomaš, T.C.; Urlep, Ž.; Moškon, M.; Mraz, M.; Rozman, D. LiverSex Computational Model: Sexual Aspects in Hepatic Metabolism and Abnormalities. Front. Physiol. 2018, 9, 360. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]

| Protein | Gene | Gene Expression Assay ID |
|---|---|---|
| Acetyl-coenzyme A carboxylase alpha | Acca | Mm01304285_m1 |
| Acetyl-coenzyme A carboxylase beta | Accb | Mm01204683_m1 |
| Agouti related neuropeptide | Agrp | Mm00475829_g1 |
| Apolipoprotein B | Apob | Mm01545150_m1 |
| Beta-2-microglobulin | B2m | Mm00437762_m1 |
| Beta-actin | Actb | Mm00607939_s1 |
| Carnitine palmitoyltransferase 1a | Cpt1a | Mm01231183_m1 |
| Carnitine palmitoyltransferase 1b | Cpt1b | Mm00487191_g1 |
| CD68 antigen | Cd68 | Mm03047343_m1 |
| Chemokine (C-C motif) ligand 3 | Ccl3 | Mm00441259_g1 |
| Corticotropin releasing hormone | Crh | Mm01293920_s1 |
| Deiodinase, iodothyronine, type II | Dio2 | Mm00515664_m1 |
| Fatty acid synthase | Fasn | Mm00662319_m1 |
| Fibroblast growth factor 21 | Fgf21 | Mm00840165_g1 |
| Glucokinase | Gck | Mm00439129_m1 |
| Glucose-6-phosphatase, catalytic | G6pc | Mm00839363_m1 |
| Hypocretin | Hcrt | Mm01964030_s1 |
| Hypoxia inducible factor 1, alpha subunit | Hif1a | Mm00468869_m1 |
| Insulin receptor | Insr | Mm01211875_m1 |
| Insulin receptor substrate 1 | Irs1 | Mm01278327_m1 |
| Insulin receptor substrate 2 | Irs2 | Mm03038438_m1 |
| Insulin-like growth factor 1 | Igf1 | Mm00439560_m1 |
| Interleukin 1 beta | Il1b | Mm00434228_m1 |
| Klotho beta | Klb | Mm00473122_m1 |
| Leptin receptor | Lepr | Mm00440181_m1 |
| Lipase, hormone sensitive | Lipe | Mm00495359_m1 |
| Lipoprotein lipase | Lpl | Mm00434764_m1 |
| Melanocortin receptor type 4 | Mc4r | Mm00457483_s1 |
| Microsomal triglyceride transfer protein | Mttp | Mm00435015_m1 |
| Neuropeptide Y | Npy | Mm01410146_m1 |
| Nuclear factor, erythroid derived 2, like 2 | Nfe2l2 | Mm00477784_m1 |
| Patatin-like phospholipase domain containing 2 (adipose triglyceride lipase (ATGL)) | Pnpla2 | Mm00503040_m1 |
| Peroxisome proliferative activated receptor, gamma, coactivator 1 alpha | Ppargc1a (Pgc1) | Mm01208835_m1 |
| Peroxisome proliferator activated receptor alpha | Ppara | Mm0040939_m1 |
| Peroxisome proliferator activated receptor gamma | Pparg | Mm00440940_m1 |
| Phosphoenolpyruvate carboxykinase 1, cytosolic | Pck1 | Mm01247058_m1 |
| Pro-opiomelanocortin | Pomc | Mm00435874_m1 |
| Pyruvate dehydrogenase kinase, isoenzyme 4 | Pdk4 | Mm01166879_m1 |
| Pyruvate kinase liver and red blood cell | Pklr | Mm00443090_m1 |
| Signal transducer and activator of transcription 5A | Stat5 | Mm03053818_s1 |
| Solute carrier family 2 (facilitated glucose transporter), member 2 (GLUT2) | Slc2a2 | Mm00446229_m1 |
| Solute carrier family 2 (facilitated glucose transporter), member 4 (GLUT4) | Slc2a4 | Mm00436615_m1 |
| Tissue inhibitor of metalloproteinase 1 | Timp1 | Mm01341361_m1 |
