Synthesis of New Phosphorus-Containing (Co)Polyesters Using Solid-Liquid Phase Transfer Catalysis and Product Characterization
Abstract
:1. Introduction
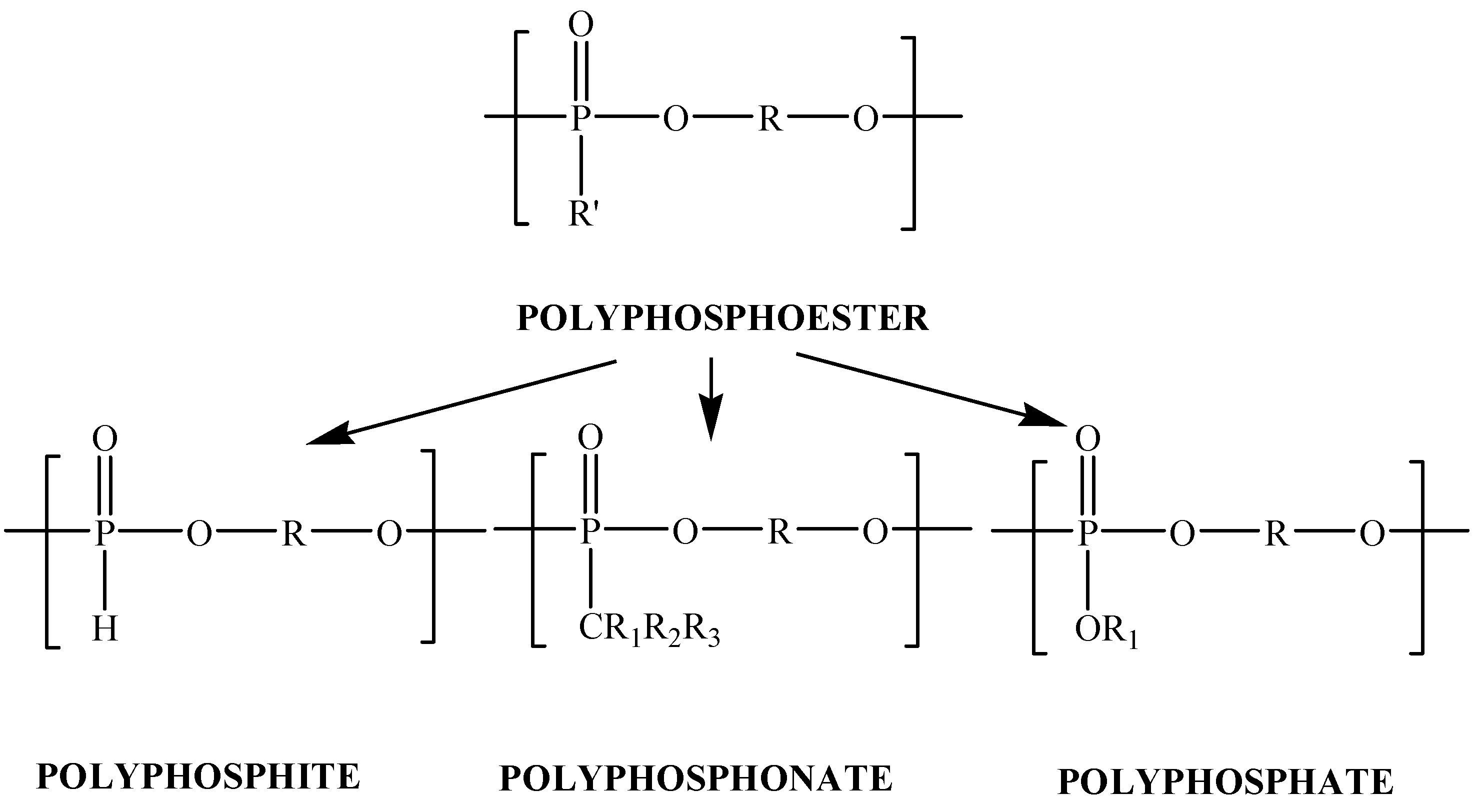
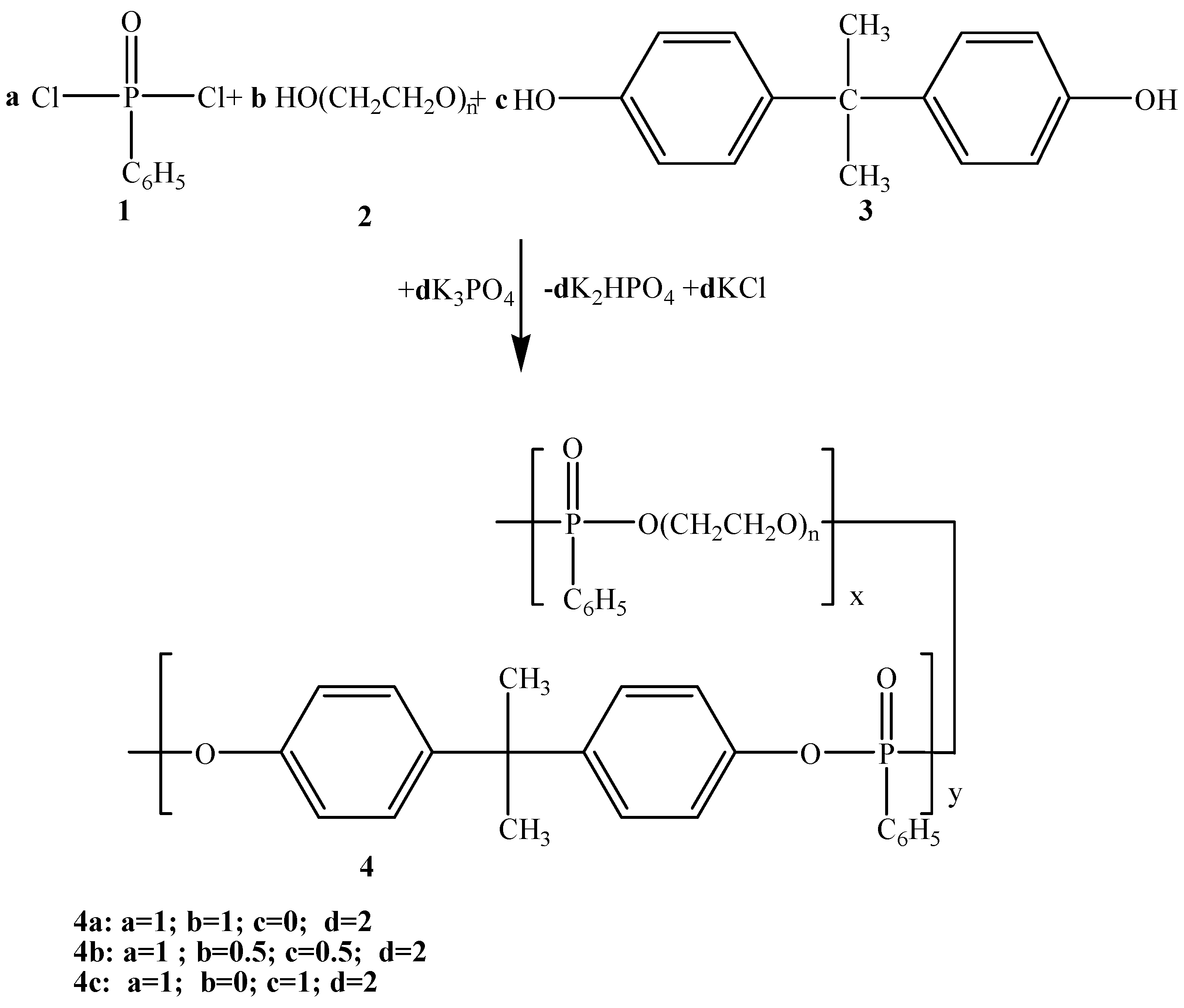
2. Results and Discussion
| (Co)polym | η, % | ηinh, b dL/g | Mn × 10−4 | Mw × 10−4 | polydisp. | P (%) c | |
|---|---|---|---|---|---|---|---|
| calc | exp | ||||||
| 4a | 85.4 | 0.58 | 2.63 | 3.0 | 1.14 | 0.25 | 0.22 |
| 4b | 86.5 | 0.55 | 2.30 | 2.77 | 1.16 | 0.25 | 0.20 |
| 4c | 88.0 | 0.32 | 0.54 | 0.62 | 1.15 | 8.85 | 8.20 |

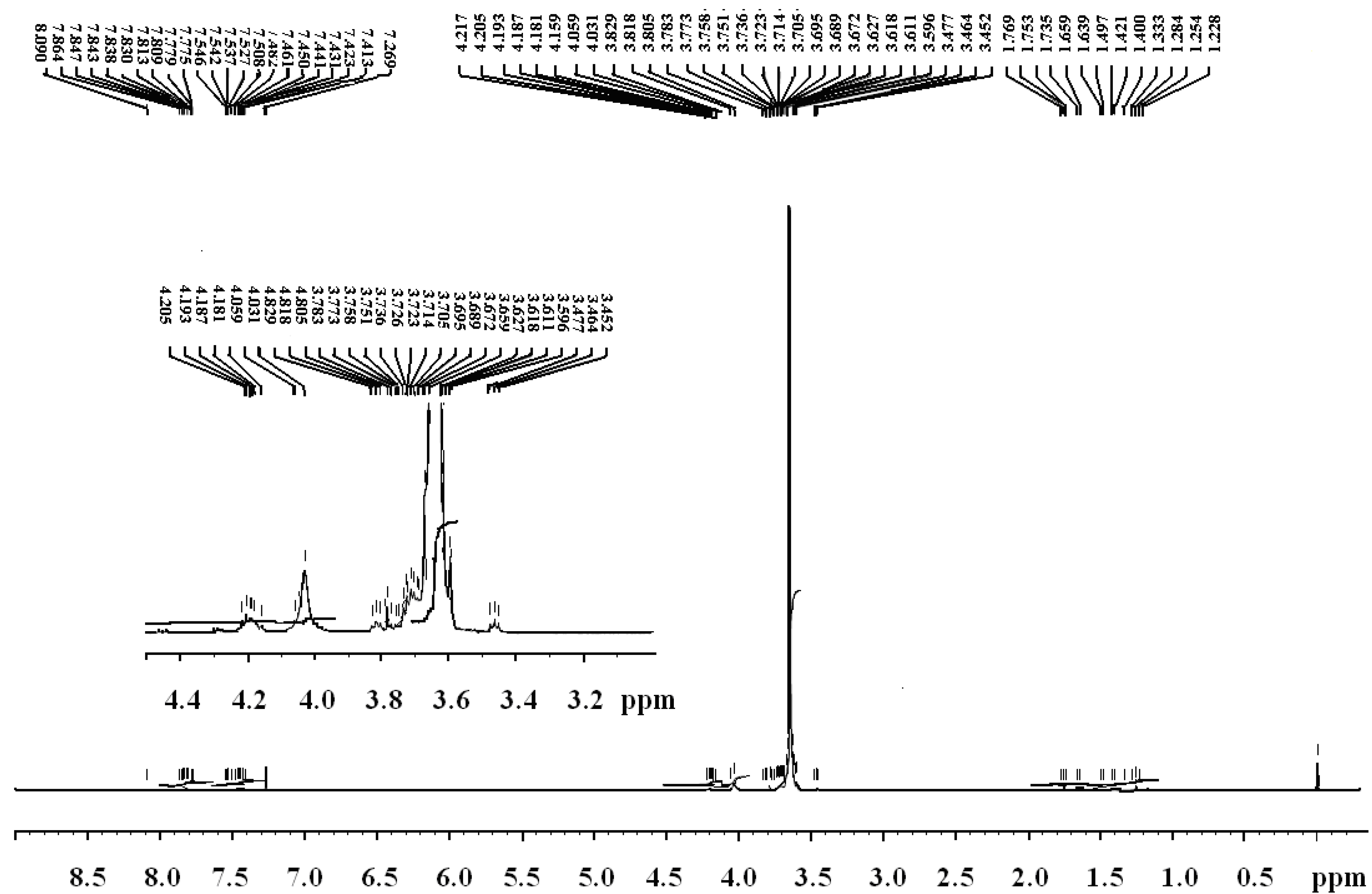
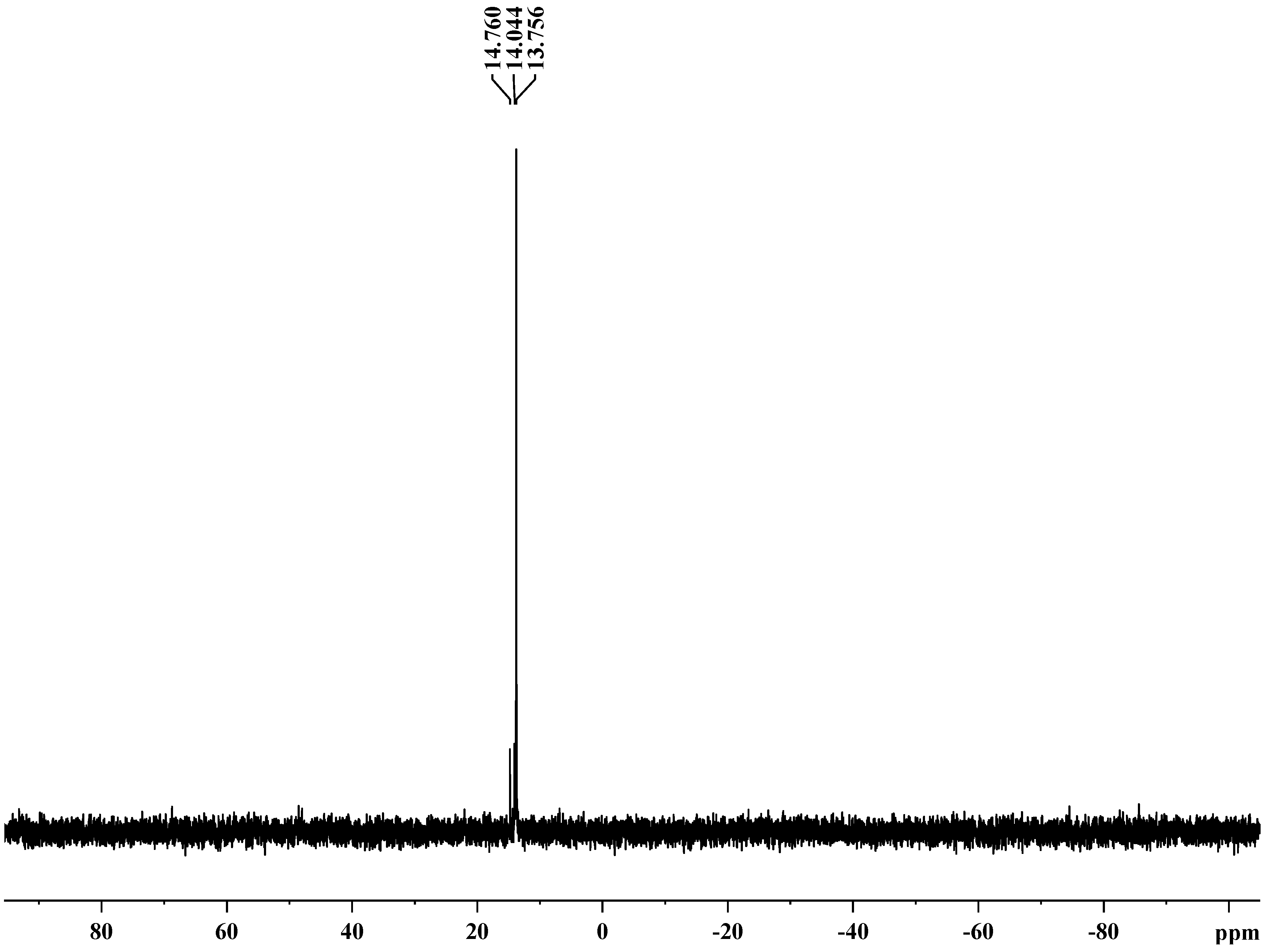
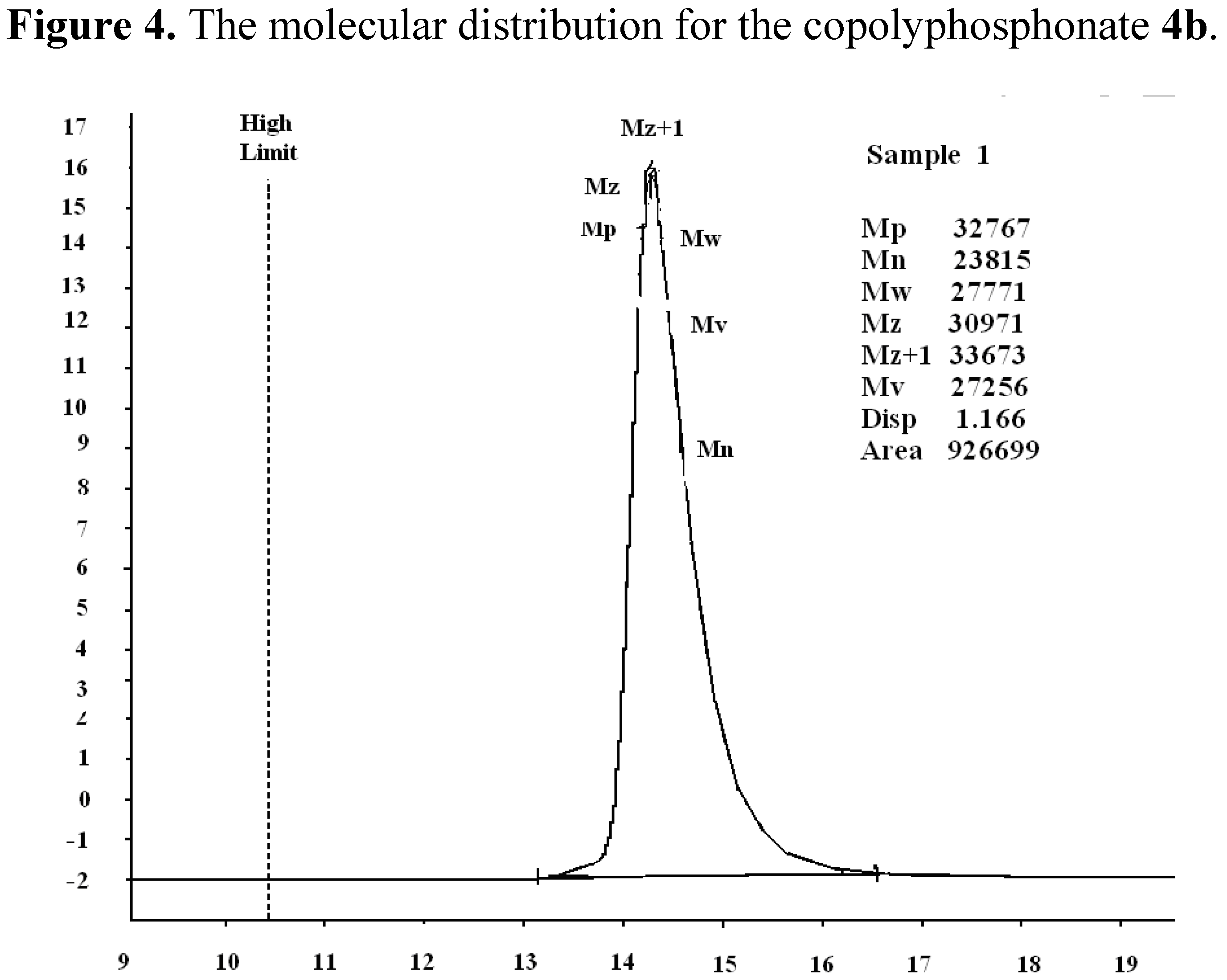
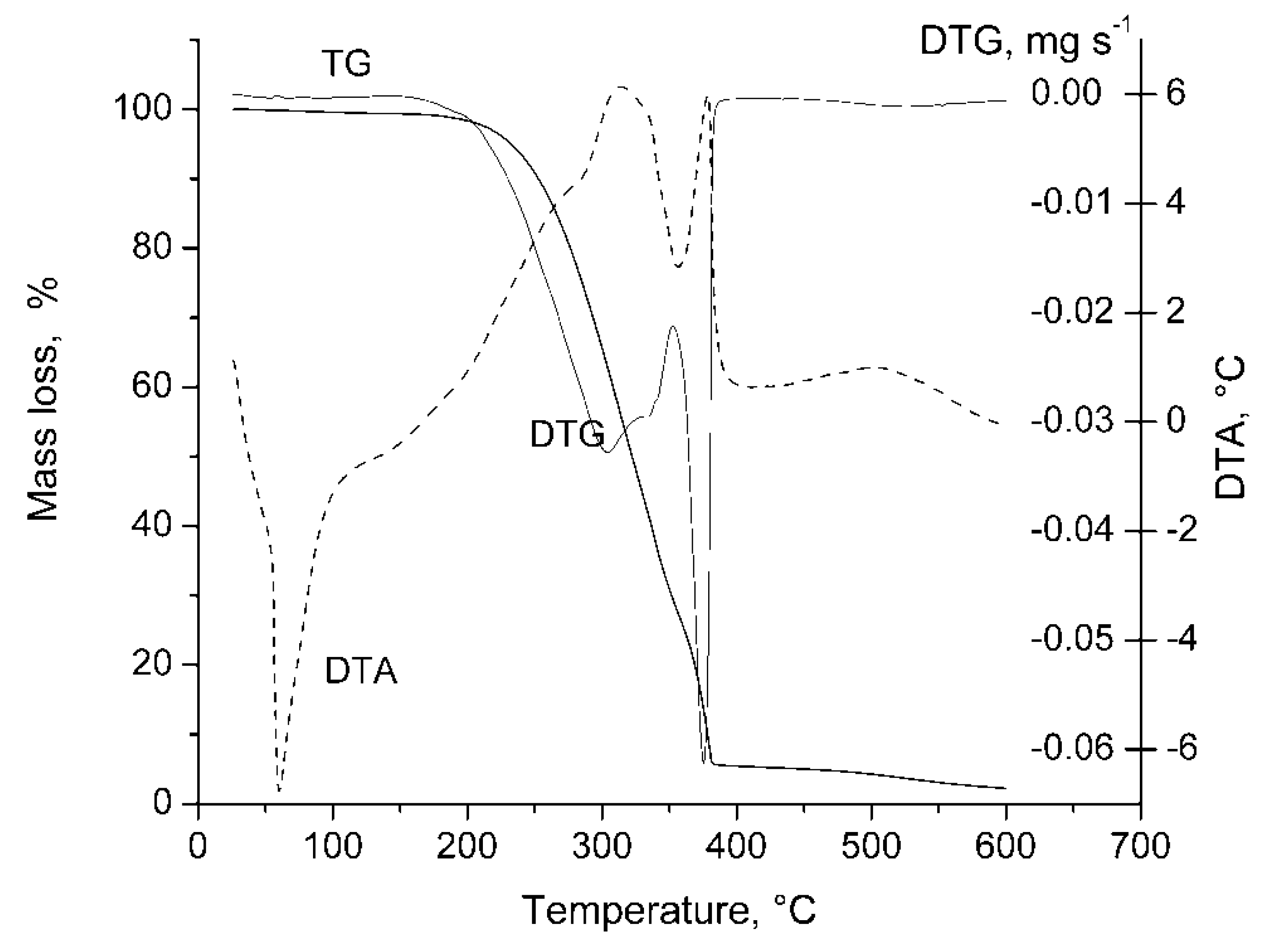
| No | Weight loss correspondence to (°C) | Tm, °C | Char yield at 550 °C, % | LOI | |
|---|---|---|---|---|---|
| 5% | 95% | ||||
| 4a | 222.2 | 378 | 55.12 | 1.8 | 28 |
| 4b | 230.6 | 380 | 59.87 | 2.3 | 30 |
| 4c | 190.4 | 420 | - | 2.6 | 38 |
| PEG | 320 | 420 | 74.63 | 0.8 | 23 |

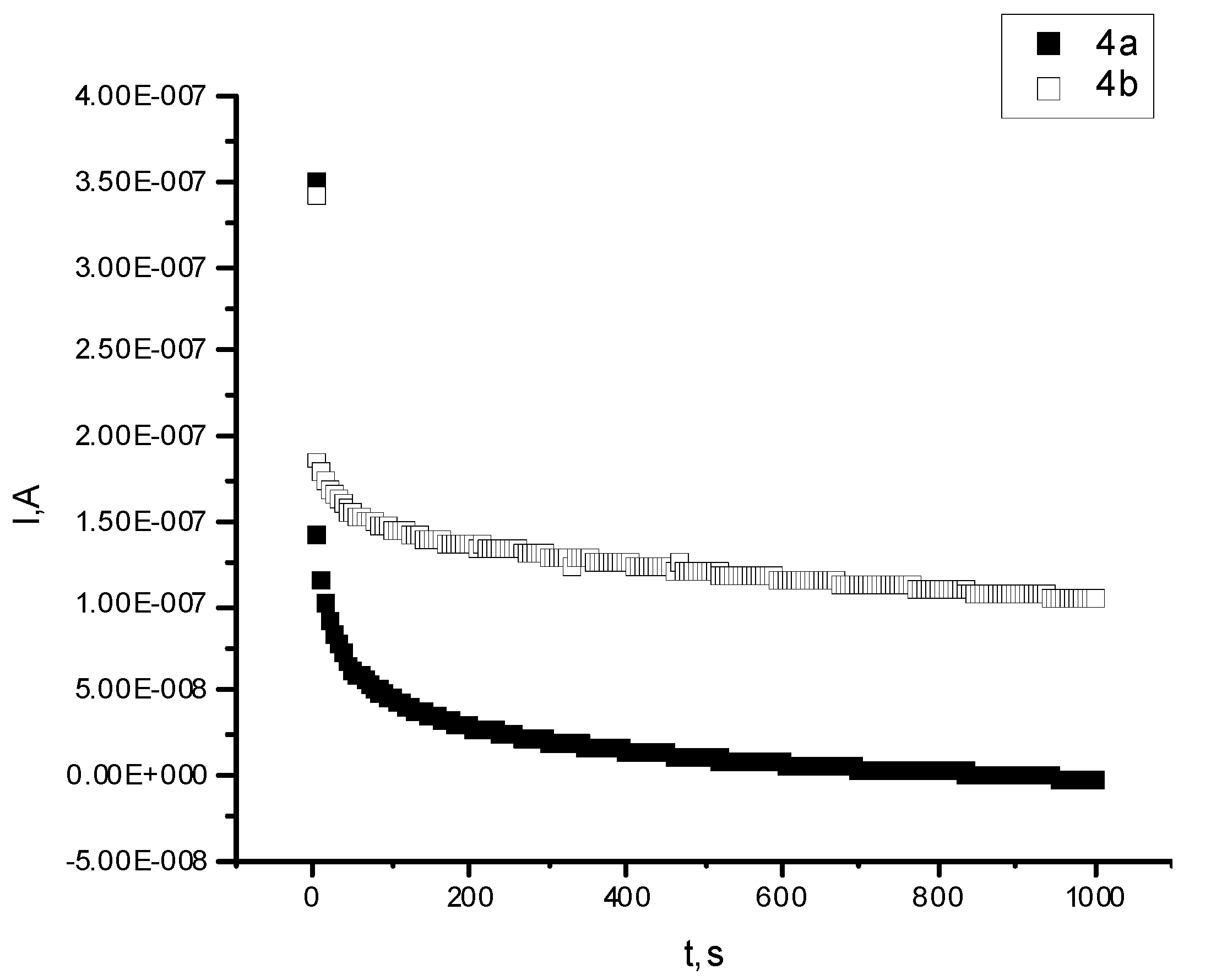
3. Experimental
3.1. Materials
3.2. Procedure
