Excitation and Circular Dichroism Spectra of (+)-(S,S)-bis(2-Methylbutyl)chalcogenides
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Stable conformations for each ground-state molecule
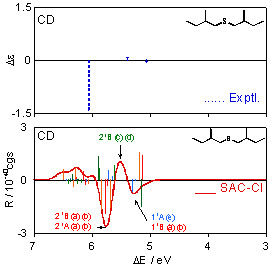
3.2. Excitation and CD spectra of bis(2-methylbutyl)sulfide
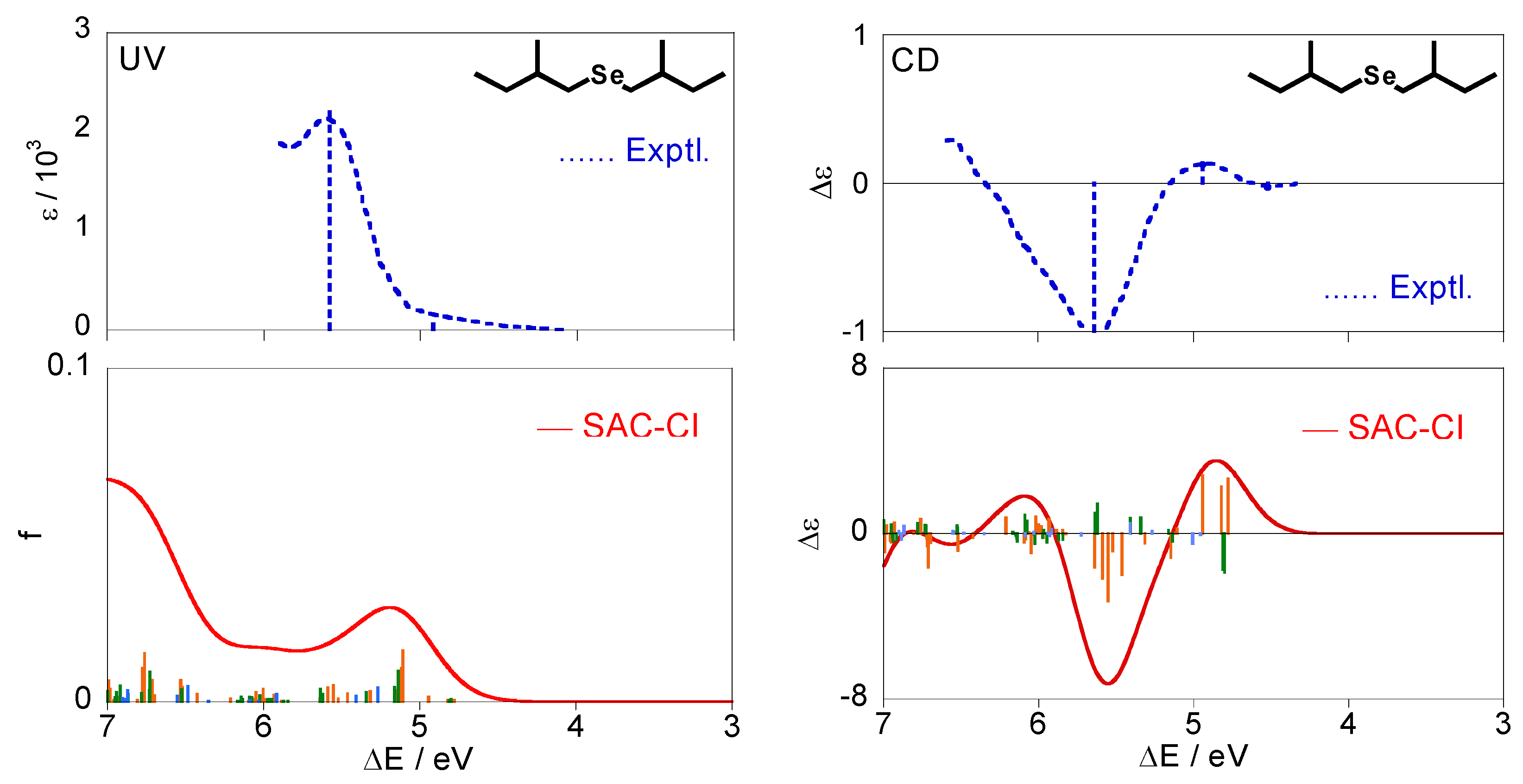
3.3. Excitation and CD spectra of bis(2-methylbutyl)selenide
3.4. Excitation and CD spectra of bis(2-methylbutyl)telluride
4. Conclusions
Acknowledgements
References and Notes
- Berova, N.; Nakanishi, K.; Woody, R.W. Circular Dichroism. Principles and Applications; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Uray, G.; Verdino, P.; Belaj, F.; Kappe, C.O.; Fabian, W.M.F. Absolute configuration in 4-Alkyl- and 4-Aryl-3,4-dihydro-2(1H)-pyrimidones: A combined theoretical and experimental investigation. J. Org. Chem. 2001, 66, 6685–6694. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.B.; Hansen, A.E. Ab initio calculation and display of the rotary strength tensor in the random phase approximation. Method and model studies. Chem. Phys. Lett. 1995, 246, 1–8. [Google Scholar] [CrossRef]
- Hansen, A.E.; Bouman, T.D. Optical activity of monoolefins: RPA calculations and extraction of the mechanisms in Kirkwood’s theory. Application to (–)-trans-cyclooctene and 3(3R)-3-methylcyclopentene. J. Am. Chem. Soc. 1985, 107, 4828–4839. [Google Scholar] [CrossRef]
- Diedrich, C.; Grimme, S. Systematic investigation of modern quantum chemical methods to predict electronic circular dichroism spectra. J. Phys. Chem. A 2003, 107, 2524–2539. [Google Scholar] [CrossRef]
- Guennic, B.L.; Hieringer, W.; Go¨rling, A.; Autschbach, J. Density functional calculation of the electronic circular dichroism spectra of the transition metal complexes [M(phen)3]2+ (M = Fe, Ru, Os). J. Phys. Chem. A 2005, 109, 4836–4846. [Google Scholar] [CrossRef] [PubMed]
- Autschbach, J.; Ziegler, T.; Gisbergen, S.J.A.; Baerends, E.J. Chiroptical properties from time-dependent density functional theory. I. Circular dichroism spectra of organic molecules. J. Chem. Phys. 2002, 116, 6930–6940. [Google Scholar] [CrossRef]
- Autschbach, J.; Jorge, F.E.; Ziegler, T. Density functional calculations on electronic circular dichroism spectra of chiral transition metal complexes. Inorg. Chem. 2003, 42, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Spassova, M.; Asselberghs, I.; Verbiest, T.; Clays, K.; Botek, E.; Champagne, B. Theoretical investigation on bridged triarylamine helicenes: UV/visible and circular dichroism spectra. Chem. Phys. Lett. 2007, 439, 213–218. [Google Scholar] [CrossRef]
