1. Introduction
Enhancing the solubility and dissolution behavior of poorly-water-soluble active pharmaceutical ingredients (APIs) is a key challenge in the development of new formulations. A successful strategy along this line are amorphous solid dispersions (ASDs) where the APIs are molecularly dissolved in a suitable polymer carrier and thus converted into an amorphous state [
1,
2]. Several tablet formulations based on this technology have been recently developed [
3]. Particularly, polymeric carriers of poly (
dl-lactic-co-glycolic acids) (PLGAs) are considered as particularly promising for a variety of medical applications like prolonged release tablets or for depot formulations [
4].
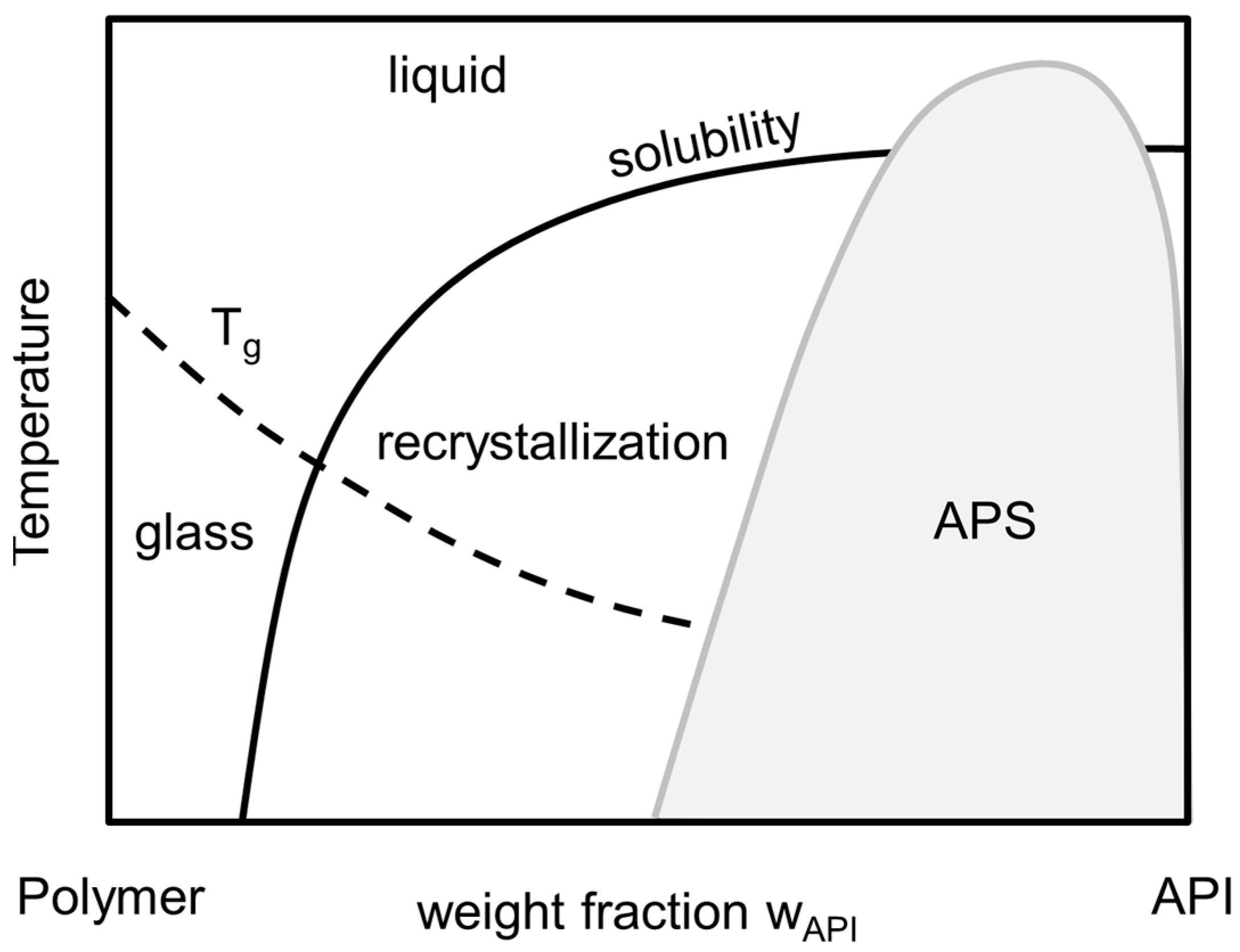
The long-term stability of these ASDs is determined by their thermodynamic phase behavior as it determines the maximum API weight fraction of the formulation (
wAPI) at which it is long-term (i.e., thermodynamically) stable against API recrystallization as well as amorphous-amorphous phase separation (APS) [
2]. The thermodynamic phase behavior of an API/polymer formulation is schematically depicted in the phase diagram, shown in
Figure 1. Here, the solubility line indicates the composition/temperature range above which an ASD is thermodynamically stable against crystallization: for a given temperature, ASDs are stable against crystallization as long as their composition is left of the solubility line. In addition, the APS region in
Figure 1 corresponds to compositions/temperatures where the formation of two liquid (amorphous) phases with different compositions of API and polymer is expected. From thermodynamic point of view, this is a liquid-liquid demixing. This immiscibility is—like crystallization—highly unwanted as it causes heterogeneities resulting in insufficient uniformity of API content in the formulation. For a given temperature, the corresponding concentrations of the two liquid phases can be found on the left and right branches of the APS curve. At conditions below both, the APS line as well as the solubility line, APS can be followed or accompanied by recrystallization, which is not considered here.
