A New Iridoid Glycoside from the Roots of Dipsacus asper
Abstract
:1. Introduction

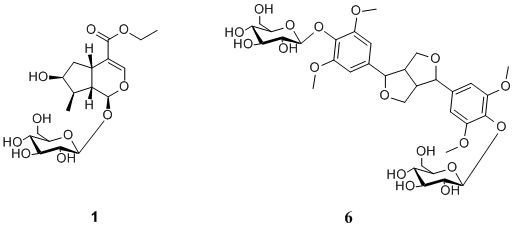
2. Results and Discussion

| Compound | Inhibition ratio (%) a | ||
|---|---|---|---|
| 10 μM | 30 μM | 100 μM | |
| 1 | 27.66 ± 1.72 b | 18.59 ± 1.10 b | 14.17 ± 0.38 b |
| 4 | 26.40 ± 2.05 b | 19.17 ± 1.34 b | 15.98 ± 2.11 b |
| 5 | 23.17 ± 1.87 b | 17.24 ± 0.87 b | 12.02 ± 1.46 b |
| 6 | 38.73 ± 2.01 | 35.24 ± 2.33 | 36.59 ± 2.33 |
| salvianolic acid B c | 18.28 ± 1.02 b | 7.28 ± 0.87 b | 4.28 ± 0.58 b |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
 −86.3(c 0.082, MeOH); UV (MeOH) λmax (log ε) 198 (4.07), 235 (4.36) nm; IR (KBr) γmax 3420, 2933, 1688, 1634, 1399, 1288, 1076 cm−1; 1H-NMR (CD3OD) δ 5.27 (1H, d, J = 4.5 Hz, H-1), 7.38 (1H, s, H-3), 3.11 (1H, q, J = 8.0 Hz, H-5), 1.62 (1H, ddd, J = 5.0, 8.0, 14.0 Hz, H-6a), 2.24 (1H, ddd, J = 1.5, 8.0, 14.0 Hz, H-6b), 4.04 (1H, m, H-7), 1.88 (1H, m, H-8), 2.03 (1H, dt, J = 4.5, 9.0 Hz, H-9), 1.09 (3H, d, J = 7.0 Hz, H-10), 4.15 (2H, m, H-12), 1.27 (3H, t, J = 7.1 Hz, H-13), 4.65 (1H, d, J = 8.0 Hz, H-1′), 3.20 (m, H-2′), 3.30 (m, H-3′), 3.28 (m, H-4′), 3.37 (1H, m, H-5′), 3.66 (1H, m, H-6′a), 3.89 (1H, dd, J = 1.7, 12.0 Hz, H-6′b); 13C-NMR (CD3OD) δ 97.7 (C-1), 151.9 (C-3), 114.2 (C-4), 32.1 (C-5), 42.8 (C-6), 75.0 (C-7), 42.1 (C-8), 46.5 (C-9), 13.4 (C-10), 169.1 (C-11), 61.0 (C-12), 14.6 (C-13), 100.1 (C-1′), 74.7 (C-2′), 78.3 (C-3′), 71.6 (C-4′), 78.0 (1H, m, C-5′), 62.8 (C-6′); negative ESI-MS m/z 449 [M+HCOO]−; negative TOF-MS m/z 449.1818 [M+HCOO]− (calcd for C19H29O12, 449.1817).
