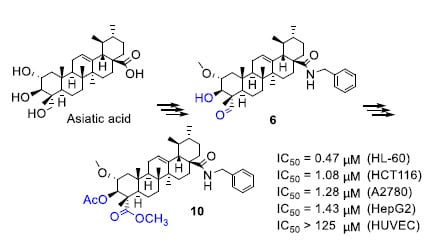
3.1. Chemistry
3.1.1. General
Asiatic acid was purchased from Xi’an Shouhe Biotechnology Co., Ltd. All reagents and solvents were obtained from Sinopharm. IR spectra were recorded on a THERMO IS5. NMR spectra (1H, 13C, DEPT, HSQC and HMBC) were recorded on a Bruker Ascend-400 400 MHz or Bruker Ascend-500 500 MHz at room temperature. HRMS were recorded on an Agilent 6530-Q-TOF mass spectrometer equipped with an Agilent 1260-HPLC.
3.1.2. α-Hydroxy-3β,23-Isopropylidenedioxy-Urs-12-Ene-28-Oic Acid (1)
Under a nitrogen atmosphere, AA (480 mg, 1 mmol) was dissolved in dry acetone (50 mL). 2,2-dimethoxypropane (150 μL, 1.2 mmol) and TsOH·H
2O (17 mg, 0.1 mmol) was added, stirring at room temperature overnight. Evaporated 2,2-dimethoxypropane and acetone, neutralized the reaction mixture with a saturated solution of sodium bicarbonate and extracted the solution with DCM (dichloromethane). The organic phase was washed with brine, dried over Na
2SO
4 and concentrated to dryness under vacuum to obtain the crude product
1 as a white solid (449 mg, 85%). IR (CH
2Cl
2, cm
−1) ν
max: 1033, 1190, 1391, 1454, 1552, 1648, 1689, 2871, 2939, 3199, and 3466 [
22].
1H-NMR (400 MHz, CDCl
3) δ 5.25 (t,
J = 3.6 Hz, 1H, H-12), 3.81 (td,
J = 10.2, 4.5 Hz, 1H, H-2), 3.58–3.44 (m, 2H, H-23), 3.34 (d,
J = 9.6 Hz, 1H, H-3), 1.27 (d,
J = 2.2 Hz, 3H), 1.11 (s, 3H), 1.07 (s, 3H), 1.06 (s, 3H), 0.87 (d,
J = 6.4 Hz, 3H), 0.76 (s, 3H).
13C-NMR (101 MHz, CDCl
3) δ 183.63 (C-28), 137.85 (C-13), 125.45 (C-12), 99.65 (3β,23-ketal), 82.10 (C-3), 72.67 (C-23), 65.26 (C-2), 52.44, 51.38, 47.87, 47.57, 46.49, 41.94, 39.55, 39.01, 38.79, 38.01, 36.92, 36.66, 32.30, 30.57, 29.74, 27.92, 23.97, 23.67, 23.15, 21.20, 19.43, 17.96, 17.50, 16.99, 16.95, 13.49. HRMS (ESI) observed C
33H
51O
5 527.3742 (M-H
−) requires 527.3742.
3.1.3. α-Hydroxy-3β,23-Isopropylidenedioxy-Urs-12-Ene-28-Benzylamide (2)
To a solution of compound 1 (1.1 g, 2 mmol) in dry DMF (30 mL), HATU (1.17 g, 3 mmol), benzyl amine (0.5 mL, 4 mmol) and DIPEA (65 μL, 4 mmol) was added, stirring at 60 °C overnight. The reaction mixture was poured into the brine, and extracted with ethyl acetate (100 mL × 3). The organic phase was washed with brine (200 mL × 3), dried over Na2SO4 and concentrated to dryness under vacuum. The crude product was purified by column chromatography over silica gel ((200–300 mesh) using hexane/AcOEt (5:2) to afford compound 2 as a white solid (766 mg, 62%). IR (CH2Cl2, cm−1) νmax: 3367, 2920 (C=C-H), 1639 (C=O), 1520 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.18 (m, 1H, NH-28), 5.24 (t, J = 3.5 Hz, 1H, C-12), 4.51 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.15 (dd, J = 14.8, 4.7 Hz, 1H, CH2-N-28), 4.10 (q, J = 7.2 Hz, 1H), 3.79 (td, J = 10.3, 4.5 Hz, 1H, H-2), 3.59–3.44 (m, 2H, H-23), 3.32 (d, J = 9.6 Hz, 1H, H-3), 2.06 (s, 1H), 1.12 (s, 3H), 1.09 (s, 3H), 1.01 (s, 3H), 0.86 (d, J = 6.5 Hz, 3H), 0.69 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.80 (C-28), 139.76, 138.31, 128.67, 127.89, 127.41, 125.39 (C-12), 99.60 (3β,23-ketal), 82.06 (C-3), 72.64 (C-23), 65.21 (C-2), 53.96, 51.34, 47.72, 47.56, 46.53, 43.68, 42.50, 39.75, 39.60, 39.06, 37.89, 37.20, 36.89, 32.19, 30.86, 29.75, 27.83, 24.80, 23.33, 23.18, 21.21, 19.41, 17.92, 17.47, 17.19, 16.90, 13.51. HRMS (ESI) observed C40H60NO4 618.4518 (MH+) requires 618.4517.
3.1.4. α-Methoxy-3β,23-Isopropylidenedioxy-Urs-12-Ene-28-Benzylamide (3)
Under a nitrogen atmosphere, compound 2 (3.3 g, 5.3 mmol) was dissolved in dry DMF (20 mL), and NaH (120 mg, 8 mmol) was added at 0 °C. After stirring for 20 min, CH3I (1.6 mL 25 mmol) was added in room temperature and stirring was continued for 30 min. The reaction mixture was poured into the brine, and extracted with ethyl acetate (100 mL × 3). The organic phase was washed with brine (200 mL × 3), dried over Na2SO4 and concentrated to dryness under vacuum to obtain the crude product 3 as a white solid (3.25 g, 97%). IR (CH2Cl2, cm−1) νmax: 3376, 2921 (C=C-H), 1640 (C=O), 1522 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.17 (t, J = 5.3 Hz, 1H, NH-28), 5.22 (t, J = 3.5 Hz, 1H, H-12), 4.52 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.18 (dd, J = 14.5, 4.5 Hz, 1H, CH2-N-28), 3.54–3.43 (m, 3H, H-23 and H-3), 3.41 (s, 3H, OCH3-2), 3.36 (td, J = 10.2, 4.2 Hz, 1H, H-2), 2.07 (dd, J = 12.6, 4.4 Hz, 1H), 1.09 (d, J = 1.6 Hz, 6H), 0.99 (s, 3H), 0.67 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.70 (C-28), 139.75, 138.35, 128.63, 127.89, 127.37, 125.33 (C-12), 99.16 (3β,23-ketal), 81.44 (C-3), 74.32 (C-2), 72.62 (C-23), 57.63 (OCH3-2), 53.92, 51.00, 47.69, 47.58, 44.91, 43.66, 42.47, 39.72, 39.55, 39.05, 37.82, 37.35, 37.22, 32.20, 30.87, 29.88, 27.83, 26.91, 24.79, 23.31, 23.18, 21.22, 19.27, 17.87, 17.35, 17.18, 16.87, 13.73. HRMS (ESI) observed C41H62NO4 632.4673 (MH+) requires 632.4673.
