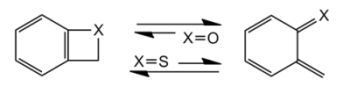
Benzoxetes and Benzothietes ¾ Heterocyclic Analogues of Benzocyclobutene
Abstract
:1. Introduction




2. Benzoxetes
2.1. Isolation of Benzoxetes


2.2. Matrix Isolation of Benzoxetes




2.3. Benzoxetes as Intermediates


2.4. Erroneous Benzoxete Structures
| Compound | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | References |
|---|---|---|---|---|---|---|---|---|---|
| 54'f | t-Bu | H | t-Bu | H | t-Bu | H | t-Bu | H | [31,36,37,39,42,43,44,47] |
| 54'g | t-Bu | H | OMe | H | t-Bu | H | OMe | H | [32,33,34,46,47] |
| 54'h | t-Bu | H | CMe2Et | H | t-Bu | H | CMe2Et | H | [35] |
| 54'i | CMe2Et | H | t-Bu | H | CMe2Et | H | t-Bu | H | [35] |
| 54'j | CMe2Et | H | CMe2Et | H | CMe2Et | H | CMe2Et | H | [36,40,42,47] |
| 54'k | t-Bu | H | CPh3 | H | t-Bu | H | CPh3 | H | [41,42,47] |
| 54'l | t-Bu | H | OC6H4-COOEt | H | t-Bu | H | OC6H4- | H | [38] |
| 54'm | t-Bu | H | t-Bu | Me | t-Bu | H | COOEt | Me | [45,47] |
| 54'n | t-Bu | H | t-Bu | Cl | t-Bu | H | t -Bu | Cl | [45,47] |
| 54'o | t-Bu | Cl | OMe | Cl | t-Bu | Cl | t -Bu | Cl | [45,47] |
| 54″p | t-Bu | H | OMe | H | t-Bu | H | OMe | OMe | [34,49] |
| 54″q | t-Bu | H | H | t-Bu | H | OMe | OEt | [34,49] |



3. Benzothietes
3.1. Preparation of Benzothietes by Ring Contraction Reactions


3.2. Benzothietes by Cycloelimination Reactions


3.3. Benzothietes by Cyclization Reactions




3.4. Benzothietes by Cycloaddition Reactions

3.5. Synthetic Applications of Benzothietes

| [2π/8π]Components | Reaction Products | References | |
|---|---|---|---|
| C=C |  | 3,4-Dihydro-2H-benzothiopyran(Thiochroman) | [14, 63, 79, 80, 81, 82, 83, 84] |
| Alkenes | |||
| Allenes | [85] | ||
 Benzo[c]furans Benzo[c]furans |  | 11,12-Dihydro-6,11-epoxy-6H-dibenzo[b,f] thiocine | [84] |
| CºC |  | 4H-1-Benzothiopyran(4H-Thiochromen) | [14] |
| Alkynes | |||
 1,4-Quinones (Oxidation) 1,4-Quinones (Oxidation) |  | 9H-Thioxanthene-1,4-dione | [82] |
| C=N |  | 3,4-Dihydro-2H-1,3-benzothiazine | |
| Azomethines | [81,86,87] | ||
| Azines | [81] | ||
| Ketenimines | [85] | ||
| Carbodiimides | [85] | ||
| Oximes and their O-derivatives | [81,88] | ||
| C=O |  | 3,1-Benzoxathian | [87,89] |
| Carbonyl Compounds | |||
| C=S |  | 1,3-Benzodithian | [90] |
| Thiocarbonyl Compounds | |||
| CºN |  | 4H-Benzo[e,1, 3]thiazine | [91] |
| Nitriles | |||
| N=C=S |   | 2-Imino-4H-1,3-Benzodithiin3,4-Dihydro-2H-1,3-Benzothiazine-2-thione | [91] |
| Isothiocyanates | |||
| N=N |  | 3,4-Dihydro-2H-1,2,3-benzothiadiazine | [87] |
| Azo Compounds | |||
| N=O |   | 3,1,2-Benzoxathiazine2,3-Dihydro-1,2-benzo=thiazol-1-oxide | [87, 92] |
| Nitroso Compounds | |||
 2-Oxonitriles 2-Oxonitriles |  | 3,1-Benzoxathian-2-carbonitrile | [91] |
| N=S=O |  | 1,2,3-Benzodithiazine-2-oxide | [85] |
| N-Sulfinylamines | |||
| P=S |  | 4H-1,3,2-Benzodithia=phosphorin-2-sulfide | [3] |
| Lawesson`s reagent |


4. Conclusions
References and Notes