−86.3(c 0.082, MeOH); UV (MeOH) λmax (log ε) 198 (4.07), 235 (4.36) nm; IR (KBr) γmax 3420, 2933, 1688, 1634, 1399, 1288, 1076 cm−1; 1H-NMR (CD3OD) δ 5.27 (1H, d, J = 4.5 Hz, H-1), 7.38 (1H, s, H-3), 3.11 (1H, q, J = 8.0 Hz, H-5), 1.62 (1H, ddd, J = 5.0, 8.0, 14.0 Hz, H-6a), 2.24 (1H, ddd, J = 1.5, 8.0, 14.0 Hz, H-6b), 4.04 (1H, m, H-7), 1.88 (1H, m, H-8), 2.03 (1H, dt, J = 4.5, 9.0 Hz, H-9), 1.09 (3H, d, J = 7.0 Hz, H-10), 4.15 (2H, m, H-12), 1.27 (3H, t, J = 7.1 Hz, H-13), 4.65 (1H, d, J = 8.0 Hz, H-1′), 3.20 (m, H-2′), 3.30 (m, H-3′), 3.28 (m, H-4′), 3.37 (1H, m, H-5′), 3.66 (1H, m, H-6′a), 3.89 (1H, dd, J = 1.7, 12.0 Hz, H-6′b); 13C-NMR (CD3OD) δ 97.7 (C-1), 151.9 (C-3), 114.2 (C-4), 32.1 (C-5), 42.8 (C-6), 75.0 (C-7), 42.1 (C-8), 46.5 (C-9), 13.4 (C-10), 169.1 (C-11), 61.0 (C-12), 14.6 (C-13), 100.1 (C-1′), 74.7 (C-2′), 78.3 (C-3′), 71.6 (C-4′), 78.0 (1H, m, C-5′), 62.8 (C-6′); negative ESI-MS m/z 449 [M+HCOO]−; negative TOF-MS m/z 449.1818 [M+HCOO]− (calcd for C19H29O12, 449.1817).3.4. Neuroprotective Effects
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Hung, T.M.; Na, M.K.; Thuong, P.T.; Su, N.D.; Sok, D.E.; Song, K.S.; Seng, Y.H.; Bae, K.H. Antioxidant activity of caffeoylquinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, Y. Phytochemicals and biological activities of Dipsacus species. Chem. Biodiv. 2011, 8, 414–430. [Google Scholar] [CrossRef]
- Sun, Y.R.; Dong, J.X.; Wu, S.G. Studies on chemical constituents from Eucommia ulmoides Oliv. J. Chin. Med. Mater. 2004, 27, 341–343. [Google Scholar]
- Li, Y.M.; Wang, T.Z.; Wang, Z.X. Studies on chemical constituents in dried buds of Lonicera similis Hemsl. China J. Chin. Mat. Med. 2001, 26, 45–47. [Google Scholar]
- Qin, S.J.; Li, H.J.; Li, P.; Tang, D. Studies on chemical constituents of aerial parts of Lonicera dasystyla Rehd. Chin. Pharm. J. 2008, 43, 662–664. [Google Scholar]
- Wei, F.; Lou, Z.C. Structure determination of sylvestroside III from Dipsacus asper Wall. China Tradit. Herbal Drugs 1996, 27, 265–266. [Google Scholar]
- Ovodov, Y.S.; Frolova, G.M.; Nefedova, M.Y.; Elyakov, G.B. Glycosides of Eleutherococcussenticosus II, the structure of eleutherosides A, B1, C and D. Khim. Prir. Soedin. 1967, 3, 63–64. [Google Scholar]
- Tomita, H.; Mouri, Y. An iridoidglucoside from dipsacus asperoides. Phytochemistry 1996, 42, 239–240. [Google Scholar]
- Zhang, Z.J.; Qian, Y.H.; Hu, H.T.; Yang, J.; Yang, G.D. The herbal medicine Dipsacus asper wall. extract reduces the cognitive deficits and overexpression of beta-amyloid protein induced by aluminum exposure. Life Sci. 2003, 73, 2443–2454. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, R.Y.; Jeon, H.J.; Kwon, Y.S.; Chun, W. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother. Res. 2005, 19, 243–245. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Yang, Z.L.; Xu, L.; Li, P.; Hu, Y.Z. Akebia saponin D, a saponin component from Dipsacus asper Wall., protects PC 12 cells against amyloid-beta induced cytotoxicity. Cell Biol. Int. 2009, 33, 1102–1110. [Google Scholar] [CrossRef]
- Lin, Y.H.; Liu, A.H.; Wu, H.L.; Westenbroek, C.; Song, Q.L.; Yu, H.M. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents Aβ25-35-induced reduction in BPRP in PC12 cells. Biochem. Bioph. Res. Commun. 2006, 348, 593–599. [Google Scholar] [CrossRef]
- Liu, C.S.; Chen, N.H.; Zhang, J.T. Protection of PC12 cells from hydrogen peroxide-induced cytotoxicity by salvianolic acid B, a new compound isolated from Radix Salviaemiltiorrhizae. Phytomedicine 2007, 14, 492–497. [Google Scholar]
- Sample Availability: Samples of the compounds 1–6 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ji, D.; Zhang, C.; Li, J.; Yang, H.; Shen, J.; Yang, Z. A New Iridoid Glycoside from the Roots of Dipsacus asper. Molecules 2012, 17, 1419-1424. https://doi.org/10.3390/molecules17021419
Ji D, Zhang C, Li J, Yang H, Shen J, Yang Z. A New Iridoid Glycoside from the Roots of Dipsacus asper. Molecules. 2012; 17(2):1419-1424. https://doi.org/10.3390/molecules17021419
Chicago/Turabian StyleJi, De, Chunfeng Zhang, Jingzhi Li, Haowei Yang, Jingyang Shen, and Zhonglin Yang. 2012. "A New Iridoid Glycoside from the Roots of Dipsacus asper" Molecules 17, no. 2: 1419-1424. https://doi.org/10.3390/molecules17021419