A Reassessment of the Barrier Effect of the Physis against Metaphyseal Osteosarcoma: A Comprehensive Pathological Study with Its Radiological and Clinical Follow-Up Correlations
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aegerter, E.; Kirkpatrick, J. Orthopaedic Diseases. In Physiology, Pathology; WB Saunders: Philadelphia, PA, USA, 1975; p. 519. [Google Scholar]
- Li, Y.-S.; Liu, Q.; Tian, J.; He, H.-B.; Luo, W. Angiogenesis Process in Osteosarcoma: An Updated Perspective of Pathophysiology and Therapeutics. Am. J. Med. Sci. 2019, 357, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Folkman, J. Inhibition of tumor angiogenesis mediated by cartilage. J. Exp. Med. 1975, 141, 427–439. [Google Scholar] [CrossRef]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Kuettner, K.E.; Pauli, B.U.; Soble, L. Morphological studies on the resistance of cartilage to invasion by osteosarcoma cells in vitro and in vivo. Cancer Res. 1978, 38, 277–287. [Google Scholar]
- Langer, R.; Brem, H.; Falterman, K.; Klein, M.; Folkman, J. Isolations of a Cartilage Factor That Inhibits Tumor Neovascularization. Science 1976, 193, 70–72. [Google Scholar] [CrossRef]
- Quan, G.M.; Ojaimi, J.; Nadesapillai, A.W.; Zhou, H.; Choong, P.F. Resistance of Epiphyseal Cartilage to Invasion by Osteosarcoma Is Likely to Be Due to Expression of Antiangiogenic Factors. Pathobiology 2002, 70, 361–367. [Google Scholar] [CrossRef]
- DeChiara, T.M.; Efstratiadis, A.; Robertsen, E.J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 1990, 345, 78–80. [Google Scholar] [CrossRef]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef]
- Panuel, M.; Gentet, J.C.; Scheiner, C.; Jouve, J.L.; Bollini, G.; Petit, P.; Bourliere-Najean, B.; Devred, P. Physeal and epiphyseal extent of primary malignant bone tumors in childhood. Correlation of preoperative MRI and the pathologic examination. Pediatr. Radiol. 1993, 23, 421–424. [Google Scholar] [CrossRef]
- Enneking, W.F.; Kagan, A. Transepiphyseal extension of osteosarcoma: Incidence, mechanism, and implications. Cancer 1978, 41, 1526–1537. [Google Scholar] [CrossRef]