- Jansík, B.; Rizzo, A.; Ågren, H.; Champagne, B. Strong two-photon circular dichroism in helicenes: A theoretical investigation. J. Chem. Theory Comp. 2008, 4, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Šebek, J.; Bouř, P. Ab initio Modeling of the Electronic Circular Dichroism Induced in Porphyrin Chromophores. J. Phys. Chem. A 2008, 112, 2920–2929. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Lin, N.; Ruud, K. Ab initio study of the one- and two-photon circular dichroism of R-(+)-3-methyl-cyclopentanone. J. Chem. Phys. 2008, 128, 164312. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Santoro, F.; Rizzo, A.; Luo, Y.; Zhao, X.; Barone, V. Theory for vibrationally resolved two-photon circular dichroism spectra. Application to (R)-(+)-3-methylcyclopentanone. J. Phys. Chem. A 2009, 113, 4198–4207. [Google Scholar] [CrossRef] [PubMed]
- Shustov, G.V.; Kachanov, A.V.; Korneev, V.A.; Kostyanovsky, R.G.; Rauk, A. Chiroptical properties of C2-symmetric N-haloaziridines. Chiral rules for the N-haloaziridine chromophore. J. Am. Chem. Soc. 1993, 115, 10267–10274. [Google Scholar] [CrossRef]
- Shustov, G.V.; Kadorkina, G.K.; Kostyanovsky, R.G.; Rauk, A. Asymmetric nitrogen. 67. Geminal systems. 41. Chiroptical properties of N-chloro and N-bromo derivatives of three-membered nitrogen heterocycles: aziridines and diaziridines. J. Am. Chem. Soc. 1988, 110, 1719–1726. [Google Scholar] [CrossRef]
- Rauk, A. Chiroptical properties of disulfides. Ab initio studies of dihydrogen disulfide and dimethyl disulfide. J. Am. Chem. Soc. 1984, 106, 6517–6524. [Google Scholar] [CrossRef]
- Rauk, A. The optical activity of the three-membered ring: oxiranes, aziridines, diaziridines, and oxaziridines. J. Am. Chem. Soc. 1981, 103, 1023–1030. [Google Scholar] [CrossRef]
- Ha, T.-K.; Cencek, W. Ab initio CI study of the optical rotatory strengths of HSSH. Chem. Phys. Lett. 1991, 182, 519–523. [Google Scholar] [CrossRef]
- Nakatsuji, H.; Hirao, K. Cluster expansion of the wavefunction. Symmetry-adapted-cluster expansion, its variational determination, and extension of open-shell orbital theory. J. Chem. Phys. 1978, 68, 2053–2065. [Google Scholar] [CrossRef]
- Nakatsuji, H. Cluster expansion of the wavefunction. Excited states. Chem. Phys. Lett. 1978, 59, 362–364. [Google Scholar] [CrossRef]