3.1.5. α-Methoxy-3β,23-Dihydroxy-Urs-12-Ene-28-Benzylamide (4)
Compound 3 (2.5 g, 4 mmol) was stirred with 40 mL of dilute aqueous HCl (1 M) for 30 min. The reaction mixture was neutralized with a saturated solution of sodium bicarbonate and extracted with DCM. The organic phase was washed with brine, dried over Na2SO4 and concentrated to dryness under vacuum to obtain the crude product. The crude product was purified by column chromatography over silica gel ((200–300 mesh) using hexane/AcOEt (3:2) to afford compound 4 as a white solid (2.32 g, 98%). IR (CH2Cl2, cm−1) νmax: 3421, 2920 (C=C-H), 1637 (C=O), 1522 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.24–6.09 (m, 1H, NH-28), 5.24 (t, J = 3.5 Hz, 1H, H-12), 4.55 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.20 (dd, J = 14.5, 4.5 Hz, 1H,) CH2-N-28, 4.13 (q, J = 7.1 Hz, 1H), 3.64 (d, J = 10.8 Hz, 1H, H-23), 3.46 (d, J = 9.5 Hz, 1H, H-23), 3.40 (s, 3H, OCH3-2), 3.39 (dd, 1H, H-3), 3.30 (ddd, J = 11.2, 9.5, 4.3 Hz, 1H, H-2), 2.75 (br s, 3H), 2.11 (dd, J = 12.4, 4.3 Hz, 1H), 2.06 (s, 1H), 1.10 (s, 3H), 1.00 (s, 3H), 0.88 (s, 3H), 0.86 (d, J = 6.5 Hz, 3H), 0.71 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.86 (C-28), 139.97, 138.32, 128.67, 127.92, 127.41, 125.33 (C-12), 78.36 (C-2), 78.36 (C-3), 69.60 (C-23), 56.58 (OCH3-2), 53.93, 48.34, 47.73, 47.42, 43.71, 42.57, 42.36, 41.93, 39.70, 39.58, 39.06, 37.88, 37.24, 32.42, 30.86, 27.80, 24.83, 23.42, 23.34, 21.21, 18.05, 17.23, 17.19, 17.07, 12.79. HRMS (ESI) observed C38H58NO4 592.4373 (MH+) requires 592.4360.
3.1.6. α-Methoxy-3,23-Dioxo-Urs-12-Ene-28-Benzylamide (5)
Fresh DCM (50 mL × 3) suspension of Dess-Martin Periodinane (283 mg × 3, 2 mmol) added dropwise in three separate times to compound 4 (1 g, 1.7 mmol) dissolved in dry DCM (20 mL), until starting material almost consumed. The reaction mixture was poured into aqueous solution of sodium thiosulfate. The organic phase was washed with brine, followed by dried over anhydrous Na2SO4 and concentrated to dryness under vacuum. The crude product was chromatographed over silica gel (200–300 mesh) using hexane/AcOEt (5:1) to give compound 5 as a white solid (130 mg, 13%). IR (CH2Cl2, cm−1) νmax: 3440, 2919 (C=C-H), 1737 (C=O), 1705 (C=O), 1645 (C=O), 1517 (C=C). 1H-NMR (400 MHz, CDCl3) δ 9.43 (s, 1H, H-23), 6.11 (t, J = 5.4 Hz, 1H, NH-28), 5.26 (t, J = 3.6 Hz, 1H, H-12), 4.54 (dd, J = 14.5, 5.8 Hz, 1H, CH2-N-28), 4.23 (dd, J = 14.6, 4.7 Hz, 1H, CH2-N-28), 3.93 (dd, J = 10.1, 6.6 Hz, 1H, H-2), 3.39 (s, 3H, OCH3-2), 2.34 (dd, J = 13.2, 6.7 Hz, 1H), 1.31 (s, 3H), 1.19 (s, 3H), 1.13 (s, 3H), 0.75 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 209.38 (C-3), 199.45 (C-23), 177.57 (C-28), 140.05, 138.36, 128.68, 127.92, 127.43, 124.82 (C-12), 78.87 (C-2), 63.33 (OCH3-2), 58.36 (C-4), 53.97, 48.81, 47.74, 47.11, 43.70, 42.64, 39.71, 39.55, 39.05, 37.28, 37.02, 31.74, 30.83, 27.81, 24.75, 23.47, 23.34, 21.17, 20.01, 17.20, 17.17, 17.00, 14.21. HRMS (ESI) observed C38H54NO4 588.4060 (MH+) requires 588.4047.
3.1.7. α-Methoxy-3β-Hydroxy-23-Oxo-Urs-12-Ene-28-Benzylamide (6)
Following the procedure given for 5, compound 6 (701 mg, 70%) was obtained as a white solid (silica gel, hexane/AcOEt, 5:2). IR (CH2Cl2, cm−1) νmax: 3420, 2918 (C=C-H), 1730 (C=O), 1646 (C=O), 1521 (C=C). 1H-NMR (400 MHz, CDCl3) δ 9.35 (s, 1H, H-23), 6.14 (t, J = 5.3 Hz, 1H, NH-28), 5.24 (t, J = 3.4 Hz, 1H, H-12), 4.51 (dd, J = 14.5, 5.8 Hz, 1H, CH2-N-28), 4.21 (dd, J = 14.5, 4.6 Hz, 1H, CH2-N-28), 3.59 (d, J = 9.5 Hz, 1H, H-3), 3.40 (s, 3H, OCH3-2), 3.34 (ddd, J = 11.0, 9.4, 4.3 Hz, 1H, H-2), 2.16 (dd, J = 12.6, 4.3 Hz, 1H), 1.12 (s, 3H), 1.11 (s, 3H), 1.01 (s, 3H), 0.69 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 205.41 (C-23), 177.65 (C-28), 139.94, 138.34, 128.66, 127.91, 127.40, 125.03 (C-12), 77.87 (C-3), 75.30 (C-2), 56.75 (OCH3-2), 54.75 (C-4), 53.87, 47.69, 47.56, 47.42, 43.69, 42.57, 41.92, 39.67, 39.62, 39.04, 37.96, 37.25, 32.07, 30.84, 27.75, 24.75, 23.41, 23.34, 21.20, 19.88, 17.19, 17.04, 10.17. HRMS (ESI) observed C38H56NO4 590.4188 (MH+) requires 590.4204.
3.1.8. α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzylamide-23-Oic Acid (7)
Compound 6 (1 g, 1.7 mmol) was dissolved in t-BuOH (12 mL) and water (4 mL), and added 2-methyl-2-butene (1 mL), sodium dihydrogen phosphate (200 mg, 5.1 mmol) and sodium chlorite (460 mg, 5.1 mmol). After stirring at room temperature for 1 h, the reaction mixture was added aqueous solution of sodium thiosulfate, and extracted with ethyl acetate (100 mL × 3). The organic phase washed with brine followed by dried over anhydrous Na2SO4 and concentrated to dryness under vacuum. The crude product was chromatographed over silica gel (200–300 mesh) using hexane/AcOEt (1:1) to afford 7 as a white solid (957 mg, 93%). IR (CH2Cl2, cm−1) νmax: 3420, 2922 (C=C-H), 1715 (C=O), 1635 (C=O), 1525 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.19 (t, J = 5.3 Hz, 1H, NH-28), 5.23 (d, J = 3.6 Hz, 1H, H-12), 4.54 (dd, J = 14.5, 5.7 Hz, 1H, CH2-N-28), 4.21 (dd, J = 14.5, 4.4 Hz, 1H, CH2-N-28), 3.89 (d, J = 9.6 Hz, 1H, H-3), 3.41 (s, 3H, OCH3-2), 3.29 (td, J = 10.1, 9.7, 4.2 Hz, 1H, H-2), 2.13 (dd, J = 12.5, 4.3 Hz, 1H), 1.19 (s, 3H), 1.10 (s, 3H), 0.99 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.69 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 181.18 (C-23), 178.00 (C-28), 139.92, 138.20, 128.67, 127.94, 127.44, 125.14 (C-12), 78.20 (C-2), 78.03 (C-3), 56.78 (OCH3-2), 53.89 (C-4), 53.15, 50.80, 47.72, 47.57, 43.79, 42.53, 42.22, 39.69, 39.56, 39.05, 37.96, 37.18, 32.25, 30.84, 27.79, 24.76, 23.40, 21.21, 20.35, 17.20, 17.13, 16.92, 12.10. HRMS (ESI) observed C38H56NO5 606.4163 (MH+) requires 606.4153.