- Ghandur-Mnaymneh, L.; Mnaymneh, W.A.; Puls, S. The Incidence and Mechanism of Transphyseal Spread of Osteosarcoma of Long Bones. Clin. Orthop. Relat. Res. 1983, 177, 210–215. [Google Scholar] [CrossRef]
- Norton, K.I.; Hermann, G.; Abdelwahab, I.F.; Klein, M.J.; Granowetter, L.F.; Rabinowitz, J.G. Epiphyseal involvement in osteosarcoma. Radiology 1991, 180, 813–816. [Google Scholar] [CrossRef]
- San-Julián, M.; Vázquez-García, B.; Aquerreta, J.D.; Idoate, M.A. Reconstruction Following Tumor Resections in Skeletally Immature Patients. J. Am. Acad. Orthop. Surg. 2017, 25, e272–e274. [Google Scholar] [CrossRef]
- San-Julian, M.; Aquerreta, J.D.; Benito, A.; Cañadell, J. Indications for Epiphyseal Preservation in Metaphyseal Malignant Bone Tumors of Children: Relationship Between Image Methods and Histological Findings. J. Pediatr. Orthop. 1999, 19, 543–548. [Google Scholar] [CrossRef]
- van der Heijden, L.; Farfalli, G.L.; Balacó, I.; Alves, C.; Salom, M.; Lamo-Espinosa, J.M.; San-Julián, M.; van de Sande, M.A. Biology and technology in the surgical treatment of malignant bone tumours in children and adolescents, with a special note on the very young. J. Child. Orthop. 2021, 15, 322–330. [Google Scholar] [CrossRef]
- Cañadell, J.; Forriol, F.; Cara, J. Removal of metaphyseal bone tumours with preservation of the epiphysis. Physeal distraction before excision. J. Bone Jt. Surgery. Br. Vol. 1994, 76-B, 127–132. [Google Scholar] [CrossRef]
- Weitao, Y.; Qiqing, C.; Songtao, G.; Jiaqiang, W. Epiphysis preserving operations for the treatment of lower limb malignant bone tumors. Eur. J. Surg. Oncol. 2012, 38, 1165–1170. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, F.; Cai, Q.; Yao, W.; Gao, S.; Wang, J.; Wang, X. Effects of metaphyseal bone tumor removal with preservation of the epiphysis and knee arthroplasty. Exp. Ther. Med. 2014, 8, 567–572. [Google Scholar] [CrossRef]
- Betz, M.; Dumont, C.E.; Fuchs, B.; Exner, U.G. Physeal Distraction for Joint Preservation in Malignant Metaphyseal Bone Tumors in Children. Clin. Orthop. Relat. Res. 2012, 470, 1749–1754. [Google Scholar] [CrossRef] [Green Version]
- Trueta, J.; Morgan, J.D. The vascular contribution to osteogenesis. J. Bone Jt. Surgery. Br. Vol. 1960, 42-B, 97–109. [Google Scholar] [CrossRef]