The glass-transition temperature (
Tg) depicts the transition temperature from the super-cooled, glassy state of the formulation to its the liquid state. It can be correlated for using empirical approaches, e.g., the Gordon-Taylor-Equation [
5] or its modification by Kwei [
6]. Although the glass-transition temperature does not mark a thermodynamic phase transition, it indicates a drastic change in mobility and viscosity which usually dramatically decreases the rate of crystallization and/or phase separation and therewith at least kinetically stabilizes ASDs even at thermodynamically-metastable conditions.
Formulations which undergo APS usually show two distinguishable glass-transition temperatures as long as the pure component glass-transition temperatures differ to a certain extend [
7]. The presence of two glass-transition temperatures is therefore considered as the main qualitative proof for the immiscibility of API/polymer formulations [
8], polymer blends [
9,
10,
11] and amorphous mixtures of APIs and small-molecule excipients, e.g., indomethacin/citric acid [
12]. However, due to the high viscosity of the formulations, demixing may take very long time and therefore the quantitative analysis of APS and determining the equilibrium compositions of the two amorphous phases is quite challenging.
Instead, cloud-point measurements have been used to evaluate APS of polymer (API-free) systems. Koningsveld cooled polymer/solvent mixtures of certain feed compositions until turbidity was detected visually [
13], which is the proof of reaching the APS region in the phase diagram. Immiscibility of solvent-free and API-free polymer blends has also been qualitatively evaluated by atomic force microscopy [
14], X-ray powder diffraction [
15], and micro Raman mapping [
11]. Purohit and Taylor applied atomic-force microscopy combined with nanoscale infrared imaging to qualitatively evaluate the immiscibility in the system itraconazole/hydroxypropyl methylcellulose [
16].
However, none of these studies quantitatively determined the API concentration in the two APS phases as a function of temperature nor even focused on the prediction of APS. For several systems, even contradictory information exists about the existence of APS. Yuan et al. experimentally found an APS in quench-cooled formulations of nifedipine and PVP [
17], which was also predicted by Donnelly et al. [
18] applying the Flory-Huggins Theory (FHT [
19]), although earlier studies [
20,
21] found complete miscibility of nifedipine and PVP. Thus, there is still an urgent need for reliably determining APS in API/polymer systems experimentally and for theoretical methods being able to correctly predict APS. Whereas the solubility of crystalline APIs was already modeled using adequate thermodynamic models, e.g., FHT [
22,
23,
24], the Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) [
25] or empirical approaches [
26], thermodynamic modeling of APS is quite rare. Lin and Huang [
7] experimentally investigated the APS in the system felodipine (FEL) and poly (acrylic acid) which they qualitatively modeled using FHT and a χ-interaction parameter fitted to the crystalline FEL solubility in poly(acrylic acid) obtained from DSC measurements. However, they considered their calculated phase diagram only as a ‘rough draft’ as FHT is a very simplified thermodynamic model for which already Flory [
19] pointed out that it is unable to correctly describe demixing phenomena (like APS).
Therefore, in this work the Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) [
27] was used instead to thermodynamically model solubility and APS in API/polymer formulations. PC-SAFT has already been applied to model the solubility of APIs in organic solvents [
28] and the dissolution of APIs in aqueous media [
29,
30,
31,
32]. Moreover, it has been shown that PC-SAFT is suitable for describing the solubility of APIs in water-free and humid ASDs [
25,
33,
34,
35]. Kiesow et al. successfully predicted the liquid-liquid demixing (oiling out) during API crystallization processes from solvents and could show that PC-SAFT is able to simultaneously model both, solubility and APS (liquid-liquid demixing) in pharmaceutical systems [
36].