- Tomioka, H.; Matsushita, T. Benzoxetene. Direct observation and theoretical studies. Chem. Lett. 1997, 26, 399–400. [Google Scholar]
- Kolshorn, H.; Meier, H. EHT calculations of valence isomerization between benzenoids and o-quinoidal systems. Z. Naturforsch. 1977, 32A, 780–782. [Google Scholar]
- Meier, H.; Mayer, A.; Gröschl, D. Benzothietes-Versatile synthons for the preparation of heterocycles. Sulfur Rep. 1994, 16, 23–56. [Google Scholar]
- Eckes, H.-L. Nucleophilic addition and cycloaddition reactions of benzothiete. Doctoral Dissertation, University of Mainz, Mainz, Germany, 1990. [Google Scholar]
- Arumugam, S.; Popik, V.V. Photochemical generation and the reactivity of o-naphthoquinone methides in aqueous solutions. J. Am. Chem. Soc. 2009, 131, 11892–11899. [Google Scholar]
- Shanks, D.; Frisell, H.; Ottoson, H.; Engman, L. Design principles for α-tocopherol analogues. Org. Biomol. Chem. 2006, 4, 846–852. [Google Scholar]
- Latulle, M.; Guenot, P.; Ripoll, J.-L. The syntheses of 6-methylene-2,4-cyclohexadien-1-imine and related o-quinonoids by FVT of 1-hetero-1,2,3,4-tetrahydronaphthalenes. Tetrahedron Lett. 1991, 32, 2013–2016. [Google Scholar]
- Quiao, G.G.H.; Lenghaus, K.; Solomon, D.H.; Reisinger, A.; Bytheway, I.; Wentrup, C. 4,6-Dimethyl-o-quinone methide and 4,6-dimethylbenzoxete. J. Org. Chem. 1998, 63, 9806–9811. [Google Scholar]
- Mao, Y.-L.; Boekelheide, V. Benzocyclobutene-o-Xylylene valence tautomerization: Oxygen and sulfur analogs. Proc. Natl. Acad. Sci. USA 1980, 77, 1732–1735. [Google Scholar]
- Cavitt, S.B.; Sarrafizadeh, H.; Gardner, P.D. The structure of o-quinone methide trimer. J. Org. Chem. 1962, 27, 1211–1216. [Google Scholar]
- Faure, R.; Thomas-David, G.; Bartnik, R.; Cebulska, Z.; Graca, E.; Lasniak, S. Tetramer of o-methylenequinone. Bull. Soc. Chim. France 1991, 378–380. [Google Scholar]
- Adam, W.; Hadjiarapoglou, L.; Peters, K.; Sauter, M. Dimethyldioxirane epoxidation of benzofurans: reversible thermal and photochemical valence isomerization between benzofuran epoxides, quinone methides, and benzoxetenes. J. Am. Chem. Soc. 1993, 115, 8603–8608. [Google Scholar]
- Van Tilborg, W.J.M.; Plomp, R. Flash vapor-phase pyrolysis of thiophene 1,1-dioxides. Recl. Trav. Chim. Pays-Bas 1977, 96, 282–286. [Google Scholar]
- Kanakarajan, K.; Meier, H. Cycloaddition reactions of benzothiete. J. Org. Chem. 1983, 48, 881–883. [Google Scholar]
- Van Tilborg, W.J.M.; Plomp, R. Synthesis of benzothiete from benzo[b]thiophene 1,1-dioxide. J. Chem. Soc. Chem. Commun. 1977, 130–131. [Google Scholar]
- Adam, W.; Sauter, M.; Zuenkler, C. Preparation of 2H-benzoxetes by photoinduced [2 + 2] cyclo addition of quinone methides, accessible by dimethyldioxirane (DMD) oxidation of 2,3-dimethyl benzofurans. Chem. Ber. 1994, 127, 1115–1118. [Google Scholar]
- Zhang, Q.; Liu, X.-T.; Liang, J.-Y.; Min, Z.-D. Chemical constituents from the stem of Caesalpinia decapetala. Zhongguo Tianran Yaowu 2008, 6, 168–171. [Google Scholar]
- See also the natural product discussed in references [19] and [20].
