Site-Specific Labeling of Proteins with Near-IR Heptamethine Cyanine Dyes
Abstract
1. Introduction
2. Results and Discussion
2.1. Syntheses of Amino-Acid-Substituted Cy-7 Dyes
2.2. Optical Properties of Amino-Acid-Substituted Cy-7 Dyes
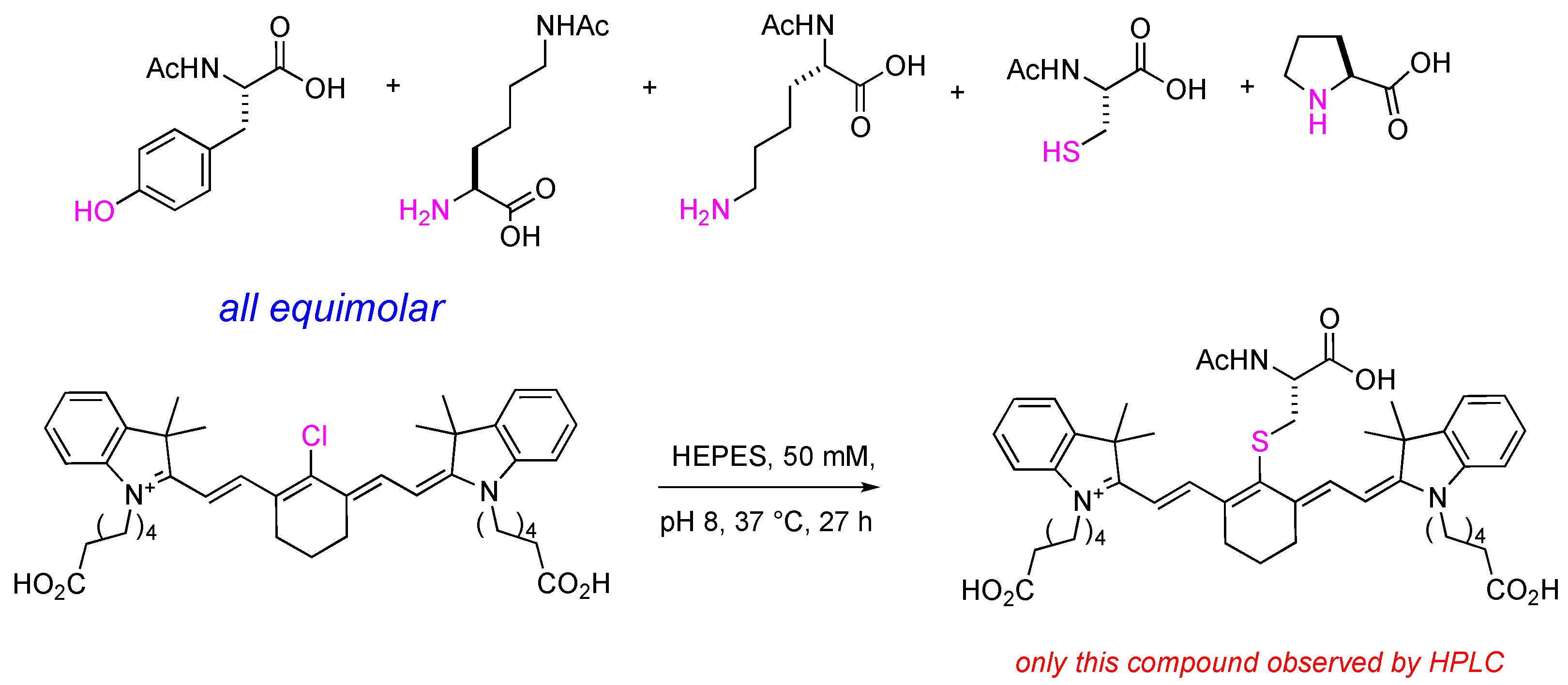
2.3. Meso-Cl Functionality of Cy-7 Dyes Is Essential for Cys-Selective Protein Labeling
2.4. Labeling of Other Proteins Using MHI-148
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization
3.3. UV-Vis and Fluorescence Analysis
3.4. NIR Gel Image Protocol
3.5. Preparation of Thiol-Blocked Vimentin
3.6. Kinetic Study of MHI-148 with Amino Acids in Aqueous Buffer
3.7. NIR Gel Image of MHI-148 with Different Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, C.; Wu Jason, B.; Pan, D. Review on near-infrared heptamethine cyanine dyes as theranostic agents for tumor imaging, targeting, and photodynamic therapy. J. Biomed. Opt. 2016, 21, 50901. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, B.E.; Mieog, J.S.D.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.J.K.; Loewik, C.W.G.M.; Frangioni, J.V.; van de Velde, C.J.H.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Patila, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Valisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, R.; Onda, N.; Yamashita, S.; Kojima, M.; Inohana, M.; Eguchi, A.; Nakamura, M.; Matsumoto, S.; Yoshida, T.; Shibutani, M. Fluorescence tumor imaging by i.v. administered indocyanine green in a mouse model of colitis-associated colon cancer. Cancer Sci. 2018, 109, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- James, N.S.; Chen, Y.; Joshi, P.; Ohulchanskyy, T.Y.; Ethirajan, M.; Henary, M.; Strekowski, L.; Pandey, R.K. Evaluation of polymethine dyes as potential probes for near infrared fluorescence imaging of tumors: Part-1. Theranostics 2013, 3, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, C.; Tong, R.; Qian, W.; Zhau, H.E.; Wang, R.; Zhu, G.; Cheng, J.; Yang, V.W.; Cheng, T.; et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin. Cancer Res. 2010, 16, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
- Henary, M.; Pannu, V.; Owens, E.A.; Aneja, R. Near infrared active heptacyanine dyes with unique cancer-imaging and cytotoxic properties. Bioorg. Med. Chem. Lett. 2012, 22, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yi, X.; Yan, F.; Wang, F.; Qin, W.; Wu, G.; Yang, X.; Shao, C.; Chung, L.W.K. Near-infrared fluorescence imaging of prostate cancer using heptamethine carbocyanine dyes. Mol. Med. Rep. 2015, 11, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, C.; Zhao, Y.; Bai, B.; An, J.; Zhang, H.; Shi, C.; Wu Jason, B. Optical imaging of gastric cancer with near-infrared heptamethine carbocyanine fluorescence dyes. Oncotarget 2016, 7, 57277–57289. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shao, C.; Wang, R.; Chu, C.-Y.; Hu, P.; Master, V.; Osunkoya, A.O.; Kim, H.L.; Zhau, H.E.; Chung, L.W.K. Optical imaging of kidney cancer with novel near infrared heptamethine carbocyanine fluorescent dyes. J. Urol. 2013, 189, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, Y.; Zhang, H.; Chen, X.; Zhao, N.; Tan, D.; Zhang, H.; Shi, C. The application of heptamethine cyanine Dye DZ-1 and indocyanine green for imaging and targeting in xenograft models of hepatocellular carcinoma. Int. J. Mol. Sci. 2017, 18, 1332. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Lin, T.-P.; Gallagher, J.D.; Kushal, S.; Chung, L.W.K.; Zhau, H.E.; Olenyuk, B.Z.; Shih, J.C. Monoamine oxidase a inhibitor-near-infrared dye conjugate reduces prostate tumor growth. J. Am. Chem. Soc. 2015, 137, 2366–2374. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Shi, C.; Chu, G.C.-Y.; Xu, Q.; Zhang, Y.; Li, Q.; Yu, J.S.; Zhau, H.E.; Chung, L.W.K. Near-infrared fluorescence heptamethine carbocyanine dyes mediate imaging and targeted drug delivery for human brain tumor. Biomaterials 2015, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhang, Y.; Xiao, L.; Li, J.; Wang, J.-P.; Chordia, M.D.; Liu, Z.-Q.; Chung, L.W.K.; Yue, W.; Pan, D. Improving therapeutic potential of farnesylthiosalicylic acid: Tumor specific delivery via conjugation with heptamethine cyanine dye. Mol. Pharm. 2017, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Yang, X.; Wang, M.; Yang, J.; Qin, Z.; Kan, Q.; Zhang, H.; Wang, Y.; Wang, D.; He, Z. Mitochondria-targeted prostate cancer therapy using a near-infrared fluorescence dye-monoamine oxidase A inhibitor conjugate. J. Control. Release 2018, 279, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Strekowski, L.; Lipowska, M.; Patonay, G. Facile derivatizations of heptamethine cyanine dyes. Synth. Commun. 1992, 22, 2593–2598. [Google Scholar] [CrossRef]
- Strekowski, L.; Lipowska, M.; Patonay, G. Substitution reactions of a nucleofugal group in heptamethine cyanine dyes. Synthesis of an isothiocyanato derivative for labeling of proteins with a near-infrared chromophore. J. Org. Chem. 1992, 57, 4578–4580. [Google Scholar] [CrossRef]