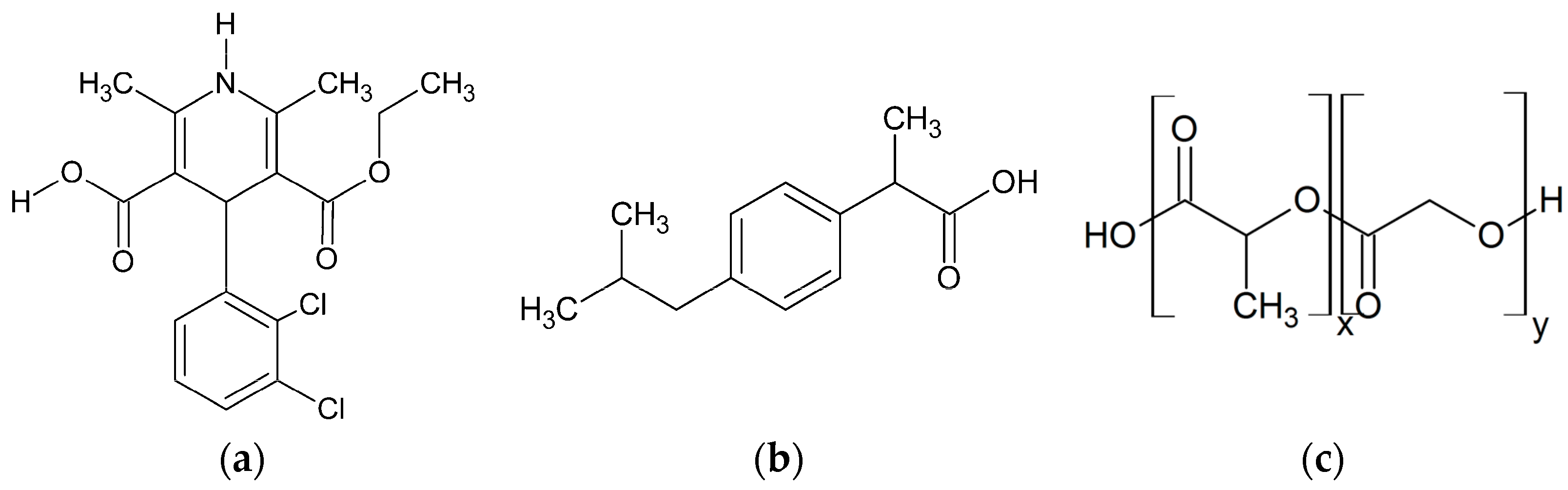
This study investigates the phase behavior of ibuprofen (IBU) and FEL formulations in PLGA-polymers with different molar ratios of (
dl)-lactic acid (LA):glycolic acid (GA)-monomers (
Figure 2).
The solubility of the crystalline APIs was determined by measuring the solubility temperature of formulations with different API weight fractions via DSC [