| Transforming growth factor, beta 1 | Tgfb1 | Mm01178820_m1 |
| Tumor necrosis factor alpha | Tnfa | Mm00443258_m1 |
| Uncoupling protein 1 (mitochondrial, proton carrier) | Ucp1 | Mm01244861_m1 |
| Uncoupling protein 3 (mitochondrial, proton carrier) | Ucp3 | Mm01163394_m1 |
| Ay Males | Ay Females | p ANOVA | |||
|---|---|---|---|---|---|
| PBS (n = 7) | FGF21 (n = 7) | PBS (n = 6) | FGF21 (n = 6) | ||
| BW (g) | 46.9 ± 0.6 | 45.7 ± 1.2 | 46.5 ± 1.45 | 48.2 ± 0.83 | ns |
| Lean mass (g) | 28.1 ± 0.4 | 28.0 ± 0.3 | 23.8 ± 0.6 # | 23.8 ± 0.5 # | s |
| Fat mass (g) | 16.7 ± 0.6 | 16.0 ± 1.0 | 20.8 ± 1.0 # | 22.5 ± 0.7 # | s |
| Liver weight (g) | 2.67 ± 0.06 | 2.15 ± 0.09 * | 1.98 ± 0.08 # | 2,12 ± 0.06 | s, e, s*e |
| iBAT weight (g) | 0.185 ± 0.025 | 0.164 ± 0.017 | 0.179 ± 0.023 | 0.201 ± 0.026 | ns |
| Ay Males | Ay Females | p ANOVA | |||
|---|---|---|---|---|---|
| PBS (n = 7) | FGF21 (n = 7) | PBS (n = 6) | FGF21 (n = 6) | ||
| Glucose (mM) | 12.6 ± 0.7 | 12.3 ± 1.3 | 10.9 ± 1.06 | 11.1 ± 1.22 | ns |
| Triglycerides (mM) | 0.64 ± 0.03 | 0.93 ± 0.13 | 0.71 ± 0.04 | 0.78 ± 0.12 | p = 0.07, e |
| Cholesterol (mM) | 4.29 ± 0.08 | 3.94 ± 0.11 | 3.85 ± 0.16 | 4.00 ± 0.12 | p = 0.06, s*e |
| Free fatty acids (mM) | 0.92 ± 0.11 | 0.64 ± 0.14 | 0.88 ± 0.11 | 0.88 ± 0.12 | ns |
| Insulin (ng/mL) | 24.2 ± 4.6 | 12.6 ± 1.3 * | 11.0 ± 1.8 # | 14.1 ± 3.4 | s*e |
| Leptin (ng/mL) | 55.6 ± 6.6 | 40.7 ± 5.4 | 46.3 ± 4.5 | 55.4 ± 4.0 | s*e |
| Adiponectin (µg/mL) | 8.2 ± 0.4 | 8.5 ± 0.2 | 13.0 ± 0.6 # | 12.8 ± 0.5 # | s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarova, E.; Kazantseva, A.; Dubinina, A.; Denisova, E.; Jakovleva, T.; Balybina, N.; Bgatova, N.; Baranov, K.; Bazhan, N. Fibroblast Growth Factor 21 (FGF21) Administration Sex-Specifically Affects Blood Insulin Levels and Liver Steatosis in Obese Ay Mice. Cells 2021, 10, 3440. https://doi.org/10.3390/cells10123440
Makarova E, Kazantseva A, Dubinina A, Denisova E, Jakovleva T, Balybina N, Bgatova N, Baranov K, Bazhan N. Fibroblast Growth Factor 21 (FGF21) Administration Sex-Specifically Affects Blood Insulin Levels and Liver Steatosis in Obese Ay Mice. Cells. 2021; 10(12):3440. https://doi.org/10.3390/cells10123440
Chicago/Turabian StyleMakarova, Elena, Antonina Kazantseva, Anastasia Dubinina, Elena Denisova, Tatiana Jakovleva, Natalia Balybina, Nataliya Bgatova, Konstantin Baranov, and Nadezhda Bazhan. 2021. "Fibroblast Growth Factor 21 (FGF21) Administration Sex-Specifically Affects Blood Insulin Levels and Liver Steatosis in Obese Ay Mice" Cells 10, no. 12: 3440. https://doi.org/10.3390/cells10123440
APA StyleMakarova, E., Kazantseva, A., Dubinina, A., Denisova, E., Jakovleva, T., Balybina, N., Bgatova, N., Baranov, K., & Bazhan, N. (2021). Fibroblast Growth Factor 21 (FGF21) Administration Sex-Specifically Affects Blood Insulin Levels and Liver Steatosis in Obese Ay Mice. Cells, 10(12), 3440. https://doi.org/10.3390/cells10123440