- Nakatsuji, H. Cluster expansion of the wavefunction. Electron correlations in ground and excited states by SAC (symmetry-adapted-cluster) and SAC CI theories. Chem. Phys. Lett. 1979, 67, 329–333. [Google Scholar] [CrossRef]
- Nakatsuji, H. SAC-CI method: Theoretical aspects and some recent topics. In Computational Chemistry, Reviews of Current Trends; Leszczynski, J., Ed.; World Scientific: Singapore, 1997; Volume 2. [Google Scholar]
- Bureekaew, S.; Hasegawa, J.; Nakatsuji, H. Electronic circular dichroism spectrum of uridine studied by the SAC-CI method. Chem. Phys. Lett. 2006, 425, 367–371. [Google Scholar] [CrossRef]
- Seino, J.; Honda, Y.; Hada, M.; Nakatsuji, H. Theoretical Study on Excitation and Circular Dichroism (CD) Spectra of Dichalcogen (S, Se, Te) Compounds Calculated by SAC-CI Method. J. Phys. Chem. A 2006, 110, 10053–10062. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Kurihara, A.; Hada, M.; Nakatsuji, H. Excitation and circular dichroism spectra of (−)-(3aS,7aS)-2-chalcogena-trans-hydrindans (Ch = S, Se, Te): SAC and SAC-CI calculations. J. Comp. Chem. 2008, 29, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Hada, M. Quantum-chemical calculations of natural circular dichroism. Comp. Lett. 2010, in press. [Google Scholar]
- Neubert, L.A.; Carmack, M. Circular dichroism of disulfides with dihedral angles of 0, 30, and 60° in the 400–185nm spectral region. J. Am. Chem. Soc. 1974, 96, 943–945. [Google Scholar] [CrossRef]
- Laur, P.H.A. Chiral selenium and tellurium compounds: Synthesis and properties. In Proceedings of the Third International Symposium on Organic Selenium and Tellurium compounds; Cagniant, D., Kirsch, G., Eds.; Universite de Metz: France, 1979; pp. 219–299. [Google Scholar]
- Laur, P.H.A.; Hauser, H.; Gurst, J.H.; Mislow, K. Optical rotatory dispersion of some cyclic sulfides. J. Org. Chem. 1967, 32, 498–500, and ibid. 3725 (Erratum). [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar]
- Huzinaga, S.; Andzelm, J.; Klobukowski, M.; Radzio-Andzelm, E.; Sakai, Y.; Tatewaki, H. Gaussian Basis Set for Molecular Calculations; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis functions for use in molecular calculations. I. Contraction of (9s5p) atomic basis sets for the first-row atoms. J. Chem. Phys. 1970, 53, 2823–2833. [Google Scholar] [CrossRef]
- Nakatsuji, H. Cluster expansion of the wavefunction, valence and Rydberg excitations, ionizations, and inner-valence ionizations of CO2 and N2O studied by the SAC and SAC CI theories. Chem. Phys. 1983, 75, 425–441. [Google Scholar] [CrossRef]
