Quorum Sensing in Halorubrum saccharovorum Facilitates Cross-Domain Signaling between Archaea and Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioreporter Strains and AHL Standards
2.2. Fermentation and Extraction from Halorubrum saccharovorum CSM52
2.3. Thin Layer Chromatography (TLC) Overlay Assays
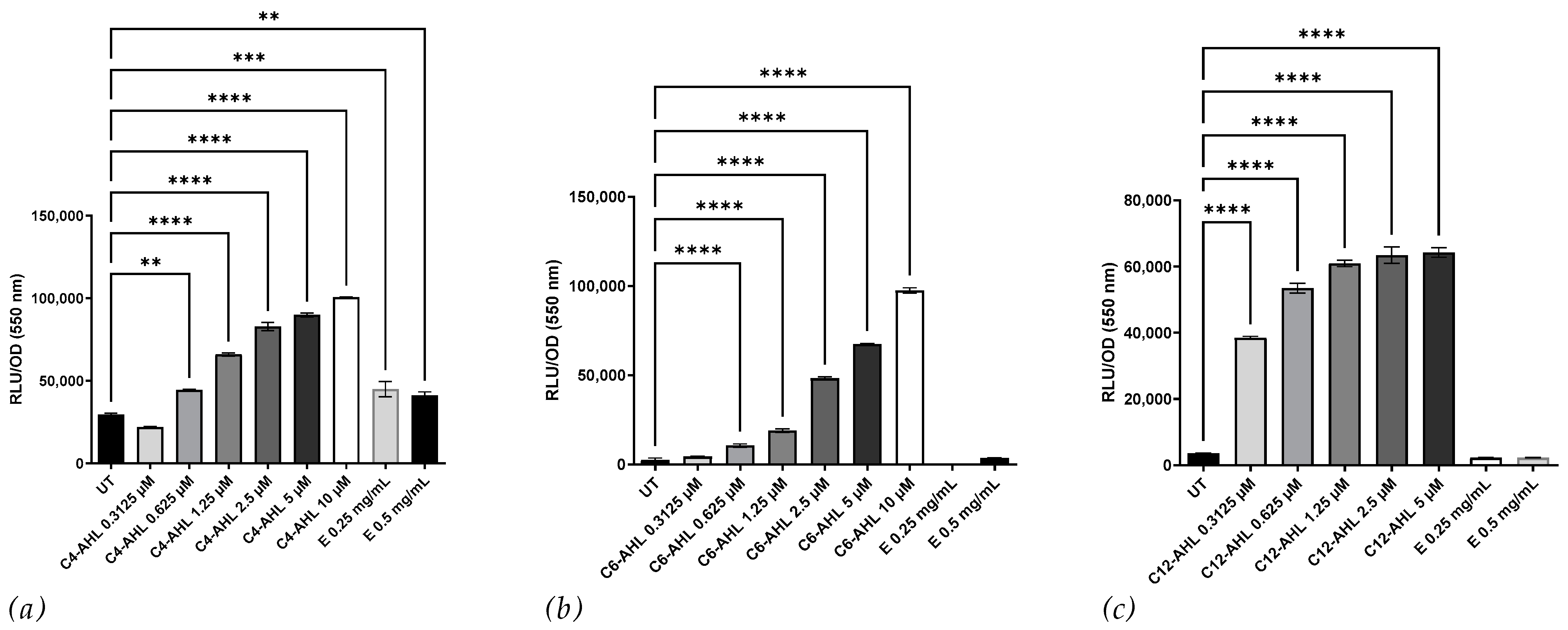
2.4. Bioluminescence Assay
2.5. Lactonolysis of AHLs
2.6. Colorimetric Quantification of Bioactive Compound(s)
2.7. Pyoverdine and Pyocyanin Production
2.8. Influence of the Extract on H. saccharovorum CSM52 Biofilm Biomass Development
2.9. Statistical Analysis
3. Results
3.1. Induction of β-Galactosidase, Violacein Production, and Biolumienescence by the Crude Extract
3.2. Investigating the Nature of the Bioactive Compound
3.3. Elucidating the Biological Function of the Extract from H. saccharovorum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassler, B.L.; Losick, R. Bacterially speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Sharif, D.I.; Gallon, J.; Smith, C.J.; Dudley, E. Quorum sensing in Cyanobacteria: N-octanoyl-homoserine lactone release and response, by the epilithic colonial cyanobacterium Gloeothece PCC6909. ISME J. 2008, 2, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, F.; Ding, G.; Li, J.; Guo, X.; Zhu, J.; Zhou, L.; Cai, S.; Liu, X.; Luo, Y. Acyl homoserine lactone-based quorum sensing in a methanogenic archaeon. ISME J. 2012, 6, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Gatsios, A.; Cuesta, S.; Lam, Y.C.; Wei, Z.; Chen, H.; Russell, R.M.; Shine, E.E.; Wang, R.; Wyche, T.P. Characterization of Autoinducer-3 Structure and Biosynthesis in E. coli. ACS Cent. Sci. 2020, 6, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Diggle, S.P.; Matthijs, S.; Wright, V.J.; Fletcher, M.P.; Chhabra, S.R.; Lamont, I.L.; Kong, X.; Hider, R.C.; Cornelis, P.; Cámara, M. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem. Biol. 2007, 14, 87–96. [Google Scholar] [CrossRef]
- Pesci, E.C.; Milbank, J.B.; Pearson, J.P.; McKnight, S.; Kende, A.S.; Greenberg, E.P.; Iglewski, B.H. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 11229–11234. [Google Scholar] [CrossRef]
- Galloway, W.R.; Hodgkinson, J.T.; Bowden, S.D.; Welch, M.; Spring, D.R. Quorum sensing in Gram-negative bacteria: Small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem. Rev. 2011, 111, 28–67. [Google Scholar] [CrossRef]
- Sitnikov, D.M.; Schineller, J.B.; Baldwin, T.O. Transcriptional regulation of bioluminesence genes from Vibrio fischeri. Mol. Microbiol. 1995, 17, 801–812. [Google Scholar] [CrossRef]
- Soo, P.C.; Horng, Y.T.; Wei, J.R.; Shu, J.C.; Lu, C.C.; Lai, H.C. Regulation of swarming motility and flhDCSm expression by RssAB signaling in Serratia marcescens. J. Bacteriol. 2008, 190, 2496–2504. [Google Scholar] [CrossRef]
- López-Martín, M.; Dubern, J.-F.; Alexander, M.R.; Williams, P. Abam regulates quorum sensing, biofilm formation, and virulence in Acinetobacter baumannii. J. Bacteriol. 2021, 203, e00635-20. [Google Scholar] [CrossRef]