3.1.9. α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzylamide-23-Oic Acid (8)
Compound 7 (300 mg, 0.5 mmol) was dissolved in pyridine (5 mL) and added acetic anhydride (0.5 mL), stirred at room temperature overnight. The reaction mixture was poured into dilute aqueous HCl (2 M), and extracted with DCM (100 mL). The organic phase was washed with dilute aqueous HCl (2 M, 200 mL), a saturated solution of sodium bicarbonate (200 mL × 2) and brine, followed by dried over anhydrous Na2SO4 and concentrated to dryness under vacuum. The crude product was chromatographed over silica gel (200–300 mesh) using hexane/AcOEt (2:1) to afford 8 as a white solid (304 mg, 94%). IR (CH2Cl2, cm−1) νmax: 3420, 2923 (C=C-H), 1739 (C=O), 1635 (C=O), 1525 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.17 (t, J = 5.2 Hz, 1H, NH-28), 5.29 (d, J = 9.9 Hz, 1H, H-3), 5.22 (d, J = 3.5 Hz, 1H, H-12), 4.53 (dd, J = 14.4, 5.6 Hz, 1H, CH2-N-28), 4.21 (dd, J = 14.5, 4.3 Hz, 1H, CH2-N-28), 3.39 (td, J = 10.9, 4.2 Hz, 1H, H-2), 3.34 (s, 3H, OCH3-2), 2.12 (dd, J = 12.7, 4.3 Hz, 1H), 2.06 (s, 3H, CH3COO-3), 1.21 (s, 3H), 1.10 (s, 3H), 1.00 (s, 3H), 0.86 (d, J = 6.3 Hz, 3H), 0.68 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 179.78 (C-23), 177.96 (C-28), 170.33 (COO-3), 139.98, 138.23, 128.67, 127.95, 127.43, 125.06 (C-12), 78.55 (C-3), 75.88 (C-2), 57.32 (OCH3-2), 53.92, 52.74, 50.34, 47.73, 47.61, 43.78, 43.51, 42.52, 39.67, 39.55, 39.05, 37.36, 37.17, 32.18, 30.84, 27.77, 24.75, 23.31, 21.20, 20.90, 20.56, 17.23, 17.03, 16.90, 12.75. HRMS (ESI) observed C40H58NO6 648.4257 (MH+) requires 648.4259.
3.1.10. α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzylamide-23-Methyl Ester (9)
Under a nitrogen atmosphere, compound 7 (600 mg, 1 mmol) was dissolved in dry DMF (2 mL), and K2CO3 (207 mg, 1.5 mmol) was added, and stirred for 20 min. Then CH3I (96 μL, 1.5 mmol) was added, and stirring was continued for 30 min at room temperature. The reaction mixture was neutralized with dilute aqueous HCl (1 M, 100 mL) and extracted with ethyl acetate (100 mL × 3). The combined organic phase was washed with a saturated solution of sodium bicarbonate and brine (200 mL × 3), followed by dried over anhydrous Na2SO4 and concentrated to dryness under vacuum.
The crude product was chromatographed over silica gel (200–300 mesh) using hexane/AcOEt (5:2) to afford compound 9 as a white solid (551 mg, 89%). IR (CH2Cl2, cm−1) νmax: 3420, 2921 (C=C-H), 1725 (C=O), 1639 (C=O), 1518 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.14 (t, J = 5.3 Hz, 1H, NH-28), 5.24 (t, J = 3.5 Hz, 1H, H-12), 4.54 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.20 (dd, J = 14.5, 4.5 Hz, 1H, CH2-N-28), 3.86 (d, J = 9.6 Hz, 1H, H-3), 3.74 (s, 3H, OCH3-23), 3.40 (s, 3H, OCH3-2), 3.28 (ddd, J = 11.2, 9.6, 4.3 Hz, 1H, H-2), 2.13 (dd, J = 12.5, 4.3 Hz, 1H), 1.19 (s, 3H), 1.11 (s, 3H), 0.99 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.69 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.75 (C-28), 177.24 (C-23), 139.95, 138.33, 128.67, 127.92, 127.41, 125.10 (C-12), 78.23 (C-2), 78.20 (C-3), 56.70 (OCH3-2), 53.91, 53.33, 52.30, 51.27, 47.71, 47.57, 43.71, 42.54, 42.07, 39.71, 39.58, 39.07, 38.01, 37.24, 32.27, 30.85, 27.80, 24.81, 23.38, 21.20, 20.43, 17.18, 16.89, 12.19. HRMS (ESI) observed C39H58NO5 620.4308 (MH+) requires 620.4310.
3.1.11. α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzylamide-23-Methyl Ester (10)
Following the procedure given for 8, compound 10 (298 mg, 90%) was obtained from compound 9 as a white solid (silica gel, hexane/AcOEt, 3:1). IR (CH2Cl2, cm−1) νmax: 3412, 2919 (C=C-H), 1735 (C=O), 1639 (C=O), 1517 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.13 (d, J = 5.2 Hz, 1H, NH-28), 5.24 (d, J = 3.6 Hz, 1H, H-3), 5.20 (d, J = 9.9 Hz, 1H, H-12), 4.54 (dd, J = 14.5, 5.5 Hz, 1H, CH2-N-28), 4.20 (dd, J = 14.4, 4.1 Hz, 1H, CH2-N-28), 3.67 (s, 3H, OCH3-23), 3.39 (ddt, J = 12.2, 6.8, 3.3 Hz, 1H, H-2), 3.34 (s, 3H, OCH3-2), 2.13 (dd, J = 12.7, 4.5 Hz, 1H), 2.05 (s, 3H, CH3COO-3), 1.22 (s, 3H), 1.12 (s, 3H), 1.00 (s, 3H), 0.69 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.74 (C-28), 175.78 (C-23), 169.96 (COO-3), 139.93, 138.34, 128.66, 127.92, 127.40, 125.06 (C-12), 78.75 (C-3), 75.86 (C-2), 57.42 (OCH3-2), 53.92, 52.97, 52.53, 50.33, 47.72, 47.59, 43.71, 43.52, 42.52, 39.70, 39.58, 39.07, 37.31, 37.26, 32.23, 30.85, 27.80, 26.91, 24.79, 23.32, 21.19, 20.95, 20.71, 17.20, 17.04, 16.87, 13.01. HRMS (ESI) observed C41H60NO6 662.4398 (MH+) requires 662.4415.
3.1.12. α-Methoxy-3-Oxo-Urs-12-Ene-28-Benzyl Amide-23-Methyl Ester (11)
Compound 9 (62 mg, 0.1 mmol) was dissolved in DCM (5 mL), added PCC (65 mg, 0.3 mmol) and stirred at room temperature overnight. The reaction mixture was added water (100 mL) and extracted with DCM. The organic phase was washed with brine, dried over anhydrous Na2SO4 and concentrated to dryness under vacuum. The crude product was chromatographed over silica gel (200–300 mesh) using hexane/AcOEt (5:1) to afford compound 10 as a white solid (28 mg, 46%). IR (CH2Cl2, cm−1) νmax: 3418, 2920 (C=C-H), 1741 (C=O), 1719 (C=O), 1640 (C=O), 1521 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.14 (t, J = 5.3 Hz, 1H, NH-28), 5.24 (t, J = 3.5 Hz, 1H, H-12), 4.54 (dd, J = 14.5, 5.8 Hz, 1H, CH2-N-28), 4.21 (dd, J = 14.6, 4.6 Hz, 1H, CH2-N-28), 3.99 (dd, J = 11.3, 6.3 Hz, 1H, H-2), 3.75 (s, 3H, OCH3-23), 3.44 (s, 3H, OCH3-2), 2.29 (td, J = 12.9, 12.1, 4.3 Hz, 2H), 1.42 (s, 3H), 1.18 (s, 3H), 1.12 (s, 3H), 0.73 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 208.25 (C-3), 177.67 (C-28), 172.83 (C-23), 140.02, 138.34, 128.67, 127.91, 127.42, 124.86 (C-12), 78.64 (C-2), 62.21 (OCH3-2), 58.43 (23-OCH3), 53.93, 52.56, 51.35, 47.72, 47.31, 46.61, 43.68, 42.58, 39.69, 39.55, 39.05, 37.26, 36.83, 31.94, 30.83, 27.84, 26.91, 24.75, 23.38, 21.18, 21.00, 17.19, 16.98, 16.76, 16.63. HRMS (ESI) observed C39H56NO5 618.4131 (MH+) requires 618.4153.
