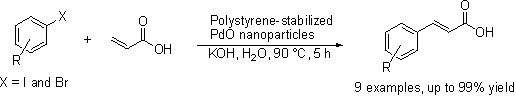Linear Polystyrene-Stabilized PdO Nanoparticle-Catalyzed Mizoroki-Heck Reactions in Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of PS-PdONPs

2.2. Coupling Conditions

| Entry | Base | Yield (%) a |
|---|---|---|
| 1 | KOH | 99 (32) b |
| 2 c | 99 (16) b | |
| 3 d | 44 | |
| 4 | K2CO3 | 27 |
| 5 | Cs2CO3 | 19 |
| 6 | CH3COONa | 20 |
| 7 | DBU | 21 |
| 8 | NEt3 | 96 |

2.3. Substrate Tolerance
| Entry | Aryl iodides | Alkenes | Yield (%) a |
|---|---|---|---|
| 1 |  |  | 99 (13) b,c |
| 2 |  |  | 99 |
| 3 |  |  | 96 |
| 4 |  |  | 99 (99) b,c |
| 5 |  |  | 92 |
| 6 |  |  | 99 |
| 7 |  |  | 99 |
| 8 |  |  | 98 |
| 9 |  |  | 59 c |
| 10 |  |  | 14 c |
2.4. Recycling Experiments