- Smith, R.S.; Iglewski, B.H. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 2003, 6, 56–60. [Google Scholar] [CrossRef]
- Mehmood, A.; Liu, G.; Wang, X.; Meng, G.; Wang, C.; Liu, Y. Fungal quorum-sensing molecules and inhibitors with potential antifungal activity: A review. Molecules 2019, 24, 1950. [Google Scholar] [CrossRef]
- Montgomery, K.; Charlesworth, J.C.; LeBard, R.; Visscher, P.T.; Burns, B.P. Quorum sensing in extreme environments. Life 2013, 3, 131–148. [Google Scholar] [CrossRef]
- Kaur, A.; Capalash, N.; Sharma, P. Quorum sensing in thermophiles: Prevalence of autoinducer-2 system. BMC Microbiol. 2018, 18, 62. [Google Scholar] [CrossRef]
- Rajput, A.; Kumar, M. Computational exploration of putative LuxR solos in Archaea and their functional implications in quorum sensing. Front. Microbiol. 2017, 8, 798. [Google Scholar] [CrossRef]
- Abbamondi, G.R.; Kambourova, M.; Poli, A.; Finore, I.; Nicolaus, B. Quorum Sensing in Extremophiles. In Quorum Sensing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 97–123. [Google Scholar]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W. Quorum sensing and Chromobacterium subtsugae: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef]
- Flynn, P.B.; Busetti, A.; Wielogorska, E.; Chevallier, O.P.; Elliott, C.T.; Laverty, G.; Gorman, S.P.; Graham, W.G.; Gilmore, B.F. Non-thermal plasma exposure rapidly attenuates bacterial AHL-dependent quorum sensing and virulence. Sci. Rep. 2016, 6, 26320. [Google Scholar] [CrossRef]
- Cha, C.; Gao, P.; Chen, Y.-C.; Shaw, P.D.; Farrand, S.K. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol. Plant Microbe Interact. 1998, 11, 1119–1129. [Google Scholar] [CrossRef]
- Yates, E.A.; Philipp, B.; Buckley, C.; Atkinson, S.; Chhabra, S.R.; Sockett, R.E.; Goldner, M.; Dessaux, Y.; Cámara, M.; Smith, H. N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 2002, 70, 5635–5646. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Lee, T.-H.; Kim, J.H.; Kim, E.J.; Joo, H.-S.; Lee, C.-S.; Kim, B.-G. High-throughput detection method of quorum-sensing molecules by colorimetry and its applications. Anal. Biochem. 2006, 356, 297–299. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter dish biofilm formation assay. JoVE (J. Vis. Exp.) 2011, 47, e2437. [Google Scholar]
- Liao, Y.; Williams, T.; Ye, J.; Charlesworth, J.; Burns, B.; Poljak, A.; Raftery, M.; Cavicchioli, R. Morphological and proteomic analysis of biofilms from the Antarctic archaeon, Halorubrum lacusprofundi. Sci. Rep. 2016, 6, 37454. [Google Scholar] [CrossRef]
- Paggi, R.A.; Martone, C.B.; Fuqua, C.; De Castro, R.E. Detection of quorum sensing signals in the haloalkaliphilic archaeon Natronococcus occultus. FEMS Microbiol. Lett. 2003, 221, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Winzer, K.; Falconer, C.; Garber, N.C.; Diggle, S.P.; Camara, M.; Williams, P. The Pseudomonas aeruginosa lectins PA-IL and PA-IIL are controlled by quorum sensing and by RpoS. J. Bacteriol. 2000, 182, 6401–6411. [Google Scholar] [CrossRef]