3.2.1. Synthesis of Phosphorus Containing (co)Polyesters by PTC Polycondensation in Solid-Liquid System [24]
3.2.2. Preparation of Phosphorus-Containing (co)Polyester Electrolytes
3.3. Analysis

4. Conclusions
Acknowledgments
References
- Kaczorowska, M.A.; Cooper, H.J. Characterization of polyphosphoesters by fourier transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 2009, 20, 2238–2247. [Google Scholar] [CrossRef] [Green Version]
- Troev, K.D. 3-Poly[alkylene(arylene(alkyl or arylphosphonate]s. In Polyphosphoesters; Elsevier: Sofia, Bulgaria, 2012; pp. 263–320. [Google Scholar]
- Chang, Y.-L.; Wang, Y.-Z.; Ban, D.-M.; Yang, B.; Zhao, G.-M. A Novel phosphorus-containing polymer as a highly effective flame retardant. Macromol. Mater. Eng. 2004, 289, 703–707. [Google Scholar] [CrossRef]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.-J. Phosphorus-containing polymers: A great opportunity for the biomedical field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef]
- Gray, F.M. Solid Polymer Electrolytes-Fundamentals and Technological Applications; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Kim, S.H.; Kim, J.Y.; Kim, H.S.; Cho, H.N. Ionic conductivity of polymer electrolytes based on phosphate and polyether copolymers. Solid State Ionics 1999, 116, 63–71. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.Y. Ionic conduction of network polymer elctrolyets based on phosphate and polyether copolymers. Solid State Ionics 1999, 124, 91–99. [Google Scholar] [CrossRef]
- Morris, S.R.; Dixon, B.G. A novel approach for development of improved polymer electrolytes for lithium batteries. J. Power Sources 2003, 119-121, 487–491. [Google Scholar] [CrossRef]
- Dixon, B.G.; Morris, R.S.; Dallek, S. Non-flammable polyphosphonate electrolytes. J. Power Sources 2004, 138, 274–276. [Google Scholar] [CrossRef]
- Minegishi, S.; Komatsu, S.; Nishikubo, T. Novel synthesis of polyphosphonates by the polyaddition of bis (epoxide) with diaryl phosphonates. J. Polym. Sci. A Polym. Chem. 2001, 37, 959–965. [Google Scholar]
- Minegisi, S.; Tsuchida, S.; Sasaki, M.; Kameyama, A.; Kudo, H.; Nishikubo, T. Synthesis of polyphosphonates containing pendant, chloromethyl groups by the polyaddition of bis (oxetane)s with phosphonic dichlorides. Polym. Sci. A Polym. Chem. 2002, 40, 3835–3846. [Google Scholar] [CrossRef]
- Avci, D.; Mathias, L.J. Synthesis and polymerization of phosphorus-containing acrylates. J. Polym. Sci. Part A-Polym. Chem. 2002, 40, 3221–3231. [Google Scholar] [CrossRef]
- Zhang, H.M.; Guo, W.Z.; Chen, T.L. Synthesis, characterization and ring-opening polymerization of cyclic (arylene phosphonate) oligomers. Chin. J. Polym. Sci. 2003, 22, 83–89. [Google Scholar]
- Myrex, R.D.; Farmer, B.; Gray, G.M. 31P and 1H-NMR studies of transesterification polymerization of polyphosphonate oligomers. Eur. Polym. J. 2003, 39, 1105–1115. [Google Scholar] [CrossRef]
- Shoba, H.K.; Johnson, H.; Sankarapandian, Y.S.; Kim, Y.S.; Rangarajan, P.; Baird, D.G.; McGrath, J.E. Synthesis of high refractive-index melt-stable aromatic polyphosphonates. J. Polym. Sci. A Polym. Chem. 2001, 39, 2904–2910. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Chen, Z.T. Synthesis, characterization, and thermal properties of phosphorus-conatining, wholly aromatic thermotropic copolyesters. J. Appl. Polym. Sci. 2002, 86, 1278–1284. [Google Scholar] [CrossRef]
- Sentil, S.; Kannan, P. Novel thermotropic liquid crystalyne polyphosphonates. Polymer 2004, 45, 3609–3615. [Google Scholar] [CrossRef]