3.1.13. α-Methoxy-3β-Acetoxy-23-Oxo-Urs-12-Ene-28-Benzyl Amide (12)
Following the procedure given for 8, compound 12 (290 mg, 91%) was obtained from compound 6 as a white solid (silica gel, hexane/AcOEt, 4:1). IR (CH2Cl2, cm−1) νmax: 3376, 2923 (C=C-H), 1734 (C=O), 1640 (C=O), 1522 (C=C). 1H-NMR (400 MHz, CDCl3) δ 9.28 (s, 1H, H-23), 6.12 (t, J = 5.3 Hz, 1H, NH-28), 5.25 (t, J = 3.5 Hz, 1H, H-12), 5.02 (d, J = 9.8 Hz, 1H, H-3), 4.52 (dd, J = 14.5, 5.7 Hz, 1H, CH2-N-28), 4.22 (dd, J = 14.5, 4.6 Hz, 1H, CH2-N-28), 3.57–3.41 (m, 1H, H-2), 3.37 (s, 3H, OCH3-2), 2.19 (dd, J = 12.8, 4.6 Hz, 1H), 2.03 (s, 3H, CH3COO-3), 1.12 (s, 3H), 1.08 (s, 3H), 1.03 (s, 3H), 0.70 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 202.69 (C-23), 177.61 (C-28), 170.31 (COO-3), 139.94, 138.36, 128.66, 127.91, 127.40, 124.95 (C-12), 75.43 (C-3), 75.25 (C-2), 57.65 (OCH3-2), 55.48 (C-4), 53.90, 47.70, 47.50, 47.45, 43.67, 42.56, 39.68, 39.59, 39.05, 37.43, 37.27, 32.05, 30.83, 27.76, 26.91, 24.76, 23.31, 21.19, 20.87, 19.95, 17.21, 16.99, 16.95, 10.35. HRMS (ESI) observed C40H58NO5 632.4316 (MH+) requires 632.4310.
3.1.14. α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzyl Amide-23-Amide (13a)
To a solution of compound 7 (60 mg, 0.1 mmol) in dry DMF (1 mL), HOBt (25 mg, 0.2 mmol), EDCI·HCl (20 mg, 0.12 mmol), DIPEA (20 μL) and NH4HCO3 (15.8 mg, 0.2 mmol) was added, stirring at 85 °C overnight. The reaction mixture was poured into the brine, and extracted with ethyl acetate (100 mL × 3). The organic phase was washed with a saturated solution of sodium bicarbonate and brine (200 mL × 3), dried over Na2SO4 and concentrated to dryness under vacuum. The crude product was purified by column chromatography over silica gel (200–300 mesh) using hexane/AcOEt (1:4) to afford compound 13a as a white solid (51 mg, 84%). IR (CH2Cl2, cm−1) νmax: 3361, 2919 (C=C-H), 1643 (C=O), 1522 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.16 (t, J = 5.4 Hz, 1H, NH-28), 6.14 (s, 1H, NH-23), 5.87 (s, 1H, NH-23), 5.23 (t, J = 3.4 Hz, 1H, H-12), 4.54 (dd, J = 14.5, 5.8 Hz, 1H, CH2-N-28), 4.19 (dd, J = 14.6, 4.4 Hz, 1H, CH2-N-28), 3.82 (d, J = 9.5 Hz, 1H, H-3), 3.40 (s, 3H, OCH3-2), 3.26 (tt, J = 11.3, 5.6 Hz, 1H, H-2), 2.70 (br s, 1H, OH-3), 2.09 (dd, J = 12.4, 4.1 Hz, 1H), 1.23 (s, 3H), 1.10 (s, 3H), 1.00 (s, 3H), 0.85 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 179.63 (C-28), 177.80 (C-23), 140.02, 138.36, 128.65, 127.89, 127.39, 125.05 (C-12), 78.34 (C-2), 77.70 (C-3), 56.75 (OCH3-2), 53.92, 52.57, 50.39, 47.71, 47.56, 43.69, 42.57, 41.90, 39.68, 39.59, 39.07, 37.79, 37.25, 32.30, 30.84, 27.78, 24.81, 23.46, 23.38, 21.19, 20.17, 17.28, 17.21, 16.95, 12.50. HRMS (ESI) observed C38H57N2O4 605.4309 (MH+) requires 605.4313.
3.1.15. α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzyl Amide-23-Methylamide (13b)
As described for 13a, compound 13b (43 mg, 70%) was obtained from methylamine hydrochloride as a white solid (silica gel, hexane/AcOEt, 1:3). IR (CH2Cl2, cm−1) νmax: 3377, 2919 (C=C-H), 1636 (C=O), 1534 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.15 (t, J = 5.2 Hz, 1H, NH-28), 6.05 (q, J = 4.8 Hz, 1H, NH-23), 5.23 (t, J = 3.5 Hz, 1H, H-12), 4.55 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.19 (dd, J = 14.5, 4.4 Hz, 1H, CH2-N-28), 3.86 (d, J = 9.7 Hz, 1H, H-3), 3.39 (s, 3H, OCH3-2), 3.34–3.18 (m, 1H, H-2), 2.85 (d, J = 4.5 Hz, 3H, CH3-N-23), 1.20 (s, 3H), 1.11 (s, 3H), 1.00 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.69 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.83 (C-28), 177.04 (C-23), 140.06, 138.36, 128.66, 127.90, 127.39, 125.07 (C-12), 78.40 (C-2), 77.59 (C-3), 56.55 (OCH3-2), 53.95, 52.36, 50.40, 47.73, 47.55, 43.70, 42.60, 41.69, 39.68, 39.60, 39.08, 37.77, 37.25, 32.34, 30.85, 27.80, 26.87, 24.83, 23.43, 23.39, 21.19, 20.17, 17.29, 17.20, 16.97, 12.19. HRMS (ESI) observed C39H59N2O4 619.4471 (MH+) requires 619.4469.
3.1.16. α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzyl Amide-23-Ethylamide (13c)
As described for 13a, compound 13c (38 mg, 60%) was obtained from ethylamine as a white solid (silica gel, hexane/AcOEt, 1:1). IR (CH2Cl2, cm−1) νmax: 3372, 2922 (C=C-H), 1636 (C=O), 1528 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.15 (t, J = 5.2 Hz, 1H, NH-28), 5.97 (t, J = 5.5 Hz, 1H, NH-23), 5.23 (t, J = 3.4 Hz, 1H, H-12), 4.55 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.19 (dd, J = 14.6, 4.4 Hz, 1H, CH2-N-28), 3.85 (d, J = 9.7 Hz, 1H, H-3), 3.39 (s, 3H, OCH3-2), 3.37–3.21 (m, 1H, H-2), 2.09 (dd, J = 12.4, 4.3 Hz, 1H), 1.11 (s, 3H), 0.99 (s, 3H), 0.85 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.85 (C-28), 176.16 (C-23), 140.05, 138.35, 128.65, 127.90, 127.39, 125.09 (C-12), 78.45 (C-2), 77.53 (C-3), 56.58 (OCH3-2), 53.94, 52.15, 50.39, 47.73, 47.55, 43.70, 42.59, 41.74, 39.68, 39.59, 39.08, 37.77, 37.24, 34.88, 32.33, 30.85, 27.79, 24.83, 23.42, 23.39, 21.19, 20.07, 17.29, 17.20, 16.96, 14.80, 12.21. HRMS (ESI) observed C40H61N2O4 633.4599 (MH+) requires 633.4626.
3.1.17. α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzyl Amide-23-Isopropylamide (13d)
As described for 13a, compound 13d (48 mg, 75%) was obtained from isopropylamine as a white solid (silica gel, hexane/AcOEt, 3:2). IR (CH2Cl2, cm−1) νmax: 3372, 2923 (C=C-H), 1637 (C=O), 1522 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.16 (t, J = 5.3 Hz, 1H, NH-28), 5.73 (d, J = 7.7 Hz, 1H, NH-23), 5.22 (t, J = 3.5 Hz, 1H, H-12), 4.54 (dd, J = 14.6, 5.9 Hz, 1H, CH2-N-28), 4.18 (dd, J = 14.6, 4.4 Hz, 1H, CH2-N-28), 3.84 (d, J = 9.7 Hz, 1H, H-3), 3.38 (s, 3H, OCH3-2), 3.34–3.20 (m, 1H, H-2), 2.42 (br s, 1H, OH-3), 2.08 (dd, J = 12.4, 4.3 Hz, 1H), 1.10 (s, 3H), 0.99 (s, 3H), 0.85 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.84 (C-28), 175.33 (C-23), 140.04, 138.36, 128.64, 127.89, 127.37, 125.09 (C-12), 78.47 (C-2), 77.49 (C-3), 56.58 (OCH3-2), 53.93, 51.96, 50.31, 47.72, 47.54, 43.69, 42.59, 41.77, 41.73, 39.68, 39.59, 39.07, 37.76, 37.24, 32.31, 30.85, 27.79, 24.82, 23.43, 23.39, 22.83, 22.59, 21.19, 20.02, 17.28, 17.20, 16.96, 12.23. HRMS (ESI) observed C41H63N2O4 647.4764 (MH+) requires 647.4782.