- Cha, J.; Nani, R.R.; Luciano, M.P.; Broch, A.; Kim, K.; Namgoong, J.-M.; Kulkarni, R.A.; Meier, J.L.; Kim, P.; Schnermann, M.J. A chemically stable fluorescent marker of the ureter. Bioorg. Med. Chem. Lett. 2018, 28, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Nani, R.R.; Shaum, J.B.; Gorka, A.P.; Schnermann, M.J. Electrophile-integrating smiles rearrangement provides previously inaccessible C4′-O-alkyl heptamethine cyanine fluorophores. Org. Lett. 2015, 17, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Vendrell, M.; Das, R.; Chang, Y.T. Development of photostable near-infrared cyanine dyes. Chem. Commun. (Camb) 2010, 46, 7406–7408. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Samanta, A.; Ha, H.H.; Chang, Y.T. Solid phase synthesis of ultra-photostable cyanine NIR dye library. RSC Adv. 2011, 1, 573–575. [Google Scholar] [CrossRef]
- Pascal, S.; Haefele, A.; Monnereau, C.; Charaf-Eddin, A.; Jacquemin, D.; Le Guennic, B.; Andraud, C.; Maury, O. Expanding the polymethine paradigm: Evidence for the contribution of a bis-dipolar electronic structure. J. Phys. Chem. A 2014, 118, 4038–4047. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhu, W.; Zhu, M.; Wu, X.; Tian, H. Near-infrared cell-permeable Hg2+-selective ratiometric fluorescent chemodosimeters and fast indicator paper for MeHg+ based on tricarbocyanines. Chem. Eur. J. 2010, 16, 14424–14432. [Google Scholar] [CrossRef] [PubMed]
- Njiojob, C.N.; Owens, E.A.; Narayana, L.; Hyun, H.; Choi, H.S.; Henary, M. Tailored near-infrared contrast agents for image guided surgery. J. Med. Chem. 2015, 58, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Song, F.; Lu, E.; Wang, Y.; Zhou, W.; Fan, J.; Gao, Y. Heptamethine cyanine dyes with a large Stokes shift and strong fluorescence: A paradigm for excited-state intramolecular charge transfer. J. Am. Chem. Soc. 2005, 127, 4170–4171. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, A.; Fichtner, I.; Garmann, D.; Jaehde, U.; Kratz, F. Synthesis and biological activity of water-soluble maleimide derivatives of the anticancer drug carboplatin designed as albumin-binding prodrugs. Bioconjugate Chem. 2004, 15, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Nanda, J.S.; Lorsch, J.R. Labeling of a protein with fluorophores using maleimide derivitization. In Methods Enzymol; Lorsch, J.R., Ed.; Elsevier: San Diego, CA, USA, 2014; Volume 536, pp. 79–86. [Google Scholar]
- Walden, H.; Podgorski, M.S.; Huang, D.T.; Miller, D.W.; Howard, R.J.; Minor, D.L., Jr.; Holton, J.M.; Schulman, B.A. The structure of the APPBP1-UBA3-NEDD8-ATP complex reveals the basis for selective ubiquitin-like protein activation by an E1. Mol. Cell 2003, 12, 1427–1437. [Google Scholar] [CrossRef]