- Roy, S.; Maiti, S. Synthesis and characterization of a new polyphosphonate for structure-flammability relationship towards group contribution approach. J. Polym. Mater. 2004, 21, 39–48. [Google Scholar]
- Senthil, S.; Kannan, P. Synthesis and characterization of liqud-liquid-chrystaline polyphosphonates containing disubstituted ferrocene esters as mesogenes. J. Polym. Sci. A Polym. Chem. 2002, 40, 2256–2263. [Google Scholar] [CrossRef]
- Narendran, N.; Kishore, K. Studies on polyphosophate esters synthesis. J. Appl. Polym. Sci. 2003, 87, 626–631. [Google Scholar] [CrossRef]
- Ranganathan, T.; Zilberman, J.; Farris, R.J.; Coughlin, E.B.; Emrick, T. Synthesis and characterization of halogen-free antiflammable polyphosphonates containing 4,4'-bishydroxybenzoin. Macromolecules 2006, 39, 5974–5975. [Google Scholar] [CrossRef]
- Iliescu, S.; Ilia, G.; Popa, A.; Dehelean, G.; Macarie, L.; Păcureanu, L. The study of the liquid-liquid interfacial polycondensation of the cyclohexylphosphonic dichloride with bisphenol A.The influnce of reaction temperature, reagents molar-ratio, phase-transfer catalyst and stirring speed. Rev. Roum. Chim. 2003, 48, 639–643. [Google Scholar]