20]. APS was detected qualitatively by hot-stage microscopy (HSM) and the detection of two glass-transition temperatures in DSC measurements. The experimentally-obtained information about APS was then compared to the PC-SAFT predictions.
6. Conclusions
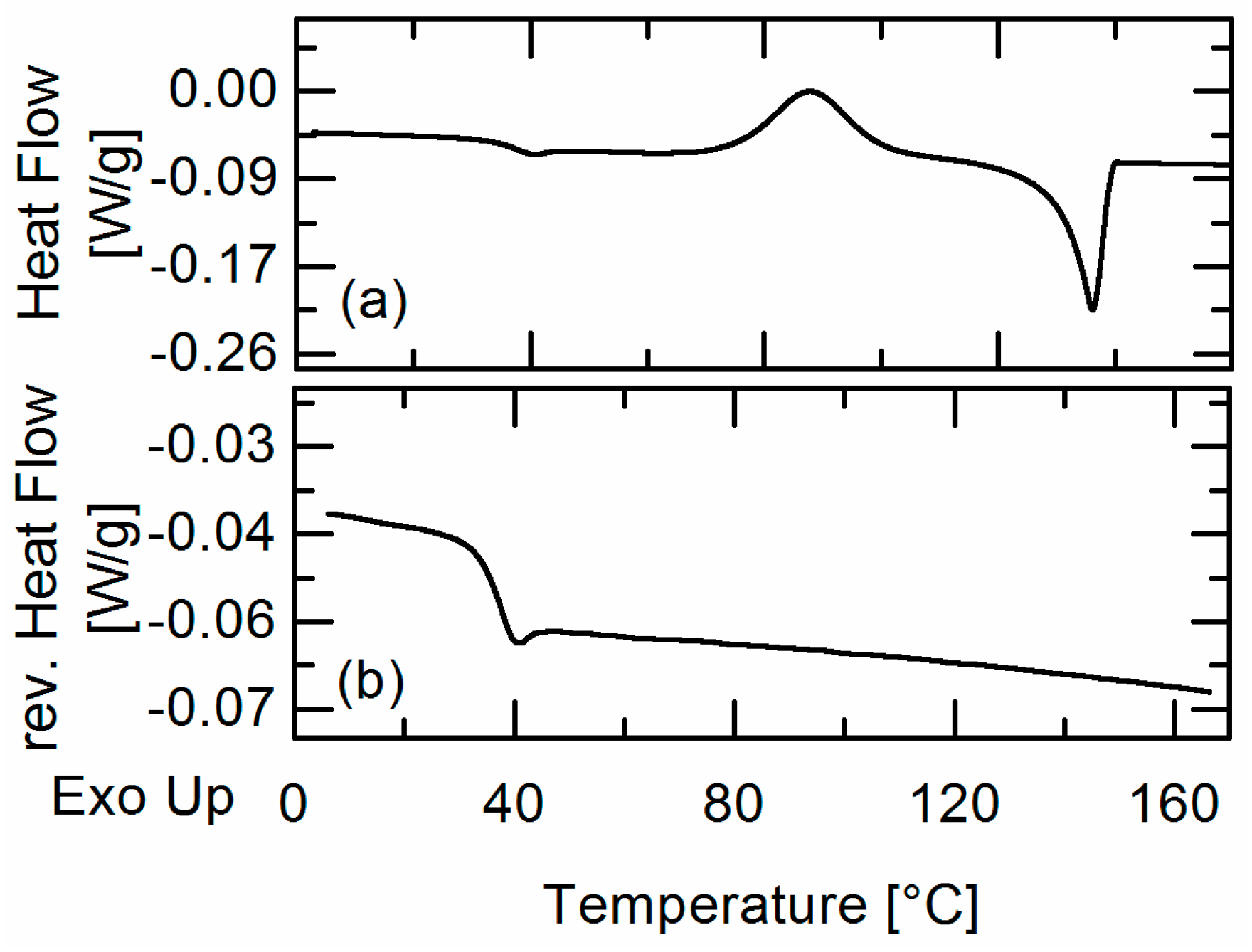
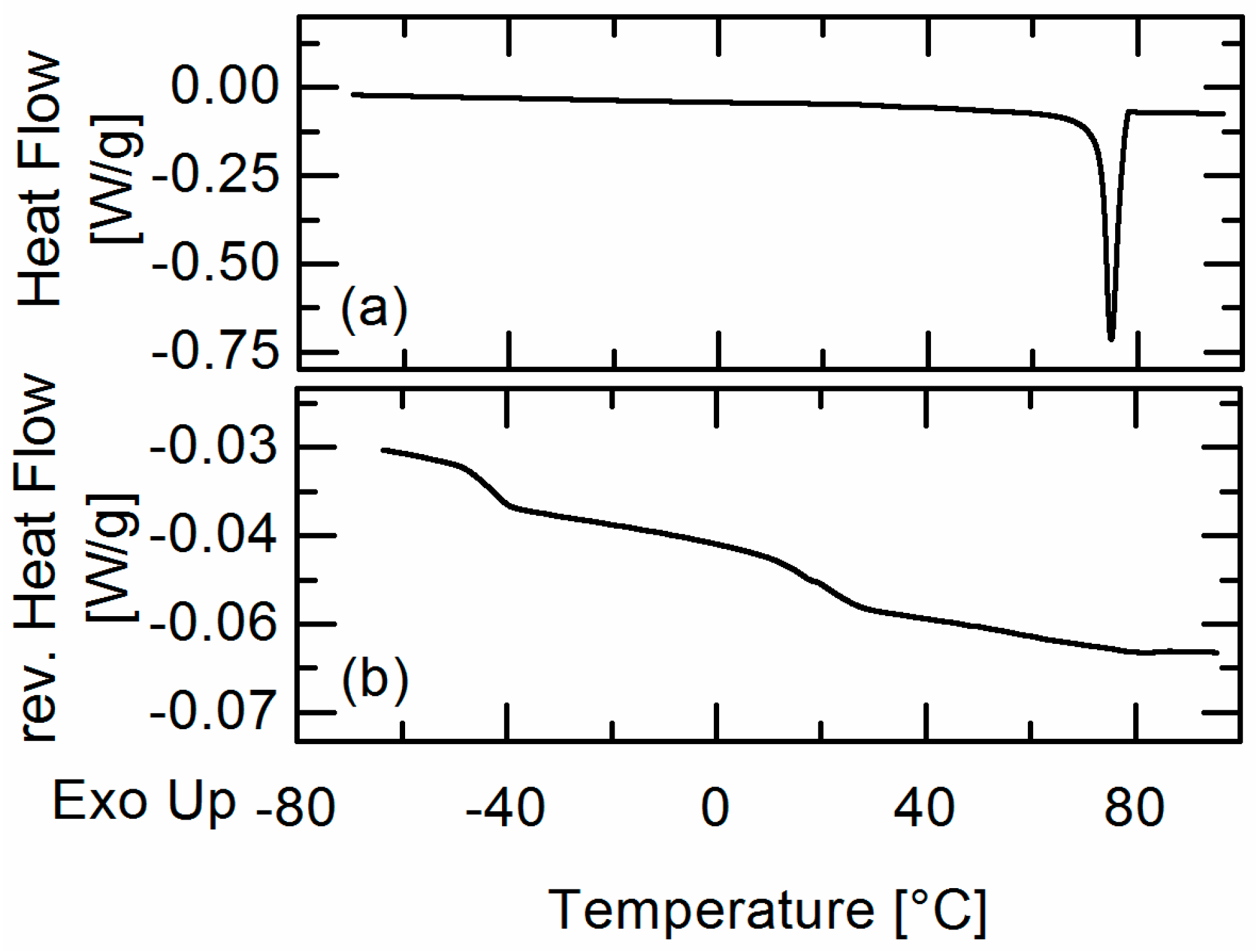
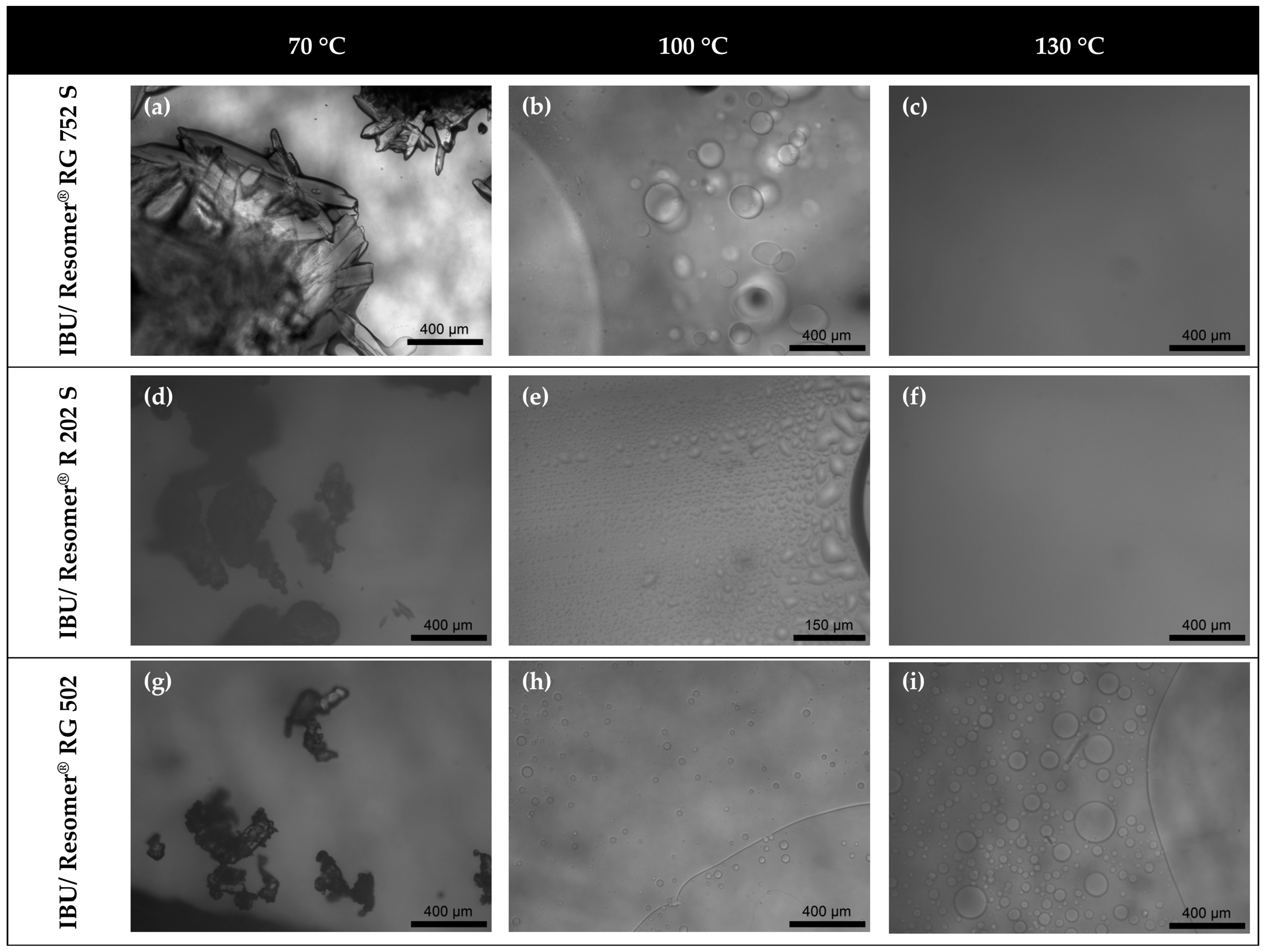
In this work, amorphous-amorphous phase separation in API/PLGA formulations was investigated via DSC measurements and HSM. Two glass-transition temperatures in the DSC measurements and visible droplets in the HSM images were detected for IBU/Resomer® systems showing these systems undergo APS in the considered temperature range. In contrast, no APS was found for FEL/Resomer® systems.