- Nakatsuji, H.; Hasegawa, J.; Hada, M. Excited and ionized states of free base porphin studied by the symmetry adapted cluster-configuration interaction (SAC-CI) method. J. Chem. Phys. 1996, 104, 2321–2329. [Google Scholar] [CrossRef]
- Tokita, Y.; Hasegawa, J.; Nakatsuji, H. SAC-CI study on the excited and ionized states of free-base porphin: Rydberg excited states and effect of polarization and Rydberg functions. J. Phys. Chem. A 1998, 102, 1843–1849. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; Millam, J.M.; Iyengar, S.S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G.A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J.E.; Hratchian, H.P.; Cross, J.B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A.J.; Cammi, R.; Pomelli, C.; Ochterski, J.W.; Ayala, P.Y.; Morokuma, K.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Zakrzewski, V.G.; Dapprich, S.; Daniels, A.D.; Strain, M.C.; Farkas, O.; Malick, D.K.; Rabuck, A.D.; Raghavachari, K.; Foresman, J.B.; Ortiz, J.V.; Cui, Q.; Baboul, A.G.; Clifford, S.; Cioslowski, J.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R.L.; Fox, D.J.; Keith, T.; Al-Laham, M.A.; Peng, C.Y.; Nanayakkara, A.; Challacombe, M.; Gill, P.M.W.; Johnson, B.; Chen, W.; Wong, M.W.; Gonzalez, C.; Pople, J.A. Gaussian 03, Revision C.2; Gaussian Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]

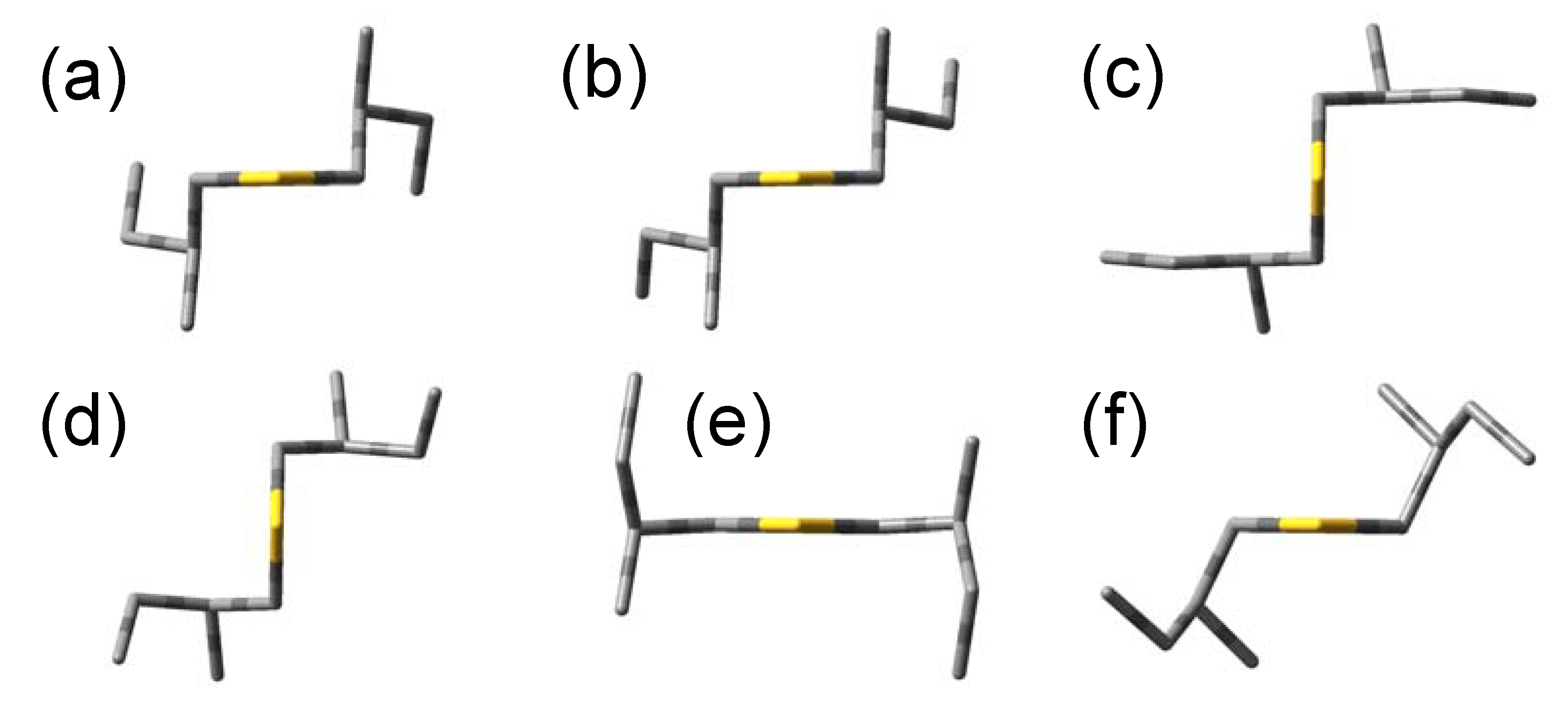




| Initial angles [deg] | Optimized angles [deg] | Relative energy [kcal/mol] | Boltzmann weight (at 298K) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ϕ1 | ϕ2 | ϕ3 | ϕ1 | ϕ2 | ϕ3 | |||||
| 60 | 60 | 60 | 98.7 | 58.4 | 55.6 | 0.727 | 0.057 | (f) | ||
| 60 | 60 | 180 | 98.5 | 63.2 | 172.9 | 1.684 | 0.011 | |||
| 60 | 60 | −60 | 98.0 | 77.7 | −66.9 | 6.347 | 0.000 | |||
| 60 | 180 | 60 | 57.9 | −174.6 | 66.8 | 0.000 | 0.195 | (a) | ||
| 60 | 180 | 180 | 57.3 | −175.6 | 175.0 | 0.052 | 0.179 | (b) | ||
| 60 | 180 | −60 | 56.9 | −172.0 | -62.1 | 1.171 | 0.027 | |||
| 60 | −60 | 60 | 94.0 | −67.1 | 87.2 | 6.536 | 0.000 | |||
| 60 | −60 | 180 | 98.7 | −62.8 | 176.3 | 1.862 | 0.008 | |||
| 60 | −60 | −60 | 98.1 | −67.5 | −69.9 | 2.836 | 0.002 | |||
| 180 | 60 | 60 | 176.7 | 57.4 | 53.9 | 0.224 | 0.134 | (e) | ||
| 180 | 60 | 180 | 178.5 | 63.6 | 171.1 | 1.877 | 0.008 | |||
| 180 | 60 | −60 | 171.9 | 71.3 | −77.8 | 5.661 | 0.000 | |||
| 180 | 180 | 60 | 172.7 | −177.3 | 61.8 | 1.849 | 0.009 | |||