- Iliescu, S.; Ilia, G.; Plesu, N.; Popa, A.; Pascariu, A. Solvent and catalyst-free synthesis of polyphosphates. Green Chem. 2006, 8, 727–730. [Google Scholar] [CrossRef]
- Iliescu, S.; Avram, E.; Visa, A.; Plesu, N.; Popa, A.; Ilia, G. New technique for the synthesis of polyphosphoesters. Macromol. Res. 2011, 19, 1186–1191. [Google Scholar] [CrossRef]
- Iliescu, S.; Ilia, G.; Pascariu, A. Novel synthesis of phosphorus containing polymers under inverse phase transfer catalysis. Polymer 2006, 47, 6509–6512. [Google Scholar] [CrossRef]
- Itoh, T.; Ikeda, M.; Hirata, N.; Moriya, Y.; Wen, Z.; Ichikawa, Y.; Kubo, M.; Yamamoto, O. Electrochemical and thermal properties of hyperbranched polymer electrolytes for batteries. Ionics 2002, 8, 44–52. [Google Scholar] [CrossRef]
- Ilia, G.; Iliescu, S.; Macarie, L.; Popa, A. Synthesis of tervalent phosphorus esters in biphasic system using potassium phosphate as unique solid base. Heteroatom Chem. 2008, 19, 360–364. [Google Scholar] [CrossRef]
- Thomas, L.C. Interpretation of the Infrared Spectra of Organophosphorus Compounds; Heydon: London, UK, 1974. [Google Scholar]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed; Chapman and Hall: London, UK, 1980; Volume 2. [Google Scholar]