3.1.18. α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzyl Amide-23-Propargylamide (13e)
As described for 13a, compound 13e (50 mg, 78%) was obtained from propargylamine as a white solid (silica gel, hexane/AcOEt, 1:1). IR (CH2Cl2, cm−1) νmax: 3372, 2923 (C=C-H), 1640 (C=O), 1520 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.28 (t, J = 5.2 Hz, 1H, NH-23), 6.15 (t, J = 5.3 Hz, 1H, NH-28), 5.22 (t, J = 3.4 Hz, 1H, H-12), 4.54 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.19 (dd, J = 14.6, 4.4 Hz, 1H, CH2-N-28), 4.16–4.10 (m, 1H), 3.99 (ddd, J = 17.5, 4.6, 2.6 Hz, 1H), 3.81 (d, J = 9.7 Hz, 1H, H-3), 3.38 (s, 3H, OCH3-2), 3.27 (td, J = 10.1, 9.5, 4.1 Hz, 1H, H-2), 2.23 (t, J = 2.5 Hz, 1H), 1.21 (s, 3H), 1.10 (s, 3H), 0.99 (s, 3H), 0.85 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.80 (C-28), 176.39 (C-23), 140.03, 138.35, 128.65, 127.89, 127.38, 125.04 (C-12), 79.80 (alkyne), 78.36 (C-2), 77.48 (C-3), 71.51 (terminal alkyne), 56.63 (OCH3-2), 53.92, 52.31, 50.58, 47.71, 47.55, 43.69, 42.57, 41.73, 39.67, 39.58, 39.06, 37.80, 37.25, 32.27, 30.84, 29.75, 27.78, 24.81, 23.41, 23.38, 21.19, 20.11, 17.29, 17.20, 16.96, 12.19. HRMS (ESI) observed C41H59N2O4 643.4473 (MH+) requires 643.4469.
3.1.19. N-(2α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzylamide-23-Oyl)-l-Alanine Methyl Ester (13f)
As described for 13a, compound 13f (45 mg, 65%) was obtained from l-alanine methyl ester as a white solid (silica gel, hexane/AcOEt, 1:1). IR (CH2Cl2, cm−1) νmax: 3437, 2918 (C=C-H), 1738 (C=O), 1637 (C=O), 1523 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.55 (d, J = 7.0 Hz, 1H, NH-23), 6.15 (m, 1H, NH-28), 5.23 (t, J = 3.4 Hz, 1H, H-12), 4.58 (d, J = 7.0 Hz, 1H), 4.55 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.19 (dd, J = 14.5, 4.4 Hz, 1H, CH2-N-28), 3.77 (d, J = 9.7 Hz, 1H, H-3), 3.76 (s, 3H, CH3-ester), 3.41 (s, 3H, OCH3-2), 3.29 (ddd, J = 11.2, 9.6, 4.3 Hz, 1H, H-2), 2.16–2.05 (m, 1H), 1.43 (d, J = 7.2 Hz, 3H), 1.25 (s, 3H), 1.10 (s, 3H), 1.01 (s, 3H), 0.85 (d, J = 6.4 Hz, 3H), 0.69 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.82 (C-28), 176.42 (C-23), 173.97 (amino acid ester), 140.04, 138.36, 128.65, 127.90, 127.38, 125.09 (C-12), 78.02 (C-2), 77.85 (C-3), 56.84 (OCH3-2), 53.93, 52.52, 52.47, 50.01, 48.73, 47.72, 47.61, 43.69, 42.58, 42.09, 39.68, 39.60, 39.07, 37.64, 37.24, 32.29, 30.85, 27.79, 24.82, 23.42, 23.37, 21.20, 20.28, 18.06, 17.32, 17.21, 16.98, 12.09. HRMS (ESI) observed C42H63N2O6 691.4665 (MH+) requires 691.4681.
3.1.20. N-(2α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzylamide-23-Oyl)-l-Valine Methyl Ester (13g)
As described for 13a, compound 13g (40 mg, 56%) was obtained from l-valine methyl ester as a white solid (silica gel, hexane/AcOEt, 1:1). IR (CH2Cl2, cm−1) νmax: 3438, 2919 (C=C-H), 1738 (C=O), 1643 (C=O), 1517 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.52 (d, J = 8.1 Hz, 1H, NH-23), 6.16 (t, J = 5.2 Hz, 1H, NH-28), 5.23 (t, J = 3.5 Hz, 1H, H-12), 4.62–4.43 (m, 1H, CH2-N-28), 4.19 (dd, J = 14.6, 4.4 Hz, 1H, CH2-N-28), 3.77 (d, J = 9.7 Hz, 1H, H-3), 3.75 (s, 3H, CH3-ester), 3.41 (s, 3H, OCH3-2), 3.28 (ddd, J = 11.3, 9.6, 4.2 Hz, 1H, H-2), 2.20 (pd, J = 6.9, 4.6 Hz, 1H), 1.11 (s, 3H), 1.02 (s, 3H), 0.85 (d, J = 6.4 Hz, 3H), 0.69 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.84 (C-28), 176.88 (C-23), 172.87 (amino acid ester), 140.07, 138.36, 128.65, 127.90, 127.38, 125.09 (C-12), 78.04 (C-2), 77.94 (C-3), 57.83, 56.88 (OCH3-2), 53.94, 52.67, 52.21, 49.78, 47.73, 47.66, 43.69, 42.60, 42.04, 39.67, 39.65, 39.07, 37.61, 37.24, 32.31, 30.84, 27.78, 24.83, 23.41, 23.38, 21.19, 20.52, 19.06, 18.07, 17.35, 17.21, 17.03, 12.21. HRMS (ESI) observed C44H67N2O6 719.4993 (MH+) requires 719.4994.
3.1.21. N-(2α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzylamide-23-oyl)-l-Phenylalanine Methyl Ester (13h)
As described for 13a, compound 13h (37 mg, 48%) was obtained from l-phenylalanine methyl ester as a white solid (silica gel, hexane/AcOEt, 2:1). IR (CH2Cl2, cm−1) νmax: 3396, 2919 (C=C-H), 1740 (C=O), 1638 (C=O), 1517 (C=C). 1H-NMR (400 MHz, CDCl3) δ 7.20–7.06 (m, 2H), 6.41 (d, J = 7.5 Hz, 1H, NH-23), 6.15 (t, J = 5.2 Hz, 1H, NH-28), 5.22 (t, J = 3.2 Hz, 1H, H-12), 4.87 (q, J = 6.7 Hz, 1H), 4.55 (dd, J = 14.6, 5.5 Hz, 1H, CH2-N-28), 4.19 (m, 1H, CH2-N-28), 3.74 (s, 3H, CH3-ester), 3.70 (d, J = 9.6 Hz, 1H, H-3), 3.39 (s, 3H, OCH3-2), 3.30–3.20 (m, 1H, H-2), 3.20–3.05 (m, 2H), 1.14 (s, 3H), 1.09 (s, 3H), 0.66 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.86 (C-28), 176.55 (C-23), 172.51(amino acid ester), 140.05, 138.34, 129.11, 128.66, 128.62, 127.91, 127.40, 127.14, 125.09 (C-12), 78.00 (C-2), 77.85 (C-3), 56.81 (OCH3-2), 53.94, 53.63, 52.55, 52.43, 49.88, 47.74, 47.60, 43.72, 42.55, 41.97, 39.68, 39.59, 39.08, 37.85, 37.59, 37.25, 32.32, 30.85, 27.81, 24.82, 23.44, 23.36, 21.20, 20.13, 17.28, 17.22, 17.00, 12.01. HRMS (ESI) observed C48H67N2O6 767.4978 (MH+) requires 767.4994.