- Guo, S.; Tang, Y.P.; Duan, J.A.; Su, S.L.; Ding, A.W. Two new terpenoids from fruits of Ziziphus jujuba. Chinese Chem. Lett. 2009, 20, 197–200. [Google Scholar]
- Chen, H.-L.; Wang, L.-W.; Su, H.-J.; Wie, B.-L.; Yang, S.-Z.; Lin, C.-N. New terpenoids from amentotaxus formosana. Org. Lett. 2006, 8, 753–756. [Google Scholar]
- Tomioka, H. Matrix isolation study of reactive o-quinoid compounds: Generation, detection and reactions. Pure Appl. Chem. 1997, 69, 837–840. [Google Scholar]
- Felix, D.; Winter, C.; Eschenmoser, A. Fragmentation of α,β-epoxyketones to acetylenic aldehydes and ketones: Preparation of 2,3-epoxycyclohexanone and its fragmentation to 5-hexynal. Org. Synth. Coll. Vol. 1988, 6, 679–682. [Google Scholar]
- Chapman, O.L.; Chang, C.C.; Kolc, J.; Rosenquist, N.R.; Tomioko, H. Photochemical method for the introduction of strained multiple bonds. Benzyne C ≡ C stretch. J. Am. Chem. Soc. 1975, 97, 6586–6588. [Google Scholar]
- Chiang, Y.; Gaplovsky, M.; Kresge, A.J.; Leung, K.H.; Ley, C.; Mac, M.; Persy, G.; Phillips, D.L.; Popik, V.V.; Roedig, C.; Wirz, J.; Zhu, Y. Photoreactions of 3-diazo-3H-benzofuran-2-one; dimerization and hydrolysis of its primary photoproduct, a quinonoid cumulenone: A study by time-resolved optical and infrared spectroscopy. J. Am. Chem. Soc. 2003, 125, 12872–12880. [Google Scholar]
- Voigt, E.; Meier, H. About the photochemistry of heteroanalogue 3-diazo-2-oxoindanes. Chem. Ber. 1977, 110, 2242–2248. [Google Scholar]
- Yoshioka, E.; Kohtani, S.; Miyabe, H. A multicomponent coupling reaction induced by insertion of arynes into the C=O bond of formamide. Angew. Chem. Int. Ed. 2011, 50, 6638–6642. [Google Scholar]
- Yoshioko, E.; Kohtani, S.; Miyabe, H. Sequential reaction of arynes via insertion into the π- bond of amides and trapping reaction with dialkylzincs. Org. Lett. 2010, 12, 1956–1959. [Google Scholar]
- Heaney, H.; McCarty, C.T. Reactions of arynes with carbonyl compounds. J. Chem. Soc. D Chem. Commun. 1970, 123. [Google Scholar]
- Heany, H.; Jablonski, J.M.; McCarty, C.T. Aryne chemistry. XXXI. Reactions of arynes with α,β-unsaturated aldehydes. J. Chem. Soc. Perkin Trans. 1 1972, 2903–2910. [Google Scholar]
- Rayne, S.; Sasaki, R.; Wan, P. Photochemical rearrangement of dibenzo [1,4] dioxins proceeds through reactive spirocyclohexadienone and biphenylquinone intermediates. Photochem. Photobiol. Sci. 2005, 4, 876–886. [Google Scholar]
- Müller, E.; Mayer, R.; Narr, B.; Rieker, A.; Scheffler, K. Oxygen radicals. XVII. Dehydrogenation of bisphenols under formation of „inner“ spirocyclic quinol ethers. Liebigs Ann. Chem. 1961, 645, 25–35. [Google Scholar]
- Müller, E.; Kaufmann, H.; Rieker, A. Synthesis of higher molecular weight compounds by phenol dehydrogenation. I. Oxidative trimerization of 4-methoxy-2,5-di-tert-butylphenol. Liebigs Ann. Chem. 1964, 671, 61–70. [Google Scholar]
- Hewgill, F.R.; Kennedy, B.R. Oxidation of alkoxy phenols. VI. A hemiacetal from 4-methoxy-2-tert-butylphenol. J. Chem. Soc. C 1966, 362-366. [Google Scholar]