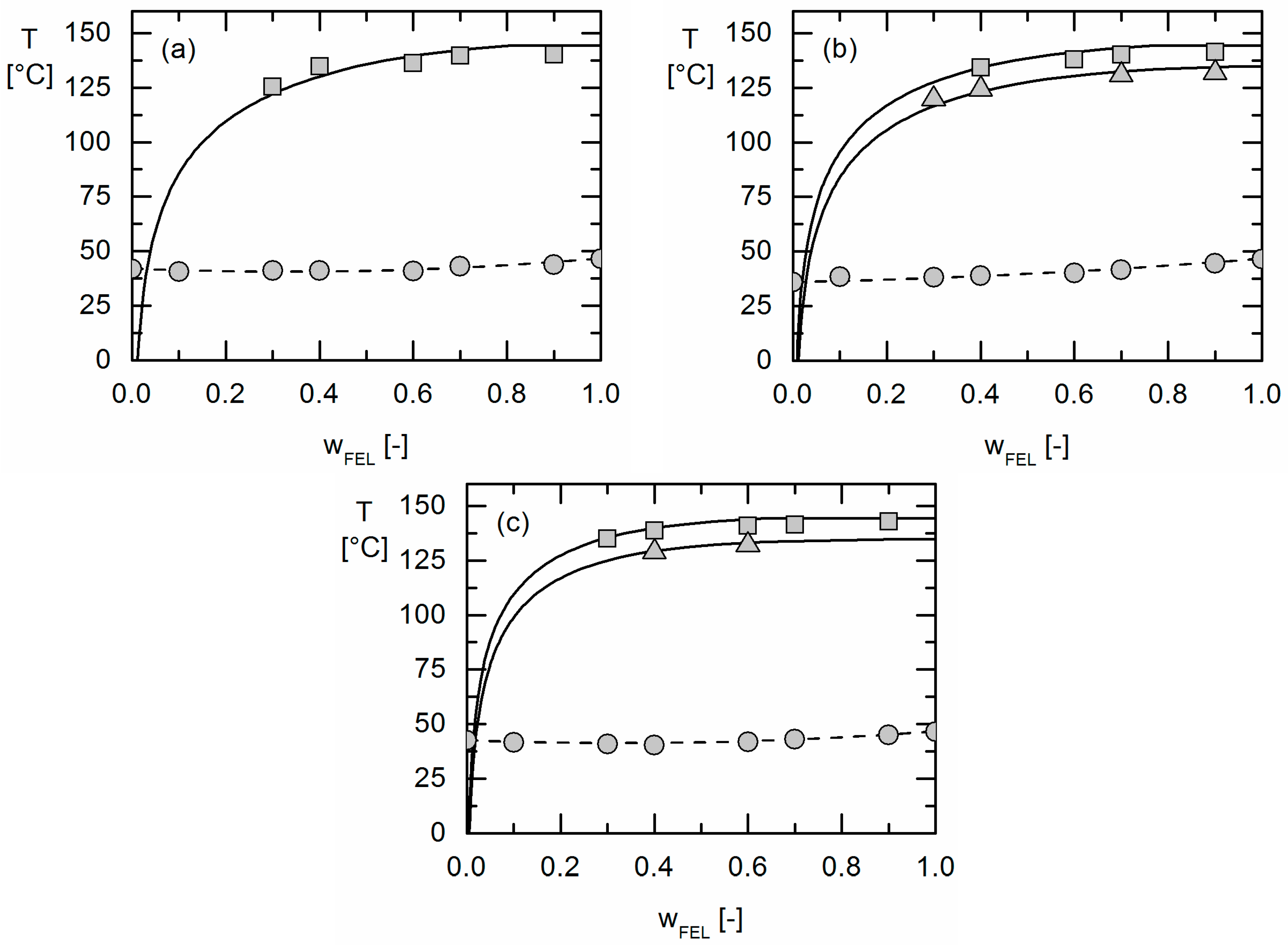
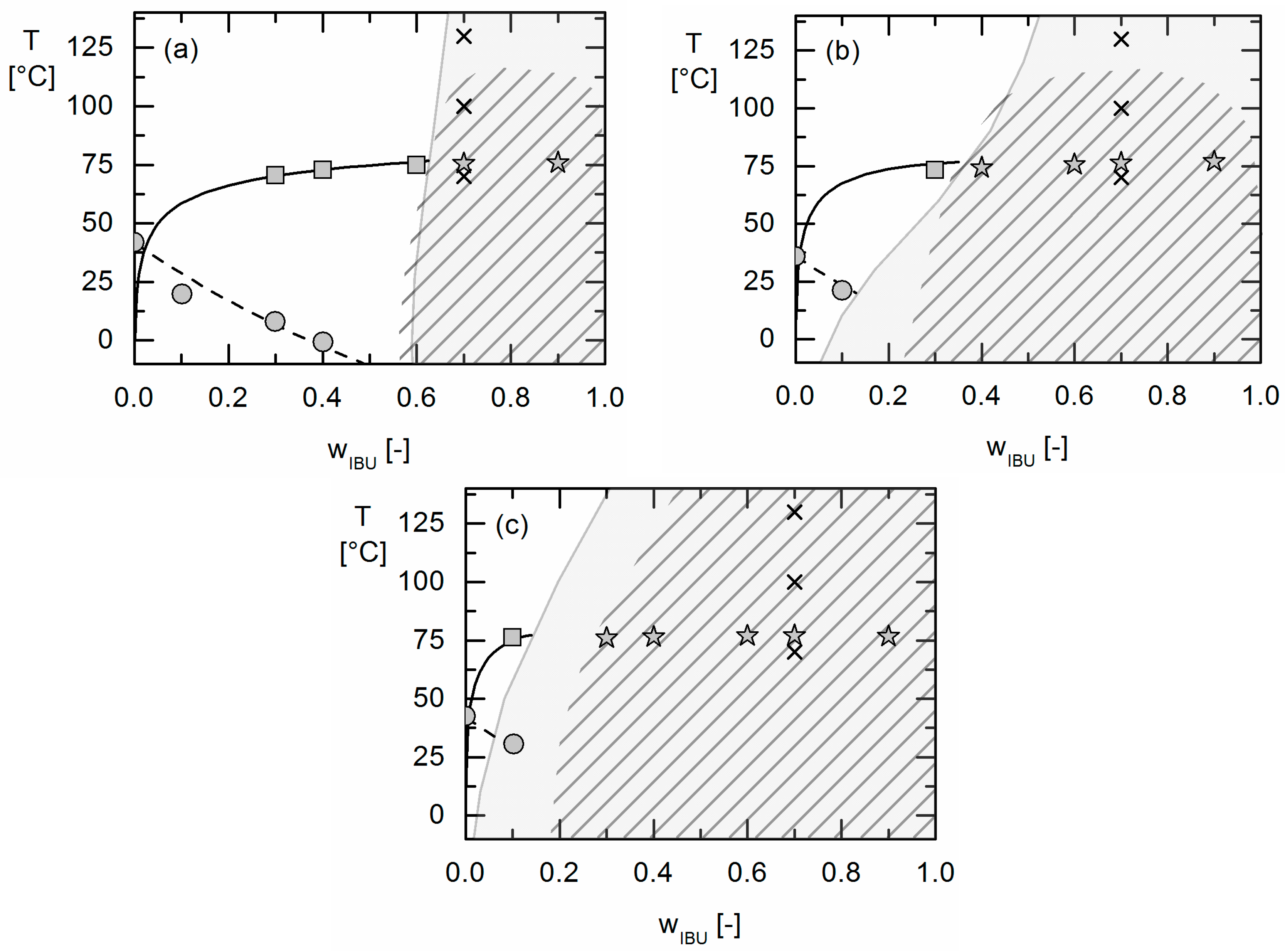
The solubility of crystalline APIs in Resomers® could be modeled precisely using the thermodynamic model PC-SAFT by fitting one binary interaction parameter to measured solubility data. Using these parameters fitted to solubility data only, the presence or absence of APS in these API/polymer formulations was purely predicted using PC-SAFT. As a result, the existence of APS was blindly predicted for the investigated IBU/Resomer® formulations whereas a homogeneous amorphous phase was predicted for the FEL/Resomer® formulations. These predictions were validated by the experimental findings of this work. This is remarkable as melting point depression caused by the Resomers® was very similar for IBU and FEL. Moreover, it was found that formulations with high IBU weight fractions undergo APS more likely than those with small IBU loadings.