3. Experimental
3.1. General
3.2. Preparation of PS-PdONPs
3.3. Determination of the Amount of Palladium
3.4. Typical Procedures for Mizoroki-Heck Reaction
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Heck, R.F. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979, 12, 146–151. [Google Scholar] [CrossRef]
- Dounay, A.B.; Overman, L.E. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem. Rev. 2003, 103, 2945–2964. [Google Scholar] [CrossRef]
- Astruc, D. Palladium nanoparticles as efficient green homogeneous and heterogeneous carbon-carbon coupling precatalysts. Inorg. Chem. 2007, 46, 1884–1894. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneouos catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Moreno-Mañas, M.; Pleixats, R. Formation of carbon-carbon bonds under catalysis by transition-metal nanoparticles. Acc. Chem. Res. 2003, 36, 638–643. [Google Scholar] [CrossRef]
- Tamami, B.; Allahyari, H.; Ghasemi, S.; Farjadian, F. Palladium nanoparticles supported on poly(N-vinylpyrrolidone)-grafted silica as new recyclable catalyst for Heck cross-coupling reactions. J. Organomet. Chem. 2011, 696, 594–599. [Google Scholar] [CrossRef]
- Ungureanu, S.; Deleuze, H.; Babot, O.; Achard, M.-F.; Sanchez, C.; Popa, M.I.; Backov, R. Palladium nanoparticles heterogeneous nucleation within organically grafted silica foams and their use as catalyst supports toward the Suzuki-Miyaura and Mizoroki-Heck coupling reactions. Appl. Catal. A: Gen. 2010, 390, 51–58. [Google Scholar] [CrossRef]
- Mieczyńska, E.; Gniewek, A.; Pryjomska-Ray, I.; Trzeciak, A.M.; Grabowska, H.; Zawadzki, M. The Heck arylation of mono- and disubstituted olefins catalyzed by palladium supported on alumina-based oxides. Appl. Catal. A: Gen. 2011, 393, 195–205. [Google Scholar] [CrossRef]
- Wang, P.; Lu, Q.; Li, J. In situ formation of palladium nanoparticles inside the pore channels of ordered mesoporous silica. Catal. Lett. 2009, 131, 444–450. [Google Scholar] [CrossRef]
- Gao, Z.; Feng, Y.; Cui, F.; Hua, Z.; Zhou, J.; Shi, J. Pd-loaded superparamagnetic mesoporous NiFe2O4 as a highly active and magnetically separable catalyst for Suzuki and Heck reactions. J. Mol. Catal. A: Chem. 2011, 336, 51–57. [Google Scholar] [CrossRef]
- Makhubela, B.C.E.; Jardine, A.; Smith, G.S. Pd nanosized particles supported on chitosan and 6-deoxy-6-amino chitosan as recyclable catalysts for Suzuki-Miyaura and Heck cross-coupling reactions. Appl. Catal. A: Gen. 2011, 393, 231–241. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, H.; Hu, J.; Liu, M.; Kuang, Y. Pd nanoparticles catalyzed ligand-free Heck reaction in ionic liquid microemulsion. Green Chem. 2009, 11, 1428–1432. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Ghaderi, A.; Ghavami, M.; Hoseini, S.J. Palladium nanoparticles supported on aminopropyl-functionalized clay as efficient catalysts for phosphine-free C-C bond formation via Mizoroki-Heck and Suzuki-Miyaura reactions. Bull. Chem. Soc. Jpn. 2011, 84, 100–109. [Google Scholar] [CrossRef]
- Lamblin, M.; Nassar-Hardy, L.; Hierso, J.-C.; Fouquet, E.; Felpin, F.-X. Recyclable heterogeneous palladium catalysts in pure water: Sustainable developments in Suzuki, Heck, Sonogashira and Tsuji-Trost reactions. Adv. Synth. Catal. 2010, 352, 33–79. [Google Scholar] [CrossRef]
- Yan, N.; Xiao, C.; Kou, Y. Transition metal nanoparticle catalysis in green solvents. Coord. Chem. Rev. 2010, 254, 1179–1218. [Google Scholar] [CrossRef]
- Grieco, P.A. Organic Synthesis in Water; Blackie Academic & Professional: London, UK, 1998. [Google Scholar]
- Leadbeater, N.E. Fast, easy, clean chemistry by using water as a solvent and microwave heating: The Suzuki coupling as an illustration. Chem. Commun. 2005, 2881–2902. [Google Scholar] [CrossRef]
- Li, C.J. Organic reactions in aqueous media with a focus on carbon-carbon bond formations: A decade update. Chem. Rev. 2005, 105, 3095–3165. [Google Scholar] [CrossRef]
- Sawoo, S.; Srimani, D.; Dutta, P.; Lahiri, R.; Sarkar, A. Size controlled synthesis of Pd nanoparticles in water and their catalytic application in C-C coupling reactions. Tetrahedron 2009, 65, 4367–4374. [Google Scholar] [CrossRef]
- Senra, J.D.; Malta, L.F.B.; da Costa, M.E.H.M.; Michel, R.C.; Aguiar, L.C.S.; Simas, A.B.C.; Antunes, O.A.C. Hydroxypropyl-α-cyclodextrin-capped palladium nanoparticles: Active scaffolds for efficient carbon-carbon bond forming cross-coupling in water. Adv. Synth. Catal. 2009, 351, 2411–2422. [Google Scholar] [CrossRef]
- Zheng, P.; Zhang, W. Synthesis of efficient and reusable palladium catalyst supported on pH-responsive colloid and its application to Suzuki and Heck reactions in water. J. Catal. 2007, 250, 324–330. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Srivastava, A.; Sengupta, S. Remarkably facile Heck and Suzuki reactions in water using a simple cationic surfactant and ligand-free palladium catalysts. Tetrahedron Lett. 2005, 46, 3557–3560. [Google Scholar] [CrossRef]
- Qiao, K.; Sugimura, R.; Bao, Q.; Tomida, D.; Yokoyama, C. An efficient Heck reaction in water catalyzed by palladium nanoparticles immobilized on imidazolium-styrene copolymers. Catal. Commun. 2008, 9, 2470–2474. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, W.; Zhang, M. Pd-catalyzed C-C cross-coupling reactions within a thermoresponsive and pH-responsive and chelating polymeric hydrogel. J. Org. Chem. 2009, 74, 1923–1931. [Google Scholar] [CrossRef]
- Ohtaka, A.; Tamaki, Y.; Igawa, Y.; Egami, K.; Shimomura, O.; Nomura, R. Polyion complex stabilized palladium nanoparticles for Suzuki and Heck reaction in water. Tetrahedron 2010, 66, 5642–5646. [Google Scholar] [CrossRef]
- Boffi, A.; Cacchi, S.; Ceci, P.; Cirilli, R.; Fabrizi, G.; Prastaro, A.; Niembro, S.; Shafir, A.; Vallribera, A. The Heck reaction of allylic alcohols catalyzed by palladium nanoparticles in water: Chemoenzymatic synthesis of (R)-(−)-rhododendrol. ChemCatChem 2011, 3, 347–353. [Google Scholar] [CrossRef]
- Ohtaka, A.; Teratani, T.; Fujii, R.; Ikeshita, K.; Kawashima, T.; Tatsumi, K.; Shimomura, O.; Nomura, R. Linear polystyrene-stabilized palladium nanoparticles catalyzed C-C coupling reaction in water. J. Org. Chem. 2011, 76, 4052–4060. [Google Scholar] [CrossRef]
- Teratani, T.; Ohtaka, A.; Kawashima, T.; Shimomura, O.; Nomura, R. Copper-free Sonogashira coupling in water with linear polystyrene-stabilized PdO nanoparticles. Synlett 2010, 2271–2274. [Google Scholar]
- Ohtaka, A.; Teratani, T.; Fujii, R.; Ikeshita, K.; Shimomura, O.; Nomura, R. Facile preparation of linear polystyrene-stabilized Pd nanoparticles in water. Chem. Commun. 2009, 7188–7190. [Google Scholar]
- Liu, C.; Yang, W. A fast and oxygen-promoted protocol for the ligand-free Suzuki reaction of 2-halogenated pyridines in aqueous media. Chem. Commun. 2009, 6267–6269. [Google Scholar]
- Sample Availability: Samples of the compounds PS-PdONPs and PS-PdNPs are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ohtaka, A.; Yamaguchi, T.; Teratani, T.; Shimomura, O.; Nomura, R. Linear Polystyrene-Stabilized PdO Nanoparticle-Catalyzed Mizoroki-Heck Reactions in Water. Molecules 2011, 16, 9067-9076. https://doi.org/10.3390/molecules16119067
Ohtaka A, Yamaguchi T, Teratani T, Shimomura O, Nomura R. Linear Polystyrene-Stabilized PdO Nanoparticle-Catalyzed Mizoroki-Heck Reactions in Water. Molecules. 2011; 16(11):9067-9076. https://doi.org/10.3390/molecules16119067
Chicago/Turabian StyleOhtaka, Atsushi, Tomohiro Yamaguchi, Takuto Teratani, Osamu Shimomura, and Ryôki Nomura. 2011. "Linear Polystyrene-Stabilized PdO Nanoparticle-Catalyzed Mizoroki-Heck Reactions in Water" Molecules 16, no. 11: 9067-9076. https://doi.org/10.3390/molecules16119067