- Brighton, C.T. The growth plate. Orthop. Clin. N. Am. 1984, 15, 571–595. [Google Scholar] [CrossRef]
- Enneking, W.F.; Kagan, A. “Skip” metastases in osteosarcoma. Cancer 1975, 36, 2192–2205. [Google Scholar] [CrossRef]
- Cui, J.; Dean, D.; Hornicek, F.J.; Chen, Z.; Duan, Z. The role of extracelluar matrix in osteosarcoma progression and metastasis. J. Exp. Clin. Cancer Res. 2020, 39, 178. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef] [Green Version]
- Verrecchia, F.; Rédini, F. Transforming Growth Factor-β Signaling Plays a Pivotal Role in the Interplay between Osteosarcoma Cells and Their Microenvironment. Front. Oncol. 2018, 8, 133. [Google Scholar] [CrossRef]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The Osteosarcoma Microenvironment: A Complex but Targetable Ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.M.; Arlt, M.J.; Kuzmanov, A.; Born, W.; Fuchs, B. Sunitinib malate (SU-11248) reduces tumour burden and lung metastasis in an intratibial human xenograft osteosarcoma mouse model. Am. J. Cancer Res. 2015, 5, 2156–2168. [Google Scholar]
- Xie, L.; Xu, J.; Sun, X.; Tang, X.; Yan, T.; Yang, R.; Guo, W. Apatinib for Advanced Osteosarcoma after Failure of Standard Multimodal Therapy: An Open Label Phase II Clinical Trial. Oncologist 2018, 24, e542–e550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assi, T.; Watson, S.; Samra, B.; Rassy, E.; Le Cesne, A.; Italiano, A.; Mir, O. Targeting the VEGF Pathway in Osteosarcoma. Cells 2021, 10, 1240. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kaneda, T.; Arakawa, T.; Morita, S.; Sato, T.; Yomada, T.; Hanada, K.; Kumegawa, M.; Hakeda, Y. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FEBS Lett. 2000, 473, 161–164. [Google Scholar] [CrossRef]
- Dahlin, D.C. Bone Tumours: General Aspects and Data on 10,165 Cases; Lippincott Williams & Wilkis: Philadelphia, PA, USA, 2009. [Google Scholar]
- Missenard, G.; Dubousset, J.; Genin, J.; Contesso, G. Confrontation anatomo-clinique pré- et post-opératoire à propos de 30 résections conservatrices pour sarcomes ostéogènes. Déductions chirurgicales [Pre- and postoperative anatomo-clinical correlations apropos of 30 conservative resections of osteogenic sarcomas. Surgical inferences]. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1986, 72, 477–483. (In French) [Google Scholar]