- Hewgill, F.R.; Hewitt, D.G. Oxidation of alkoxyphenols. X. The reaction of 2,2'-dihydroxy- 5,5'-dimethoxy-3,3'-di-tert-butylbiphenyl with lead tetraacetate. J. Chem. Soc. C 1967, 8, 726–730. [Google Scholar]
- Karpov, V.V.; Puchkov, V.A.; Khidekel, M.L. Synthesis of spirocyclic trimers by the oxidative coupling of phenols in the presence of complex catalysts. Zhurnal Org. Khim. 1968, 4, 1594–1603. [Google Scholar]
- Karpov, V.V.; Khidekel, M.L. Oxidative di- and trimerization during the oxidation of phenols by oxygen in the presence of copper-containing complexes. Zhurnal Org. Khim. 1968, 4, 861–869. [Google Scholar]
- Bowman, D.F.; Hewgill, F.R. Radical coupling in the reaction of o-halophenols with base. Chem. Commun. 1968, 524–525. [Google Scholar]
- Hewitt, D.G. Phenol oxidation mechanism. Search for phenoxylium intermediates. J. Chem. Soc. C 1971, 1750–1757. [Google Scholar]
- Claus, P.; Schilling, P.; Gratzl, J.S.; Kratzl, K. Preparation and oxidation of hydroxybenzyl alcohols. Monatsh. Chem. 1972, 103, 1178–1193. [Google Scholar]
- Becker, H.D.; Gustafsson, K. Formation and photochemical isomerization of arylated 1,3- dihydro-2H-azepin-2-ones. Tetrahedron Lett. 1976, 17, 1705–1708. [Google Scholar]
- Becker, H.D.; Gustafsson, K. On the formation of spiro-substituted benzoxetes by phenol oxidation: preparation and valence isomerization of 3,3'-di-tert-butyl-5,5'-ditrityl-2,2'-dipheno quinone. Tetrahedron Lett. 1976, 17, 4883–4886. [Google Scholar]
- Becker, H.D.; Gustafsson, K. Nucleophilic addition of amines to benzo-substituted oxetenes. Formation of 6-amino-2,4-cyclohexadienones and their ring expansion. J. Org. Chem. 1977, 42, 2966–2973. [Google Scholar]
- Oleinik, E.P.; Mamysheva, O.N.; Gorbunova, L.V. Characteristics of reactions of shielded germanium-containing phenols in the presence of oxygen under one-electron oxidation conditions. Doklady Akad. Nauk SSSR 1986, 289, 1137–1139. [Google Scholar]
- Jamois, D.; Tessier, M.; Marechal, E. Preparation of amphiphilic polyisobutylenes-β-polyethylen amines by Mannich reaction. II. Study of Mannich reaction on model systems. J. Polym. Sci. A 1993, 31, 1941–1958. [Google Scholar]
- Müller, E.; Rieker, A. unpublished work, Chemistry Department, University of Tübingen: Germany.
- Baltes, J.; Volbert, F. Mechanism of antioxidant action. II. 3,3'-Di-tert-butyl-5,5'-dimethoxybi phenyl-2,2'-peroxide, a dehydrogenation product of 2-tert-butyl-4-methoxyphenol and its rearrangement to bis[4-methoxy-6-tert-butylbenzene-(2)]indigo. Seifen Anstrichmittel 1955, 57, 660–666. [Google Scholar]
- Meier, H.; Schneider, H.P.; Rieker, A.; Hitchcock, P.B. Sterically hindered “benzoxetes”-the first isolated oxepins. Angew. Chem. Int. Ed. 1978, 90, 128–129, Angew. Chem. Int. Ed. Engl. 1978, 17, 121-123.. [Google Scholar]
- Schneider, H.P. Oxepines from o,o'-diphenoquinones, isomerization of α-keto-o-quinone methides. Doctoral Dissertation, University of Tübingen, Tübingen, Germany, 1981. [Google Scholar]