3.1.22. N-(2α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzylamide-23-Oyl)-4-Aminobutyric Ethyl Ester (13i)
As described for 13a, compound 13i (32 mg, 45%) was obtained from 4-aminobutyric ethyl ester as a white solid (silica gel, hexane/AcOEt, 1:2). IR (CH2Cl2, cm−1) νmax: 3361, 2924 (C=C-H), 1732 (C=O), 1634 (C=O), 1526 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.33 ((t, J = 5.5 Hz, 1H, NH-23), 6.15 (t, J = 5.3 Hz, 1H, NH-28), 5.22 (d, J = 3.6 Hz, 1H, H-12), 4.53 (dd, J = 14.5, 5.7 Hz, 1H, CH2-N-28), 4.25–4.04 (m, 3H), 3.80 (d, J = 9.5 Hz, 1H, H-3), 3.38 (s, 3H, OCH3-2), 2.64 (br s, 1H, OH-3), 2.43–2.29 (m, 2H), 2.08 (dd, J = 12.5, 4.1 Hz, 1H), 1.18 (s, 3H), 0.84 (d, J = 6.4 Hz, 3H), 0.67 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.98 (C-28), 176.67 (C-23), 173.65 (amino acid ester), 139.96, 138.22, 128.65, 127.88, 127.40, 125.13 (C-12), 78.42 (C-2), 77.55 (C-3), 60.56, 56.63 (OCH3-2), 53.90, 52.37, 50.33, 47.71, 47.54, 43.73, 42.55, 41.80, 39.66, 39.57, 39.04, 37.73, 37.19, 32.29, 31.82, 30.82, 27.74, 24.78, 24.32, 23.37, 21.18, 20.15, 17.29, 17.18, 16.92, 14.23, 12.12. HRMS (ESI) observed C44H67N2O6 719.4978 (MH+) requires 719.4994.
3.1.23. α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzyl Amide-23-Dimethylamide (13j)
As described for 13a, compound 13j (51 mg, 80%) was obtained from dimethyamine hydrochloride as a white solid (silica gel, hexane/AcOEt, 2:3). IR (CH2Cl2, cm−1) νmax: 3372, 2921 (C=C-H), 1630 (C=O), 1522 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.16 (t, J = 5.3 Hz, 1H, NH-28), 5.22 (t, J = 3.4 Hz, 1H, H-12), 4.53 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.18 (dd, J = 14.5, 4.5 Hz, 1H, CH2-N-28), 4.10 (d, J = 9.5 Hz, 1H, H-3), 3.39 (s, 3H, OCH3-2), 3.28 (ddd, J = 11.2, 9.4, 4.2 Hz, 1H, H-2), 3.07 (s, 6H, dimethyl-23), 2.09 (dd, J = 12.6, 4.5 Hz, 1H), 1.33 (s, 3H), 1.08 (s, 3H), 0.84 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.82 (C-28), 177.49 (C-23), 139.97, 138.36, 128.65, 127.90, 127.38, 125.15 (C-12), 79.22 (C-2), 78.42 (C-3), 56.62 (OCH3-2), 54.27, 53.93, 51.76, 47.73, 47.56, 43.69, 42.61, 42.09, 39.70, 39.67, 39.41, 39.07, 38.08, 37.24, 32.29, 30.85, 27.79, 24.82, 23.41, 23.33, 21.19, 20.79, 17.20, 17.11, 16.98, 15.99. HRMS (ESI) observed C40H61N2O4 633.4620 (MH+) requires 633.4626.
3.1.24. α-Methoxy-3β-Hydroxy-Urs-12-Ene-28-Benzylamide-23- (4-Methyl-1-Piperazinyl)-Amide (13k)
As described for 13a, compound 13k (48 mg, 70%) was obtained from methylpiperazine as a white solid (silica gel, DCM/MeOH, 30:1). IR (CH2Cl2, cm−1) νmax: 3393, 2922 (C=C-H), 1630 (C=O), 1523 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.13 (t, J = 5.2 Hz, 1H, NH-28), 5.24 (t, J = 3.5 Hz, 1H, H-12), 4.55 (dd, J = 14.6, 5.9 Hz, 1H, CH2-N-28), 4.20 (dd, J = 14.6, 4.5 Hz, 1H, CH2-N-28), 3.96 (d, J = 9.5 Hz, 1H, H-3), 3.72 (s, 4H), 3.40 (s, 3H, OCH3-2), 3.27 (ddd, J = 11.1, 9.4, 4.2 Hz, 1H, H-2), 2.44 (q, J = 4.9 Hz, 4H), 2.32 (s, 3H), 2.10 (dd, J = 12.4, 4.2 Hz, 1H), 1.12 (s, 3H), 1.02 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.71 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 177.79 (C-28), 177.79 (C-23), 140.04, 138.40, 128.68, 127.92, 127.41, 125.14 (C-12), 78.52 (C-2), 78.52 (C-3), 56.66 (OCH3-2), 55.35, 54.17, 53.95, 51.34, 47.83, 47.74, 46.18, 45.92, 45.83, 43.70, 42.67, 41.91, 39.69, 39.51, 39.08, 38.03, 37.27, 32.37, 30.86, 27.80, 24.85, 23.41, 23.35, 21.20, 20.88, 17.21, 17.12, 15.44, 8.77. HRMS (ESI) observed C43H66N3O4 688.5028 (MH+) requires 688.5048.
3.1.25. α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzyl Amide-23-Amide (14a)
As described for 13a, compound 14a (52 mg, 80%) was obtained from 8 (65 mg, 0.1 mmol) and NH4HCO3 as a white solid (silica gel, hexane/AcOEt, 2:3). IR (CH2Cl2, cm−1) νmax: 3451, 2917 (C=C-H), 1735 (C=O), 1654 (C=O), 1537 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.15 (t, J = 5.3 Hz, 1H, NH-28), 6.15 (br s, 1H, NH-23), 5.85 (br s, 1H, NH-23), 5.28 (d, J = 9.9 Hz, 1H, H-3), 5.23 (t, J = 3.6 Hz, 1H, H-12), 4.53 (dd, J = 14.5, 5.8 Hz, 1H, CH2-N-28), 4.20 (dd, J = 14.5, 4.4 Hz, 1H, CH2-N-28), 3.39 (td, J = 10.7, 4.3 Hz, 1H, H-2), 3.32 (s, 3H, OCH3-2), 2.11 (dd, J = 12.7, 4.4 Hz, 1H), 2.05 (s, 3H, CH3COO-3), 1.09 (s, 3H), 1.02 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.73 (C-28), 177.72 (C-23), 169.93 (COO-3), 139.98, 138.36, 128.65, 127.88, 127.39, 124.98 (C-12), 78.54 (C-3), 76.19 (C-2), 57.07 (OCH3-2), 53.88, 52.43, 51.23, 47.70, 47.58, 43.68, 43.18, 42.51, 39.67, 39.54, 39.05, 37.60, 37.26, 32.26, 30.82, 27.77, 24.78, 23.40, 23.36, 21.20, 21.03, 19.97, 17.29, 17.24, 16.87, 13.22. HRMS (ESI) observed C40H59N2O5 647.4413 (MH+) requires 647.4418.
3.1.26. α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzyl Amide-23-Methylamide (14b)
As described for 13a, compound 14b (49 mg, 74%) was obtained from 8 (65 mg, 0.1 mmol) and methylamine hydrochloride as a white solid (silica gel, hexane/AcOEt, 2:3). IR (CH2Cl2, cm−1) νmax: 3404, 2920 (C=C-H), 1735 (C=O), 1640 (C=O), 1529 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.15 (t, J = 5.3 Hz, 1H, NH-28), 6.05 (q, J = 4.7 Hz, 1H, NH-23), 5.28–5.19 (m, 2H, H-3 and H-12), 4.53 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.19 (dd, J = 14.5, 4.5 Hz, 1H, CH2-N-28), 3.39 (td, J = 10.7, 4.3 Hz, 1H, H-2), 3.31 (s, 3H, OCH3-2), 2.78 (d, J = 4.5 Hz, 3H, CH3-N-23), 2.10 (dd, J = 12.7, 4.4 Hz, 1H), 2.03 (s, 3H, CH3COO-3), 1.24 (s, 3H), 1.09 (s, 3H), 1.01 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.67 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.77 (C-28), 175.26 (C-23), 169.76 (COO-3), 139.99, 138.34, 128.66, 127.88, 127.40, 125.01 (C-12), 78.63 (C-3), 76.22 (C-2), 57.03 (OCH3-2), 53.91, 52.40, 51.12, 47.71, 47.57, 43.68, 43.13, 42.53, 39.67, 39.55, 39.06, 37.50, 37.27, 32.30, 30.83, 27.78, 26.98, 24.79, 23.39, 23.35, 21.19, 21.01, 20.07, 17.31, 17.22, 16.87, 13.02. HRMS (ESI) observed C41H61N2O5 661.4560 (MH+) requires 661.4575.