- Liu, X.; Bimerew, M.; Ma, Y.; Müller, H.; Ovadis, M.; Eberl, L.; Berg, G.; Chernin, L. Quorum-sensing signaling is required for production of the antibiotic pyrrolnitrin in a rhizospheric biocontrol strain of Serratia plymuthica. FEMS Microbiol. Lett. 2007, 270, 299–305. [Google Scholar] [CrossRef]
- Holden, M.T.; Ram Chhabra, S.; De Nys, R.; Stead, P.; Bainton, N.J.; Hill, P.J.; Manefield, M.; Kumar, N.; Labatte, M.; England, D. Quorum-sensing cross talk: Isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol. Microbiol. 1999, 33, 1254–1266. [Google Scholar] [CrossRef]
- Tommonaro, G.; Abbamondi, G.R.; Iodice, C.; Tait, K.; De Rosa, S. Diketopiperazines produced by the halophilic archaeon, Haloterrigena hispanica, activate AHL bioreporters. Microb. Ecol. 2012, 63, 490–495. [Google Scholar] [CrossRef]
- de Carvalho, M.P.; Abraham, W.-R. Antimicrobial and biofilm inhibiting diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef]
- Pikuta, E.V.; Hoover, R.B.; Tang, J. Microbial extremophiles at the limits of life. Crit. Rev. Microbiol. 2007, 33, 183–209. [Google Scholar] [CrossRef]
- Decho, A.W.; Visscher, P.T.; Ferry, J.; Kawaguchi, T.; He, L.; Przekop, K.M.; Norman, R.S.; Reid, R.P. Autoinducers extracted from microbial mats reveal a surprising diversity of N-acylhomoserine lactones (AHLs) and abundance changes that may relate to diel pH. Environ. Microbiol. 2009, 11, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, M.; Lee, K.M.; Greenberg, E. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 13904–13909. [Google Scholar] [CrossRef] [PubMed]
- Toder, D.; Gambello, M.; Iglewski, B. Pseudomonas aeruginosa LasA: A second elastase under the transcriptional control of lasR. Mol. Microbiol. 1991, 5, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Gambello, M.J.; Kaye, S.; Iglewski, B.H. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect. Immun. 1993, 61, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Toder, D.; Ferrell, S.; Nezezon, J.; Rust, L.; Iglewski, B. lasA and lasB genes of Pseudomonas aeruginosa: Analysis of transcription and gene product activity. Infect. Immun. 1994, 62, 1320–1327. [Google Scholar] [CrossRef]
- Seed, P.C.; Passador, L.; Iglewski, B.H. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: An autoinduction regulatory hierarchy. J. Bacteriol. 1995, 177, 654–659. [Google Scholar] [CrossRef]
- Pearson, J.P.; Passador, L.; Iglewski, B.H.; Greenberg, E.P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1995, 92, 1490–1494. [Google Scholar] [CrossRef]
- Winson, M.K.; Camara, M.; Latifi, A.; Foglino, M.; Chhabra, S.R.; Daykin, M.; Bally, M.; Chapon, V.; Salmond, G.; Bycroft, B.W. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1995, 92, 9427–9431. [Google Scholar] [CrossRef]