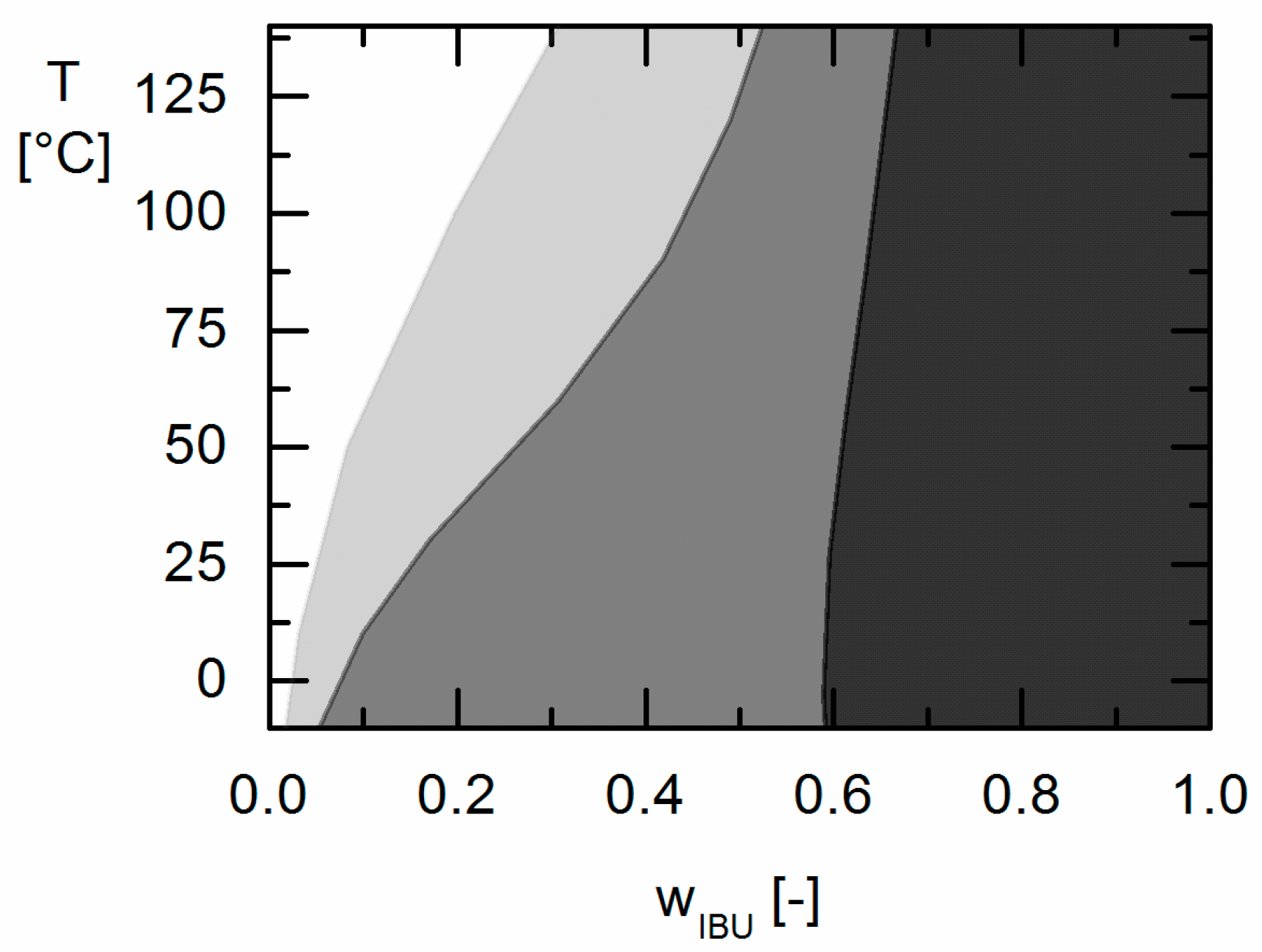
Moreover, the influence of different GA contents in the Resomers® on APS formation was predicted in qualitative and even almost quantitative agreement with the experimental data. While the increasing GA content influences the solubility (crystal formation) at room temperature only slightly, it strongly affects the APS region leading to more extended APS regions with increasing GA content in the Resomer® (biggest for Resomer® RG 502).
Using thermodynamic phase diagrams allows for estimating the processability window of API/PLGA formulations as well its long-term behaviour during storage. Processing formulations with API loadings in the APS region might cause APS already at extruder conditions or after long-term storage (even at dry conditions) resulting in non-homogeneous formulations with undesired release performance and unpredictable bioavailability. By applying thermodynamic modeling using PC-SAFT, APS can be estimated at conditions relevant for processing (above the melting point of the API in the case of extrusion processes) or long-term storage (25 °C and 40 °C).
As a result of this work evidently low the GA contents in the copolymer (as for Resomer® R 202 S) and low IBU contents (e.g., below wIBU = 0.595 in IBU/Resomer® R 202 S formulations) in IBU/Resomer® prevent APS during processing and/or long-term storage.