3.1.27. α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzyl Amide-23-Ethylamide (14c)
As described for 13a, compound 14c (47 mg, 70%) was obtained from 8 (65 mg, 0.1 mmol) and ethylamine as a white solid (silica gel, hexane/AcOEt, 3:2). IR (CH2Cl2, cm−1) νmax: 3372, 2924 (C=C-H), 1740 (C=O), 1639 (C=O), 1526 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.14 (t, J = 5.3 Hz, 1H, NH-28), 5.88 (t, J = 5.5 Hz, 1H, NH-23), 5.32–5.13 (m, 2H, H-3 and H-12), 4.54 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.19 (dd, J = 14.6, 4.5 Hz, 1H, CH2-N-28), 3.39 (td, J = 10.8, 10.4, 4.2 Hz, 1H, H-2), 3.32 (s, 3H, OCH3-2), 3.26–3.15 (m, 1H), 2.11 (dd, J = 12.7, 4.4 Hz, 1H), 2.04 (s, 3H, CH3COO-3), 1.23 (s, 3H), 1.02 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.78 (C-28), 174.30 (C-23), 169.66 (COO-3), 140.01, 138.36, 128.65, 127.89, 127.38, 125.00 (C-12), 78.53 (C-3), 76.19 (C-2), 57.03 (OCH3-2), 53.91, 52.26, 51.04, 47.71, 47.64, 43.68, 43.20, 42.53, 39.68, 39.56, 39.07, 37.46, 37.26, 34.86, 32.32, 30.84, 27.78, 24.80, 23.36, 21.19, 20.96, 20.03, 17.33, 17.23, 16.89, 14.82, 12.98. HRMS (ESI) observed C41H61N2O5 675.4712 (MH+) requires 675.4731.
3.1.28. α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzyl Amide-23-Isopropylamide (14d)
As described for 13a, compound 14d (58 mg, 84%) was obtained from 8 (65 mg, 0.1 mmol) and isopropylamine as a white solid (silica gel, hexane/AcOEt, 2:1). IR (CH2Cl2, cm−1) νmax: 3403, 2921 (C=C-H), 1740 (C=O), 1637 (C=O), 1526 (C=C).1 H-NMR (400 MHz, CDCl3) δ 6.15 (t, J = 5.3 Hz, 1H, NH-28), 5.61 (d, J = 7.7 Hz, 1H, NH-23), 5.25–5.19 (m, 2H, H-3 and H-12), 4.54 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28), 4.19 (dd, J = 14.6, 4.5 Hz, 1H, CH2-N-28), 4.13–3.97 (m, 1H), 3.44–3.33 (m, 1H, H-2), 3.32 (s, 3H, OCH3-2), 2.11 (dd, J = 12.7, 4.4 Hz, 1H), 2.03 (s, 3H, CH3COO-3), 1.21 (s, 3H), 1.01 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 177.78 (C-28), 173.42 (C-23), 169.56 (COO-3), 140.01, 138.37, 128.64, 127.88, 127.37, 125.01 (C-12), 78.46 (C-3), 76.17 (C-2), 57.02 (OCH3-2), 53.91, 52.13, 51.00, 47.71, 47.67, 43.67, 43.22, 42.52, 41.63, 39.67, 39.56, 39.06, 37.43, 37.25, 32.34, 30.84, 27.77, 24.79, 23.34, 22.73, 22.55, 21.19, 20.92, 20.02, 17.33, 17.22, 16.89, 12.92. HRMS (ESI) observed C42H65N2O5 689.4893 (MH+) requires 689.4888.
3.1.29. α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzyl Amide-23-Propargylamide (14e)
As described for 13a, compound 14e (54 mg, 79%) was obtained from 8 (65 mg, 0.1 mmol) and propargylamine as a white solid (silica gel, hexane/AcOEt, 3:1). IR (CH2Cl2, cm−1) νmax: 3379, 2922 (C=C-H), 1737 (C=O), 1643 (C=O), 1521 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.16 (t, J = 5.3 Hz, 1H, NH-28), 6.15 (t, J = 5.2 Hz, 1H, NH-23), 5.34–5.16 (m, 2H, H-3 and H-12), 4.54 (dd, J = 14.5, 5.8 Hz, 1H, CH2-N-28), 4.19 (dd, J = 14.5, 4.4 Hz, 1H, CH2-N-28), 4.08 (ddd, J = 17.5, 5.5, 2.6 Hz, 1H), 3.95 (ddd, J = 17.5, 4.6, 2.5 Hz, 1H), 3.39 (td, J = 10.8, 4.4 Hz, 1H, H-2), 3.32 (s, 3H, OCH3-2), 2.22 (t, J = 2.5 Hz, 1H), 2.11 (dd, J = 12.7, 4.4 Hz, 1H), 2.04 (s, 3H, CH3COO-3), 1.10 (s, 3H), 1.02 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.77 (C-28), 174.49 (C-23), 169.72 (COO-3), 140.01, 138.35, 128.66, 127.89, 127.40, 124.97 (C-12), 79.70 (alkyne), 78.39 (C-3), 76.10 (C-2), 71.46 (terminal alkyne), 57.08 (OCH3-2), 53.92, 52.47, 51.04, 47.72, 47.61, 43.69, 43.15, 42.54, 39.68, 39.55, 39.07, 37.49, 37.26, 32.24, 30.84, 29.77, 27.79, 24.79, 23.37, 21.20, 21.00, 20.07, 17.34, 17.22, 16.89, 12.89. HRMS (ESI) observed C43H61N2O5 685.4553 (MH+) requires 685.4575.
3.1.30. N-(2α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzylamide-23-oyl)-l-Alanine Methyl Ester (14f)
As described for 13a, compound 14f (62 mg, 84%) was obtained from 8 (65 mg, 0.1 mmol) and l-alanine methyl ester as a white solid (silica gel, hexane/AcOEt, 5:2). IR (CH2Cl2, cm−1) νmax: 3391, 2921 (C=C-H), 1742 (C=O), 1640 (C=O), 1525 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.39 (d, J = 7.2 Hz, 1H, NH-23), 6.14 (t, J = 5.4 Hz, 1H, NH-28), 5.23 (t, J = 3.9 Hz, 1H, H-12), 5.19 (d, J = 9.9 Hz, 1H, H-3), 4.56 (ddd, J = 14.4, 11.3, 6.6 Hz, 2H), 4.19 (dd, J = 14.6, 4.4 Hz, 1H, CH2-N-28), 3.74 (s, 3H, CH3-ester), 3.39 (td, J = 10.8, 4.3 Hz, 1H, H-2), 3.33 (s, 3H, OCH3-2), 2.04 (s, 3H, CH3COO-3), 1.03 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.68 (d, J = 2.3 Hz, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.80 (C-28), 174.11 (C-23), 173.62 (amino acid ester), 169.85 (COO-3), 140.04, 138.36, 128.65, 127.90, 127.39, 124.98 (C-12), 78.30 (C-3), 76.12 (C-2), 57.13 (OCH3-2), 53.93, 52.40, 52.23, 50.95, 48.30, 47.72, 43.69, 43.24, 42.55, 39.68, 39.57, 39.07, 37.43, 37.25, 32.24, 30.84, 27.77, 24.80, 23.35, 21.19, 20.96, 20.10, 18.35, 17.30, 17.23, 16.92, 12.84. HRMS (ESI) observed C44H65N2O7 733.4770 (MH+) requires 733.4786.