- Levi, G.C.; Lichter, R.I.; Nelson, G.I. Nuclear Magnetic Resonance Spectroscopy, 2nd ed; John Wiley and Sons Inc.: Hoboken, NJ, USA, 1980. [Google Scholar]
- Ewing, G.C. 13C substituent effect in monosubstituted benzenes. Org. Mag. Res. 1979, 12, 499–524. [Google Scholar] [CrossRef]
- Van Wazer, J.R. Topics in Phosphorus Chemistry; Grayson, F., Griffin, M., Eds.; John Wiley: London, UK, 1967; Volume 5. [Google Scholar]
- Van Wazer, J.R.; Callins, C.F.; Shoolery, J.N.; Jones, R.C. Principles of phosphorus chemistry. II.Nuclear magnetic resonance measurement. J. Am. Chem. Soc. 1956, 78, 5715–5726. [Google Scholar] [CrossRef]
- Carty, P.; White, S. The importance of char-forming reactions in thermoplastic polymers. Fire Mater. 1994, 18, 151–166. [Google Scholar] [CrossRef]
- Annakutty, S.; Kishore, K. A novel approach to structure—Flammability correlation in polyphosphate esters. Polymer 1988, 29, 1273–1276. [Google Scholar] [CrossRef]
- Singh, T.H.J.; Bhat, S.V. Morphology and conductivity studies of a new solid polymer eelctrolyte: (PEG)xLiClO4. Bull. Mater. Sci. 2003, 26, 707–714. [Google Scholar]