- Schneider, H.P.; Winter, W.; Rieker, A. Oxepines. Part V. The reaction of oxepino benzofurans with alcohols; the structure of Hewgill’s “trialkoxyspirobenzoxetes”. J. Chem. Res. Synopses 1978, 336–337. [Google Scholar]
- Hagen, H.; Becke, F. BASF AG) Spiro[benzoxete-2,2'-imidazolidines] and corresponding hexhydropyrimidines. Ger. Offen. DE 2034758 A 1972 0120, 1972. [Google Scholar]
- Aroyan, A.A.; Iradyan, M.A. Synthesis of some tetrasubstituted ethylenediamines. Armyanskii Khimicheskii Zhurnal 1966, 19, 784–792. [Google Scholar]
- Klosa, J. Synthesis of spasmolytically active substances. XXI. Synthesis of α-alkoxybenzilic acid hydrazides. J. Prakt. Chem. 1966, 31, 20–33. [Google Scholar]
- Osman, A.M.; Bassiouni, I. 2-Aryinaphthoxazoles and some other condensed oxazoles. J. Org. Chem. 1962, 27, 558–561. [Google Scholar]
- Mastagli, P.; Metayer, M.; de Bievre-Gallin, G. Action of formamide on substituted benz aldehydes. Bull. Soc. Chim. France 1948, 662–665. [Google Scholar]
- Von Euler, H.; Adler, E.; Cedwall, J.O. Reaction of phenols with C2H2. Arkiv. Kemi Mineral. Geol. 1942, 15A, 10. [Google Scholar]
- Darapsky, A.; Berger, H.; Neuhaus, A. Action of hydrazine hydrate on lactones. J. Prakt. Chem. 1936, 147, 145–160. [Google Scholar]
- Cox, E.H. Mechanism and application of the Fries reaction. J. Am. Chem. Soc. 1930, 52, 352–358. [Google Scholar]
- Meier, H.; Issa, A.; Merkle, U. 5aH-, 1OaH-,15aH-Tribenzo[b,f,j,1,4,7]trioxa[9b]azaphenalenes- alledged benzoxetes. Z. Naturforsch. 1979, 34b, 290–296. [Google Scholar]
- Voigt, E.; Meier, H. Synthesis of the 2H-1-thiacyclobutabenzene system. Angew. Chem. 1976, 88, 94, Angew. Chem. Int. Ed. Engl. 1976, 15, 117.. [Google Scholar]
- Voigt, E.; Meier, H. Photochemistry of heteroanalogue 3-diazo-2-oxoindanes. Chem. Ber. 1977, 110, 2242–2248. [Google Scholar]
- Raasch, M.S. Syntheses with halogen derivatives of thiophene and benzothiophene. J. Org. Chem. 1980, 45, 2151–2155. [Google Scholar]
- Schulz, R.; Schweig, A. Theory and application of photoelectron spectroscopy. Part 87. Elucidation of thermal reaction by variable temperature photoelectron spectroscopy. A new synthesis of benzothiete and first direct evidence for transient benzothiete ketene. Tetrahedron Lett. 1980, 21, 343–346. [Google Scholar]
- Meier, H.; Mayer, A. Synthetic equivalents for benzo- and naphthothietes. Synthesis 1996, 1996, 327–329. [Google Scholar]
- Kolmakov, K.A.; Kresge, A.J. Synthesis of possible o-thioquinone methide precursors. Canad. J. Chem. 2008, 86, 119–123. [Google Scholar]
- van Tilborg, W.J.M.; Plomb, R. Synthesis of benzothiete from benzo[b]thiophene 1,1-dioxide. J. Chem. Soc. Chem. Commun. 1977, 130–131. [Google Scholar]
- van Tilborg, W.J.M.; Plomb, R. Flash vapor-phase pyrolysis of thiophene 1,1-dioxides. Recl. Trav. Chim. Pays-Bas 1977, 96, 282–286. [Google Scholar]
- Hortmann, A.G.; Aron, A.J.; Bhattacharya, A.K. 3H-1,2-Benzodithiole oxides: Studies directed toward the generation of o-thiobenzoquinone methide and benzo[b]thiete. J. Org. Chem. 1978, 43, 3374–3378. [Google Scholar]
- The pyrolysis zone can be filled with quartz particles for this purpose.