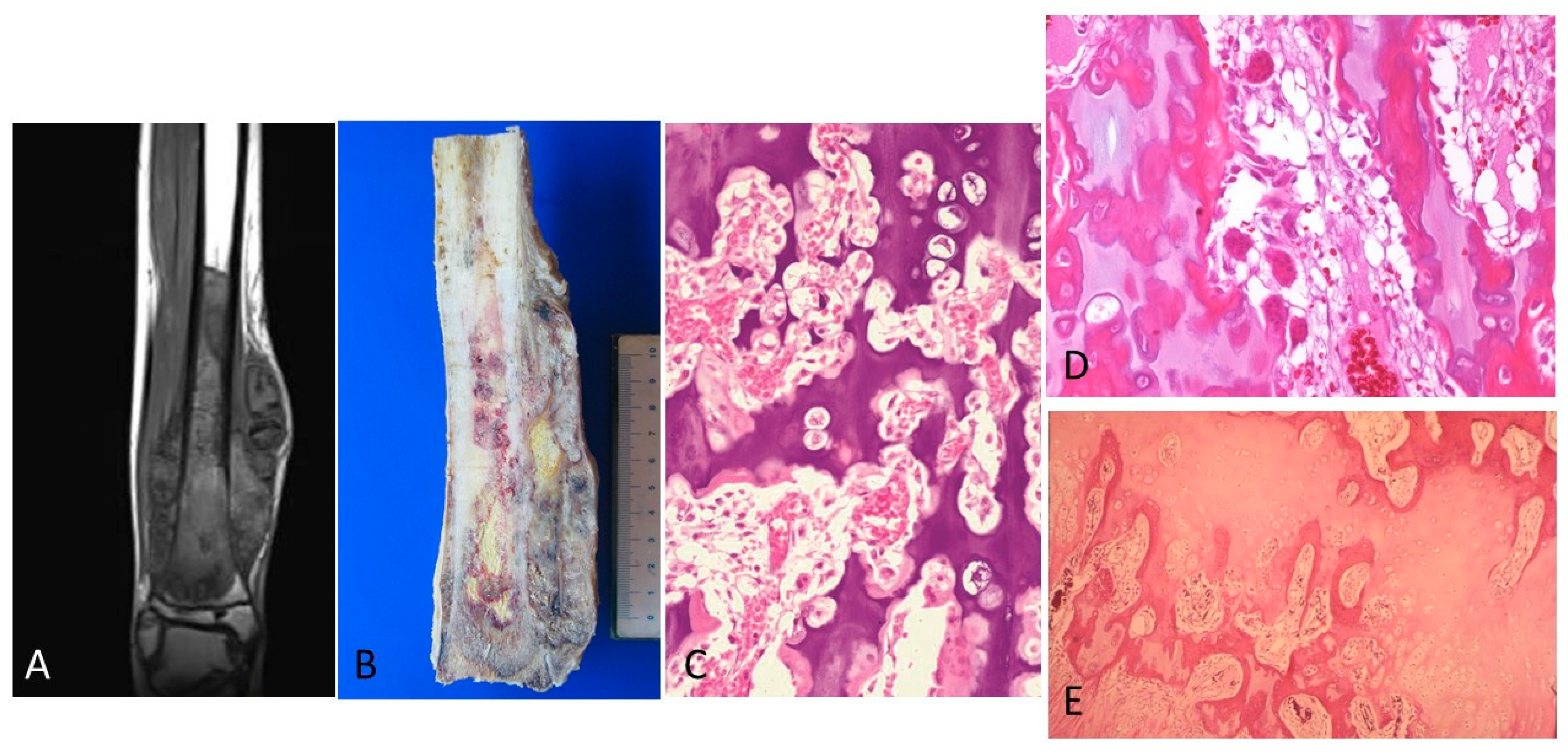

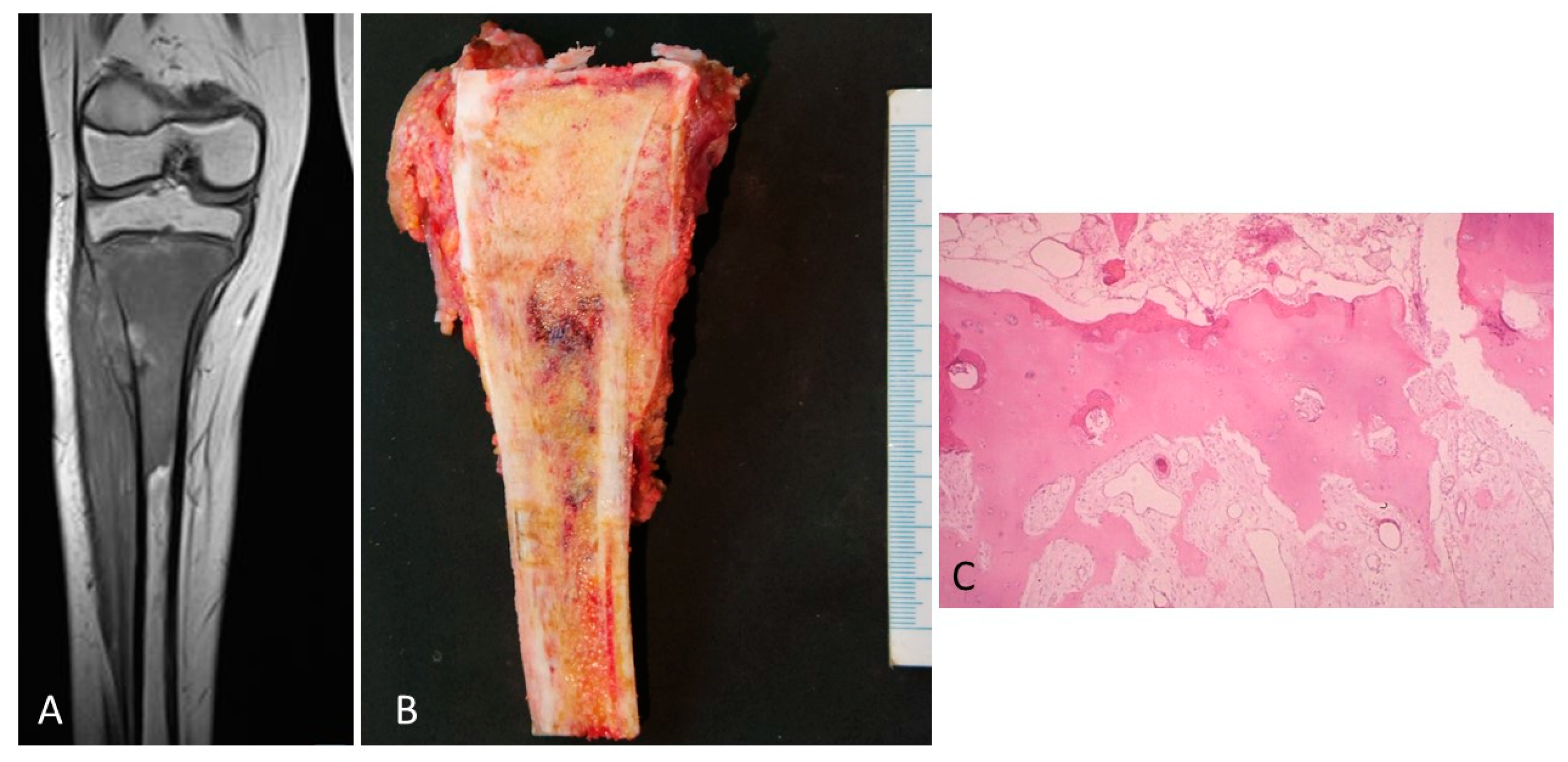


| Items | Group I (n = 152) | Group II (n = 130) |
|---|---|---|
| Physeal Invasion | 93% | 0% |
| Time elapsed between onset of symptoms and treatment | 4 months | 2 months |
| Mean age (mean and range) | 14.5 y.o (9–17) | 12.5 y.o (2–15) |
| Anatomical Location (n) | ||
| Distal fémur | 51 cases | 44 cases |
| Proximal tibia | 40 cases | 37 cases |
| Proximal humerus | 23 cases | 14 cases |
| Proximal femur | 18 cases | 6 cases |
| Distal tibia | 14 cases | 11 cases |
| Anatomical Location of Recurrence | Soft tissue | soft tissue close to diaphysis/metaphysis |
| Treatment | 62 osteoarticular allograft 89 prostheses 1 amputation | 97 Epiphysiolisis before resection + graft reconstruction 33 intraepiphyseal osteotomy + graft reconstruction |
| Frequency of Local Recurrence | 12% | 8% |
| Frequency of Long-term recurrences | 2% | 0% |
| Metastases at diagnosis | 38% | 22% |
| Disease free survival (%), at a mean follow-up of 18 years | 59% | 73% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idoate, M.Á.; Aquerreta, J.D.; Lamo-Espinosa, J.M.; San-Julian, M. A Reassessment of the Barrier Effect of the Physis against Metaphyseal Osteosarcoma: A Comprehensive Pathological Study with Its Radiological and Clinical Follow-Up Correlations. Diagnostics 2022, 12, 450. https://doi.org/10.3390/diagnostics12020450
Idoate MÁ, Aquerreta JD, Lamo-Espinosa JM, San-Julian M. A Reassessment of the Barrier Effect of the Physis against Metaphyseal Osteosarcoma: A Comprehensive Pathological Study with Its Radiological and Clinical Follow-Up Correlations. Diagnostics. 2022; 12(2):450. https://doi.org/10.3390/diagnostics12020450
Chicago/Turabian StyleIdoate, Miguel Á., Jesús Dámaso Aquerreta, José María Lamo-Espinosa, and Mikel San-Julian. 2022. "A Reassessment of the Barrier Effect of the Physis against Metaphyseal Osteosarcoma: A Comprehensive Pathological Study with Its Radiological and Clinical Follow-Up Correlations" Diagnostics 12, no. 2: 450. https://doi.org/10.3390/diagnostics12020450