| 180 | 180 | 180 | 177.1 | −171.6 | 175.7 | 1.786 | 0.010 | |||
| 180 | 180 | −60 | 180.0 | −167.1 | −61.0 | 2.584 | 0.002 | |||
| 180 | −60 | 60 | −171.5 | −84.9 | 62.6 | 5.047 | 0.000 | |||
| 180 | −60 | 180 | −174.7 | −63.2 | 176.5 | 1.805 | 0.009 | |||
| 180 | −60 | −60 | −174.6 | −56.1 | −54.9 | 1.341 | 0.020 | |||
| −60 | 60 | 60 | −69.6 | 71.6 | 54.7 | 24.901 | 0.000 | |||
| −60 | 60 | 180 | −98.2 | 64.5 | 174.0 | 1.617 | 0.013 | |||
| −60 | 60 | −60 | −83.6 | 103.1 | −71.0 | 13.944 | 0.000 | |||
| −60 | 180 | 60 | −97.3 | −175.2 | 62.5 | 2.324 | 0.004 | |||
| −60 | 180 | 180 | −98.6 | −172.9 | 174.5 | 2.018 | 0.006 | |||
| −60 | 180 | −60 | −98.0 | −169.1 | −63.6 | 2.913 | 0.001 | |||
| −60 | −60 | 60 | −53.2 | −74.3 | 72.0 | 4.837 | 0.000 | |||
| −60 | −60 | 180 | −57.0 | −59.8 | 174.2 | 0.136 | 0.155 | (c) | ||
| −60 | −60 | −60 | −58.0 | −52.9 | −56.7 | 0.162 | 0.149 | (d) | ||
| Compound | Initial angles [deg] | Optimized angles [deg] | Relative energy [kcal/mol] | Boltzmann weight (at 298K) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ϕ1 | ϕ2 | ϕ3 | ϕ1 | ϕ2 | ϕ3 | |||||
| Ch = S | 60 | 180 | 60 | 57.9 | −174.6 | 66.8 | 0.000 | 0.195 | (a) | |
| 60 | 180 | 180 | 57.3 | −175.6 | 175.0 | 0.052 | 0.179 | (b) | ||
| −60 | −60 | 180 | −57.0 | −59.8 | 174.2 | 0.136 | 0.155 | (c) | ||
| −60 | −60 | −60 | −58.0 | −52.9 | −56.7 | 0.162 | 0.149 | (d) | ||
| 180 | 60 | 60 | 176.7 | 57.4 | 53.9 | 0.224 | 0.134 | (e) | ||
| 60 | 60 | 60 | 98.7 | 58.4 | 55.6 | 0.727 | 0.057 | (f) | ||
| Ch = Se | 60 | 180 | 60 | 57.4 | −172.5 | 66.8 | 0.000 | 0.233 | (a) | |
| 60 | 180 | 180 | 56.3 | −172.4 | 175.5 | 0.160 | 0.178 | (b) | ||
| −60 | −60 | 180 | −56.1 | −63.1 | 173.9 | 0.310 | 0.138 | (c) | ||
| −60 | −60 | −60 | −56.8 | −54.9 | −56.3 | 0.427 | 0.113 | (d) | ||
| 60 | 60 | 60 | 97.7 | 58.4 | 54.8 | 0.499 | 0.100 | (f) | ||
| 180 | 60 | 60 | 174.1 | 56.8 | 54.1 | 0.671 | 0.075 | (e) | ||
| Ch = Te | 60 | 60 | 60 | 95.9 | 58.8 | 54.3 | 0.000 | 0.232 | (f) | |
| 60 | 180 | 60 | 56.3 | −170.3 | 66.2 | 0.106 | 0.194 | (a) | ||
| 60 | 180 | 180 | 54.6 | −167.8 | 175.9 | 0.339 | 0.131 | (b) | ||
| −60 | −60 | 180 | −54.8 | −67.9 | 173.5 | 0.542 | 0.093 | (c) | ||
| −60 | −60 | −60 | −55.2 | −58.0 | −55.8 | 0.773 | 0.063 | (d) | ||
| 180 | 60 | 60 | 167.4 | 56.1 | 54.9 | 0.837 | 0.056 | (e) | ||
| SAC-CI | Experiments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Statea | Main configurations (|c|>0.4) | Nature | ΔE | f | R | −e<r2> | ΔE(ε)b | ΔE(Δε) | |