- Sample Availability: Samples of the compounds 4a–4c are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iliescu, S.; Augusti, M.-G.; Fagadar-Cosma, E.; Plesu, N.; Fagadar-Cosma, G.; Macarie, L.; Popa, A.; Ilia, G. Synthesis of New Phosphorus-Containing (Co)Polyesters Using Solid-Liquid Phase Transfer Catalysis and Product Characterization. Molecules 2012, 17, 9090-9103. https://doi.org/10.3390/molecules17089090
Iliescu S, Augusti M-G, Fagadar-Cosma E, Plesu N, Fagadar-Cosma G, Macarie L, Popa A, Ilia G. Synthesis of New Phosphorus-Containing (Co)Polyesters Using Solid-Liquid Phase Transfer Catalysis and Product Characterization. Molecules. 2012; 17(8):9090-9103. https://doi.org/10.3390/molecules17089090
Chicago/Turabian StyleIliescu, Smaranda, Maite-Gyl Augusti, Eugenia Fagadar-Cosma, Nicoleta Plesu, Gheorghe Fagadar-Cosma, Lavinia Macarie, Adriana Popa, and Gheorghe Ilia. 2012. "Synthesis of New Phosphorus-Containing (Co)Polyesters Using Solid-Liquid Phase Transfer Catalysis and Product Characterization" Molecules 17, no. 8: 9090-9103. https://doi.org/10.3390/molecules17089090