- Huang, D.T.; Miller, D.W.; Mathew, R.; Cassell, R.; Holton, J.M.; Roussel, M.F.; Schulman, B.A. A unique E1-E2 interaction required for optimal conjugation of the ubiquitin-like protein NEDD8. Nat. Struct. Mol. Biol. 2004, 11, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Danley, D.E.; Geoghegan, K.F.; Griffor, M.C.; Hawkins, J.L.; Subashi, T.A.; Varghese, A.H.; Ammirati, M.J.; Culp, J.S.; Hoth, L.R.; et al. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat. Struct. Mol. Biol. 2007, 14, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Llinas, P.; Le Du, M.H.; Gardsvoll, H.; Dano, K.; Ploug, M.; Gilquin, B.; Stura, E.A.; Menez, A. Crystal structure of the human urokinase plasminogen activator receptor bound to an antagonist peptide. Embo J. 2005, 24, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, H.; Ishitani, R.; Nureki, O.; Fukai, S.; Yamanaka, M.; Kim, J.-H.; Saito, K.; Sakamoto, A.; Inoue, M.; Shirouzu, M.; et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 2002, 110, 775–787. [Google Scholar] [CrossRef]
- Usama, S.M.; Thavornpradit, S.; Burgess, K. Optimized Heptamethine Cyanines for Photodynamic Therapy. ACS Appl. Bio Mater. 2018, 1, 1195–1205. [Google Scholar] [CrossRef]
- Usama, S.M.; Lin, C.-M.; Burgess, K. On the mechanisms of update of tumor-seeking cyanine dyes. Bioconjugate Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Gorka, A.P.; Nagaya, T.; Michie, M.S.; Nakamura, Y.; Nani, R.R.; Coble, V.L.; Vasalatiy, O.V.; Swenson, R.E.; Choyke, P.L.; et al. Effect of charge localization on the in vivo optical imaging properties of near-infrared cyanine dye/monoclonal antibody conjugates. Mol. BioSyst. 2016, 12, 3046–3056. [Google Scholar] [CrossRef] [PubMed]
- Nani, R.R.; Gorka, A.P.; Nagaya, T.; Kobayashi, H.; Schnermann, M.J. Near-IR light-mediated cleavage of antibody-drug conjugates using cyanine photocages. Angew. Chem. Int. Ed. 2015, 54, 13635–13638. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1a–d are available from the authors. |







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-M.; Usama, S.M.; Burgess, K. Site-Specific Labeling of Proteins with Near-IR Heptamethine Cyanine Dyes. Molecules 2018, 23, 2900. https://doi.org/10.3390/molecules23112900
Lin C-M, Usama SM, Burgess K. Site-Specific Labeling of Proteins with Near-IR Heptamethine Cyanine Dyes. Molecules. 2018; 23(11):2900. https://doi.org/10.3390/molecules23112900
Chicago/Turabian StyleLin, Chen-Ming, Syed Muhammad Usama, and Kevin Burgess. 2018. "Site-Specific Labeling of Proteins with Near-IR Heptamethine Cyanine Dyes" Molecules 23, no. 11: 2900. https://doi.org/10.3390/molecules23112900
APA StyleLin, C.-M., Usama, S. M., & Burgess, K. (2018). Site-Specific Labeling of Proteins with Near-IR Heptamethine Cyanine Dyes. Molecules, 23(11), 2900. https://doi.org/10.3390/molecules23112900