| 11A (a) | 0.57(44-46)-0.52(44-57)-0.47(44-80) | n-σ* | 5.14 | 0.0006 | 7.04 | −219.21 | |||
| 11A (d) | 0.60(44-46)-0.40(44-55)-0.40(44-81) | n-σ* | 5.15 | 0.0006 | −8.11 | −217.48 | 5.06(−0.05) | ||
| 11A (e) | 0.60(44-45)-0.40(44-54) | n-σ* | 5.15 | 0.0013 | −10.33 | −216.65 | |||
| 11A (b) | 0.59(44-46)-0.51(44-57)-0.43(44-79) | n-σ* | 5.18 | 0.0007 | 8.81 | −220.17 | |||
| 11A (f) | 0.64(44-45)-0.45(44-55) | n-σ* | 5.21 | 0.0042 | 32.65 | −218.11 | |||
| 11B (c) | 0.78(44-45) | n-Ryd(pz) | 5.26 | 0.0001 | −0.37 | −237.69 | |||
| 11B (a) | 0.78(44-45) | n-Ryd(pz) | 5.30 | 0.0083 | −3.39 | −261.84 | 5.34(170) | ||
| 11A (c) | 0.47(44-77) | n-σ* | 5.30 | 0.0002 | −5.08 | −200.37 | |||
| 11B (b) | 0.73(44-45) | n-Ryd(pz) | 5.31 | 0.0089 | −4.78 | −257.90 | |||
| 11B (f) | 0.69(44-47)+0.47(44-53) | n-Ryd(pz) | 5.32 | 0.0061 | −23.36 | −242.12 | |||
| 11B (d) | 0.74(44-45) | n-Ryd(pz) | 5.33 | 0.0097 | 2.62 | −256.91 | |||
| 11B (e) | 0.59(44-47)-0.54(44-46) | n-Ryd(pz) | 5.35 | 0.0050 | 5.55 | −247.79 | |||
| 21B (f) | 0.79(44-46) | n-Ryd(s) | 5.50 | 0.0078 | −7.24 | −252.23 | |||
| 21B (e) | 0.61(44-46)+0.53(44-47)-0.42(44-56) | n-Ryd(s) | 5.53 | 0.0094 | 6.37 | −246.23 | 5.39(+0.06) | ||
| 21B (d) | 0.64(44-48) | n-Ryd(s) | 5.62 | 0.0047 | 5.33 | −241.83 | |||
| 21B (c) | 0.71(44-47) | n-Ryd(s) | 5.65 | 0.0126 | 1.58 | −242.14 | |||
| 21B (b) | 0.62(44-48)-0.40(44-53)-0.39(44-45) | n-Ryd(s) | 5.68 | 0.0056 | −8.65 | −245.20 | |||
| 21A (c) | 0.79(44-46) | n-Ryd(pxy) | 5.72 | 0.0090 | 3.98 | −258.59 | |||
| 21B (a) | 0.60(44-48)-0.51(44-53) | n-Ryd(s) | 5.74 | 0.0031 | −1.62 | −253.72 | |||
| 21A (a) | 0.78(44-47) | n-Ryd(pxy) | 5.76 | 0.0050 | −9.85 | −270.95 | 6.26(1850) | 6.05(−1.40) | |
| 21A (b) | 0.78(44-47) | n-Ryd(pxy) | 5.84 | 0.0064 | −9.29 | −273.38 | |||
| 21A (d) | 0.81(44-47) | n-Ryd(pxy) | 5.86 | 0.0076 | 4.08 | −275.85 | |||
| 21A (f) | 0.49(44-48)-0.49(44-45) | n-Ryd(pxy) | 5.87 | 0.0010 | −12.30 | −253.23 | |||
| 21A (e) | 0.70(44-48)+0.47(44-45) | n-Ryd(pxy) | 5.89 | 0.0055 | 9.08 | −271.98 | |||
| SAC-CI | Experiments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| State a | Main configurations (|c|>0.4) | Nature | ΔE | f | R | −e<r2> | ΔE(ε) b | ΔE(Δε) | |
| 11A (a) | 0.57(44-54)+0.55(44-72)-0.41(44-45) | n-σ* | 4.78 | 0.0009 | 28.48 | −215.39 | 4.92(140) | 4.94(+0.13) | |
| 11A (d) | 0.57(44-54)-0.46(44-45)+0.42(44-73) | n-σ* | 4.80 | 0.0025 | −41.14 | −213.95 | |||