- Ochsner, U.A.; Koch, A.K.; Fiechter, A.; Reiser, J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J. Bacteriol. 1994, 176, 2044–2054. [Google Scholar] [CrossRef]
- Latifi, A.; Winson, M.K.; Foglino, M.; Bycroft, B.W.; Stewart, G.S.; Lazdunski, A.; Williams, P. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 1995, 17, 333–343. [Google Scholar] [CrossRef]
- Ochsner, U.A.; Reiser, J. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1995, 92, 6424–6428. [Google Scholar] [CrossRef] [PubMed]
- Latifi, A.; Foglino, M.; Tanaka, K.; Williams, P.; Lazdunski, A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 1996, 21, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Pesci, E.C.; Pearson, J.P.; Seed, P.C.; Iglewski, B.H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1997, 179, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.P.; Kelly, S.A.; Skvortsov, T.; Plunkett, G.; Ruffell, A.; Hallsworth, J.E.; Hopps, J.; Gilmore, B.F. Microbiology of a NaCl stalactite ‘salticle’ in Triassic halite. Environ. Microbiol. 2021, 23, 3881–3895. [Google Scholar] [CrossRef]
- Wilmes, P.; Trezzi, J.-P.; Aho, V.; Jäger, C.; Schade, S.; Janzen, A.; Hickl, O.; Kunath, B.; Thomas, M.; Schmit, K.; et al. An archaeal compound as a driver of Parkinson’s disease pathogenesis. Under Rev. Nat. Portf. 2022, in press. [Google Scholar]
- Miller, E.L.; Kjos, M.; Abrudan, M.I.; Roberts, I.S.; Veening, J.-W.; Rozen, D.E. Eavesdropping and crosstalk between secreted quorum sensing peptide signals that regulate bacteriocin production in Streptococcus pneumoniae. ISME J. 2018, 12, 2363–2375. [Google Scholar] [CrossRef]
- Fröls, S. Archaeal biofilms: Widespread and complex. Biochem. Soc. Trans. 2013, 41, 393–398. [Google Scholar] [CrossRef]
- Megaw, J.; Gilmore, B.F. Archaeal persisters: Persister cell formation as a stress response in Haloferax volcanii. Front. Microbiol. 2017, 8, 1589. [Google Scholar] [CrossRef]
- Wu, H.; Song, Z.; Hentzer, M.; Andersen, J.B.; Molin, S.; Givskov, M.; Høiby, N. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J. Antimicrob. Chemother. 2004, 53, 1054–1061. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, T.P.; Busetti, A.; Gilmore, B.F. Quorum Sensing in Halorubrum saccharovorum Facilitates Cross-Domain Signaling between Archaea and Bacteria. Microorganisms 2023, 11, 1271. https://doi.org/10.3390/microorganisms11051271
Thompson TP, Busetti A, Gilmore BF. Quorum Sensing in Halorubrum saccharovorum Facilitates Cross-Domain Signaling between Archaea and Bacteria. Microorganisms. 2023; 11(5):1271. https://doi.org/10.3390/microorganisms11051271
Chicago/Turabian StyleThompson, Thomas P., Alessandro Busetti, and Brendan F. Gilmore. 2023. "Quorum Sensing in Halorubrum saccharovorum Facilitates Cross-Domain Signaling between Archaea and Bacteria" Microorganisms 11, no. 5: 1271. https://doi.org/10.3390/microorganisms11051271