- Davis, F.A.; Awad, S.B.; Jenkins, R.H., Jr.; Billmers, R.L.; Jenkins, L.A. Chemistry of sulfenic acids. 5. A novel rearrangement of 2,4,6-trineopentylbenzenesulfenic acid to 2-tert-butyl-4,6-dineopentylbenzo[b]thiete and 3,3-dimethyl-4,4-dihydro-6,8-dineopentylbenzo[b]thiopyran. Synthesis of thiete sulfoxides. J. Org. Chem. 1983, 48, 3071–3074. [Google Scholar]
- Pohl, E.; Herbst-Irmer, R.; Sheldrick, G.M. Structure of 4,6-bis(trifluoromethyl)-2,2-bis[2,4,6- tris(trifluoromethyl)phenylthio]-1-thiabenzocyclobutene. Acta Crystallogr. C 1993, C49, 1026–1028. [Google Scholar]
- Hagen, H.; Amann, A.; Becke, F. BASF AG) Spiro[benzothiete-2,2`-imidazolidines] and corresponding hexahydropyrimidines. Ger. Offen. DE 203 4987, 1972. [Google Scholar]
- Hagen, H.; Amann, A.; Giertz, H. (BASF AG) 2-(o-Alkylthiophenyl)-1,3-diazocycloalkene hydrohalides. U.S. Patent 4,122,263, 1978. [Google Scholar]
- Korobov, M.S.; Minkin, V.I.; Nivorozhkin, L.E. Benzoid-quinoid tautomerism of azomethines and their structural analogs. XX. Imines of 5-nitrothiosalicylaldehyde. Zhur. Organ. Khimii 1975, 11, 836–842. [Google Scholar]
- Goldfarb, Y.L.; Skorova, A.E.; Kirmalova, M.L. Action of sodium in liquid ammonia on diethyl acetal of 6-methylthio-3-methylbenzaldehyde. Izv. Akad. Nauk. SSSR Seriya Khim. 1966, 1426–1432. [Google Scholar]
- Goldfarb, Y.L.; Skorova, A.E.; Kirmalova, M.L. Action of sodium in liquid ammonia on 6- methylthio-3-methylbenzaldehyde. Izv. Akad. Nauk SSSR Seriya. Khim. 1966, 1421–1425. [Google Scholar]
- Nakayama, J.; Horikoshi, R.; Ishii, A.; Hoshino, M.; Kobayashi, H. Reaction of benzyne with thiophosgene. Phosphorus Sulfur Silicon Relat. Elem. 1983, 16, 195–199. [Google Scholar]
- Okuma, K.; Shiki, K.; Sonoda, S.; Koga, Y.; Shioji, K.; Kitamura, T.; Fujiwara, Y.; Yokomori, Y. Reaction of electronically stabilized thiones with benzyne. The isolation of thiobenzophenone- benzyne and thiopivalophenone-benzyne adducts. Bull. Chem. Soc. Jpn. 2000, 73, 155–161. [Google Scholar]
- Okuma, K.; Shiki, K.; Shioji, K. Reaction of thiopivalophenones with benzyne. Formation of 2H-benzo[b]thietes. Chem. Lett. 1998, 79-80. [Google Scholar]
- Meier, H.; Eckes, H.-L.; Niedermann, H.-P.; Kolshorn, H. A nonspecific Diels-Alder reaction. Angew. Chem. Int. Ed. 1987, 99, 1040–1042, Angew. Chem. Int. Ed. Engl. 1987, 26, 1046-1048.. [Google Scholar]
- Meier, H.; Schmidt, M.; Eckes, H.-L. Cycloaddition of benzothiete and 4-substituted styrenes. Chem. Ber. 1989, 122, 1545–1550. [Google Scholar]
- Meier, H.; Saul, K.; Jacob, D. Reactions of benzothiete and imines. Liebigs Ann. Chem. 1993, 313–319. [Google Scholar]
- Gröschl, D.; Mayer, A.; Schmidt, M.; Meier, H. Synthesis of benzo[b]thioxanthenes. J. Prakt. Chem. Chem. Ztg. 1995, 337, 379–384. [Google Scholar]
- Meier, H.; Gröschl, D. Reaction of 2H-1-benzothiete with diazo compounds in the presence of (II) rhodium acetate. Tetrahedron Lett. 1995, 36, 6047–6050. [Google Scholar]
- Gröschl, D.; Meier, H. Cycloaddition reactions of 2H-benzo[b]thiete and conjugated cyclic dienes. J. Heterocycl. Chem. 1996, 33, 1727–1729. [Google Scholar]
- Gröschl, D.; Niedermann, H.-P.; Meier, H. Cycloadditions of 2H-benzo[b]thietes and compounds with cumulated double bonds. Chem. Ber. 1994, 127, 955–958. [Google Scholar]