| 11A (c) | 0.58(44-54)-0.51(44-73)-0.46(44-45) | n-σ* | 4.81 | 0.0011 | −31.28 | −215.51 | |||
| 11A (b) | 0.58(44-54)+0.50(44-73)-0.44(44-45) | n-σ* | 4.82 | 0.0012 | 32.16 | −217.17 | |||
| 11A (f) | 0.51(44-55)-0.48(44-45)+0.40(44-73) | n-σ* | 4.94 | 0.0073 | 70.37 | −213.65 | |||
| 11B (e) | 0.72(44-45) | n-Ryd(pz) | 4.96 | 0.0006 | −1.40 | −245.28 | |||
| 11A (e) | 0.56(44-74) | n-σ* | 5.01 | 0.0002 | −15.80 | −203.06 | |||
| 11B (a) | 0.69(44-46) | n-Ryd(pz) | 5.11 | 0.0328 | 2.49 | −264.46 | 5.58(2250) | ||
| 11B (b) | 0.65(44-46)+0.43(44-53) | n-Ryd(pz) | 5.12 | 0.0281 | 0.28 | −263.14 | |||
| 11B (c) | 0.64(44-46) | n-Ryd(pz) | 5.14 | 0.0334 | −6.81 | −260.92 | |||
| 11B (f) | 0.61(44-47)-0.49(44-53) | n-Ryd(s) | 5.15 | 0.0172 | −28.69 | −252.52 | |||
| 11B (d) | 0.63(44-47)+0.40(44-53) | n-Ryd(pz) | 5.16 | 0.0195 | 3.71 | −256.16 | |||
| 21B (e) | 0.58(44-48)+0.43(44-54) | n-Ryd(s) | 5.27 | 0.0280 | 4.47 | −243.12 | |||
| 21B (f) | 0.71(44-46)-0.51(44-54) | n-Ryd(pz) | 5.32 | 0.0145 | −11.57 | −255.00 | |||
| 21B (d) | 0.61(44-46)-0.50(44-55) | n-Ryd(s) | 5.34 | 0.0124 | 17.07 | −253.21 | |||
| 21B (c) | 0.56(44-48)+0.42(44-53)+0.41(44-55) | n-Ryd(s) | 5.41 | 0.0042 | 13.46 | −252.77 | |||
| 21A (e) | 0.64(44-46)-0.49(44-56)+0.44(44-47) | n-Ryd(pxy) | 5.41 | 0.0107 | 16.90 | −266.72 | |||
| 21B (b) | 0.52(44-48)-0.48(44-55)-0.45(44-53) | n-Ryd(s) | 5.46 | 0.0068 | −27.91 | −255.66 | 5.64(−1.1) | ||
| 21B (a) | 0.55(44-53)+0.48(44-48)+0.42(44-55) | n-Ryd(s) | 5.52 | 0.0016 | −9.03 | −261.24 | |||
| 21A (a) | 0.67(44-47)+0.44(44-45)-0.40(44-56) | n-Ryd(pxy) | 5.55 | 0.0107 | −34.88 | −279.68 | |||
| 21A (b) | 0.66(44-47)+0.46(44-45) | n-Ryd(pxy) | 5.59 | 0.0117 | −30.13 | −280.76 | |||
| 21A (d) | 0.57(44-48)-0.53(44-45) | n-Ryd(pxy) | 5.62 | 0.0096 | 31.61 | −283.49 | |||
| 21A (c) | 0.72(44-47)+0.41(44-56) | n-Ryd(pxy) | 5.64 | 0.0140 | 17.82 | −283.72 | |||
| 21A (f) | 0.66(44-45) | n-Ryd(pxy) | 5.64 | 0.0040 | −40.63 | −261.18 | |||
| SAC-CI | Experiments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Statea | Main configurations (|c|>0.4) | Nature | ΔE | f | R | −e<r2> | ΔE(ε)b | ΔE(Δε) | |
| 11A (d) | 0.55(44-66)-0.52(44-54) | n-σ* | 3.73 | 0.0006 | −41.43 | −209.26 | 4.31(160) | ||
| 11A (a) | 0.56(44-67)+0.53(44-54) | n-σ* | 3.75 | 0.0002 | 20.45 | −211.68 | |||
| 11A (c) | 0.64(44-67)-0.53(44-54) | n-σ* | 3.75 | 0.0003 | −21.90 | −210.83 | |||
| 11A (b) | 0.69(44-67)+0.53(44-54) | n-σ* | 3.76 | 0.0002 | 17.56 | −211.81 | |||
| 11A (e) | 0.74(44-67) | n-σ* | 3.89 | 0.0000 | 8.91 | −201.61 | |||
| 11A (f) | 0.67(44-66) | n-σ* | 3.92 | 0.0025 | 70.25 | −205.00 | 3.97(+0.13) | ||
| 11B (e) | 0.61(44-45)-0.53(44-54) | n-Ryd(s) | 4.56 | 0.0058 | −6.85 | −248.11 | |||
| 11B (a) | 0.60(44-46)-0.48(44-55) | n-Ryd(pz) | 4.70 | 0.0608 | 18.29 | −270.91 | 5.21(4600) | ||
| 21B (e) | 0.51(44-48)-0.40(44-62) | n-Ryd(pz) | 4.71 | 0.0467 | 3.96 | −249.24 | |||
| 11B (d) | 0.51(44-47)-0.49(44-55) | n-Ryd(pz) | 4.71 | 0.0537 | −13.81 | −262.71 | |||
| 11B (c) | 0.57(44-46)-0.50(44-55) | n-Ryd(pz) | 4.71 | 0.0645 | −22.54 | −266.70 | |||
| 11B (b) | 0.61(44-46)-0.43(44-55) | n-Ryd(pz) | 4.72 | 0.0629 | 23.57 | −271.86 | |||
| 11B (f) | 0.59(44-47)+0.43(44-55) | n-Ryd(s) | 4.74 | 0.0451 | −1.62 | −253.90 | |||
| 21B (d) | 0.52(44-47) | n-Ryd(s) | 4.85 | 0.0056 | 33.54 | −257.85 | |||
| 21B (f) | 0.65(44-45)-0.44(44-55) | n-Ryd(pz) | 4.87 | 0.0063 | −47.96 | −264.49 | 5.19(−0.48) | ||
| 21B (c) | 0.57(44-47) | n-Ryd(s) | 4.90 | 0.0099 | 15.20 | −253.02 | |||
| 21B (a) | 0.51(44-48)-0.40(44-71)-0.39(44-55) | n-Ryd(s) | 4.94 | 0.0116 | −15.70 | −253.73 | |||
| 21B (b) | 0.51(44-48)-0.45(44-55)-0.42(44-70) | n-Ryd(s) | 4.95 | 0.0075 | −31.50 | −249.63 | |||
| 21A (e) | 0.53(44-46)+0.52(44-55)-0.45(44-47) | n-Ryd(pxy) | 5.05 | 0.0103 | 6.78 | −282.66 | |||
| 21A (f) | 0.69(44-46)-0.44(44-54) | n-Ryd(pxy) | 5.12 | 0.0144 | −44.02 | −285.02 | |||
| 21A (a) | 0.51(44-45)-0.51(44-47)+0.46(44-56) | n-Ryd(pxy) | 5.16 | 0.0138 | −28.89 | −288.98 | |||
| 21A (d) | 0.65(44-45) | n-Ryd(pxy) | 5.16 | 0.0145 | 30.47 | −290.54 | |||
| 21A (b) | 0.58(44-45)+0.47(44-56)-0.42(44-47) | n-Ryd(pxy) | 5.17 | 0.0173 | −22.83 | −285.36 | |||
| 21A (c) | 0.54(44-45)+0.47(44-48)-0.44(44-56) | n-Ryd(pxy) | 5.19 | 0.0160 | 12.21 | −287.75 | |||
© 2010 by the authors;
Share and Cite
Honda, Y.; Kurihara, A.; Kenmochi, Y.; Hada, M. Excitation and Circular Dichroism Spectra of (+)-(S,S)-bis(2-Methylbutyl)chalcogenides. Molecules 2010, 15, 2357-2373. https://doi.org/10.3390/molecules15042357
Honda Y, Kurihara A, Kenmochi Y, Hada M. Excitation and Circular Dichroism Spectra of (+)-(S,S)-bis(2-Methylbutyl)chalcogenides. Molecules. 2010; 15(4):2357-2373. https://doi.org/10.3390/molecules15042357
Chicago/Turabian StyleHonda, Yasushi, Atsushi Kurihara, Yusuke Kenmochi, and Masahiko Hada. 2010. "Excitation and Circular Dichroism Spectra of (+)-(S,S)-bis(2-Methylbutyl)chalcogenides" Molecules 15, no. 4: 2357-2373. https://doi.org/10.3390/molecules15042357