- Jacob, D.; Meier, H. Cycloaddition reactions of benzothiete with azomethines. J. Heterocycl. Chem. 1986, 23, 1085–1086. [Google Scholar]
- Jacob, D.; Niedermann, H.-P.; Meier, H. Cycloaddition reactions of benzothiete and hetero dienophiles for the synthesis of heterocyclic systems. Tetrahedron 1986, 27, 5703–5706. [Google Scholar]
- Meier, H.; Saul, K.; Mengel, R.; Niedermann, H.-P. Cycloaddition of benzothiete and oximes, oxime ethers and oxime esters. J. Heterocycl. Chem. 1991, 28, 843–848. [Google Scholar]
- Schmidt, M.; Meier, H.; Niedermann, H.-P.; Mengel, R. 4H-3,1-Benzoxathiines from benzothiete and carbonyl compounds. Chem. Ber. 1990, 123, 1143–1148. [Google Scholar]
- Meier, H.; Gröschl, D.; Beckert, R.; Weiß, D. Cycloaddition reaction of 2H-benzo[b]thiete and thiocarbonyl compounds. Liebigs Ann. 1997, 1997, 1603–1605. [Google Scholar]
- Schmidt, M.; Meier, H.; Saleh, S.-A. Cycloaddition of benzothiete and electron-deficient nitriles. J. Heterocycl. Chem. 1991, 28, 573–575. [Google Scholar]
- Saul, K.; Eckes, H.-L.; Jacob, D.; Meier, H. Cycloadditions of benzothiete and aromatic nitroso compounds. Chem. Ber. 1993, 126, 775–758. [Google Scholar]
- Meier, H.; Schmidt, M.; Mayer, A.; Schollmeyer, D.; Beile, B. Stereoselective synthesis of polycyclic thiopyrans. J. Heterocycl. Chem. 2012. [Google Scholar]
- Ohmo, M.; Kojima, S.; Shirakawa, Y.; Eguchi, S. Hetero-Diels-Alder reaction of fullerene: Synthesis of thiochroman-fused C60 with o-thioquinone methide and oxidation to its S-oxides. Tetrahedron Lett. 1995, 36, 6899–6902. [Google Scholar]
- Kanakarajan, K.; Meier, H. Ring enlargement of benzothiete to 2,3-dihydrobenz[d]isothiazoles. Angew. Chem. Int. Ed. 1984, 96, 220, Angew. Chem. Int. Ed. Engl. 1984, 23, 244.. [Google Scholar]
- Niedermann, H.-P.; Eckes, H.-L.; Meier, H. Reactions of benzothiete with phosphorus- nucleophiles-A novel type of Arbuzov rearrangement. Tetrahedron 1989, 30, 155–158. [Google Scholar]
- Eckes, H.-L.; Niedermann, H.-P.; Meier, H. Addition of phosphorous nucleophiles to benzothiete. Chem. Ber. 1991, 124, 377–381. [Google Scholar]
- Meier, H. Benzothiete, a versatile reagent in heterocyclic syntheses. J. Prakt. Chem. Chem. Ztg. 1996, 338, 383–385. [Google Scholar]
- Rokita, S.E. Quinone Methides; Wiley: San Francisco, CA, USA, 2009. [Google Scholar]
- Drohm, C.; Meyer, H.; Schweig, A. Kinetics of a monomolecular photoreaction in a strongly light-absorbing solid solution exemplified by the forward reaction of the optically switchable o-thiobenzoquinone methide. Chem. Phys. Lett. 1995, 245, 529–533. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Meier, H. Benzoxetes and Benzothietes ¾ Heterocyclic Analogues of Benzocyclobutene. Molecules 2012, 17, 1548-1570. https://doi.org/10.3390/molecules17021548
Meier H. Benzoxetes and Benzothietes ¾ Heterocyclic Analogues of Benzocyclobutene. Molecules. 2012; 17(2):1548-1570. https://doi.org/10.3390/molecules17021548
Chicago/Turabian StyleMeier, Herbert. 2012. "Benzoxetes and Benzothietes ¾ Heterocyclic Analogues of Benzocyclobutene" Molecules 17, no. 2: 1548-1570. https://doi.org/10.3390/molecules17021548