3.1.31. N-(2α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzylamide-23-Oyl)-l-Valine Methyl Ester (14g)
As described for 13a, compound 14g (57 mg, 75%) was obtained from 8 (65 mg, 0.1 mmol) and l-valine methyl ester as a white solid (silica gel, hexane/AcOEt, 4:1). IR (CH2Cl2, cm−1) νmax: 3420, 2959, 2921 (C=C-H), 1741 (C=O), 1638 (C=O), 1515 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.28 (d, J = 8.3 Hz, 1H, NH-23), 6.15 (t, J = 5.2 Hz, 1H, NH-28), 5.24 (t, J = 3.4 Hz, 1H, H-12), 5.17 (d, J = 9.9 Hz, 1H, H-3), 4.62–4.44 (m, 2H), 4.19 (dd, J = 14.6, 4.4 Hz, 1H, CH2-N-28), 3.73 (s, 3H, CH3-ester), 3.39 (td, J = 10.8, 4.3 Hz, 1H, H-2), 3.33 (s, 3H, OCH3-2), 2.04 (s, 3H, CH3COO-3), 1.12 (s, 3H), 1.03 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.84 (C-28), 174.63 (C-23), 172.74 (amino acid ester), 169.76 (COO-3), 140.07, 138.35, 128.65, 127.90, 127.39, 124.99 (C-12), 78.26 (C-3), 76.19 (C-2), 57.41, 57.09 (OCH3-2), 53.95, 52.52, 52.11, 50.66, 47.73, 47.71, 43.69, 43.12, 42.57, 39.68, 39.58, 39.07, 37.35, 37.24, 32.19, 31.14, 30.84, 27.76, 24.81, 23.34, 21.19, 20.96, 20.29, 18.98, 17.83, 17.31, 17.23, 16.94, 12.80. HRMS (ESI) observed C46H69N2O7 761.5092 (MH+) requires 761.5099.
3.1.32. N-(2α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzylamide-23-Oyl)-l-Phenylalanine Methyl Ester (14h)
As described for 13a, compound 14h (47 mg, 58%) was obtained from 8 (65 mg, 0.1 mmol) and l-phenylalanine methyl ester as a white solid (silica gel, hexane/AcOEt, 4:1). IR (CH2Cl2, cm−1) νmax: 3373, 2953, 2921 (C=C-H), 1742 (C=O), 1640 (C=O), 1525 (C=C). 1H-NMR (400 MHz, CDCl3) δ 7.17–7.07 (m, 3H, ), 6.16 (d, J = 7.5 Hz, 1H, NH-23), 6.15 (t, J = 5.2 Hz, 1H, NH-28), 5.23 (t, J = 3.5 Hz, 1H, H-12), 5.14 (d, J = 10.0 Hz, 1H, H-3), 4.90 (dt, J = 7.9, 6.1 Hz, 1H), 4.55 (dd, J = 14.5, 5.9 Hz, 1H, CH2-N-28 ), 4.20 (dd, J = 14.5, 4.4 Hz, 1H, CH2-N-28), 3.72 (s, 3H, CH3-ester), 3.36 (dt, J = 10.8, 5.4 Hz, 1H, H-2), 3.31 (s, 3H, OCH3-2), 3.20–3.00 (m, 3H), 2.08 (dd, J = 12.7, 4.4 Hz, 1H), 2.00 (s, 3H, CH3COO-3), 1.18 (s, 3H), 0.99 (s, 3H), 0.67 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.81 (C-28), 174.22 (C-23), 172.17 (amino acid ester), 169.74 (COO-3), 140.06, 138.37, 135.94, 129.18, 128.65, 128.57, 127.90, 127.39, 127.11, 124.97 (C-12), 78.23 (C-3), 76.00 (C-2), 57.07 (OCH3-2), 53.94, 52.97, 52.29, 52.26, 50.28, 47.74, 47.63, 43.69, 43.08, 42.53, 39.68, 39.55, 39.09, 38.03, 37.26, 32.24, 31.51, 30.85, 30.14, 27.80, 24.81, 23.39, 23.32, 21.20, 20.89, 20.03, 17.24, 16.94, 12.60. HRMS (ESI) observed C50H69N2O7 809.5068 (MH+) requires 809.5099.
3.1.33. N-(2α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzylamide-23-Oyl)-4-Aminobutyric Ethyl Ester (14i)
As described for 13a, compound 14i (49 mg, 64%) was obtained from 8 (65 mg, 0.1 mmol) and 4-aminobutyric ethyl ester as a white solid (silica gel, hexane/AcOEt, 3:2). IR (CH2Cl2, cm−1) νmax: 3372, 2924 (C=C-H), 1737 (C=O), 1639 (C=O), 1525 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.26 (t, J = 5.5 Hz, 1H, NH-23), 6.14 (t, J = 5.4 Hz, 1H, NH-28), 5.30–5.15 (m, 2H, H-3 and H-12), 4.54 (dd, J = 14.5, 5.8 Hz, 1H, CH2-N-28), 4.29–3.98 (m, 3H, CH2-N-28 and CH2-ester), 3.38 (m, 1H, H-2), 3.32 (s, 3H, OCH3-2), 3.19 (dq, J = 13.0, 6.6 Hz, 1H), 2.34 (t, J = 7.0 Hz, 2H), 2.11 (dd, J = 12.7, 4.4 Hz, 1H), 1.10 (s, 3H), 1.01 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.68 (s, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.76 (C-28), 174.70 (C-23), 173.66 (amino acid ester), 169.67 (COO-3), 140.01, 138.36, 128.65, 127.89, 127.38, 125.01 (C-12), 78.57 (C-3), 76.19 (C-2), 60.59, 57.05 (OCH3-2), 53.92, 52.37, 51.02, 47.71, 47.63, 43.68, 43.20, 42.53, 39.67, 39.56, 39.06, 37.46, 37.25, 32.30, 31.86, 30.83, 27.78, 24.79, 24.27, 23.34, 21.18, 20.95, 20.09, 17.29, 17.22, 16.89, 14.22, 12.89. HRMS (ESI) observed C46H69N2O7 761.5096 (MH+) requires 761.5099.
3.1.34. α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzyl Amide-23-Dimethylamide (14j)
As described for 13a, compound 14j (42 mg, 62%) was obtained from 8 (65 mg, 0.1 mmol) and dimethyamine hydrochloride as a white solid (silica gel, hexane/AcOEt, 1:1). IR (CH2Cl2, cm−1) νmax: 3411, 2920 (C=C-H), 1740 (C=O), 1632 (C=O), 1522 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.12 (t, J = 5.2 Hz, 1H, NH-28), 5.58 (d, J = 9.3 Hz, 1H, H-3), 5.24 (q, J = 6.0, 4.7 Hz, 1H, H-12), 4.53 (dd, J = 14.6, 5.7 Hz, 1H, CH2-N-28), 4.21 (dd, J = 14.5, 4.6 Hz, 1H, CH2-N-28), 3.36 (s, 3H, OCH3-2), 3.16 (s, 4H), 2.05 (s, 3H, CH3COO-3), 1.11 (s, 3H), 1.04 (s, 3H), 0.87 (d, J = 6.4 Hz, 3H), 0.70 (s, 3H). 13C-NMR (101 MHz, MeOD) δ 178.37 (C-28), 174.31 (C-23), 170.73 (COO-3), 138.94, 138.64, 128.03, 127.57, 126.68, 125.18 (H-12), 76.87 (C-3), 76.54 (C-2), 65.24, 56.67 (OCH3-2), 53.67, 52.88, 49.25, 47.88, 42.99, 41.95, 39.42, 39.15, 38.89, 37.36, 32.45, 30.57, 30.37, 29.47, 27.55, 23.82, 23.10, 22.80, 20.30, 19.53, 18.91, 16.42, 16.15, 15.04, 12.79. HRMS (ESI) observed C42H63N2O5 670.4709 (MH+) requires 675.4731.
3.1.35. α-Methoxy-3β-Acetoxy-Urs-12-Ene-28-Benzylamide-23-(4-Methyl-1-Piperazinyl)-Amide (14k)
As described for 13a, compound 14k (33 mg, 45%) was obtained from 8 (65 mg, 0.1 mmol) and methylpiperazine as a white solid (silica gel, hexane/AcOEt, 1:3). IR (CH2Cl2, cm−1) νmax: 3383, 2925 (C=C-H), 1740 (C=O), 1635 (C=O), 1522 (C=C). 1H-NMR (400 MHz, CDCl3) δ 6.13 (t, J = 5.4 Hz, 1H, NH-28), 5.51 (d, J = 9.3 Hz, 1H, H-3), 5.23 (d, J = 3.7 Hz, 1H, H-12), 4.50 (ddd, J = 14.7, 6.0, 2.5 Hz, 1H, CH2-N-28), 4.20 (dd, J = 14.5, 4.5 Hz, 1H, CH2-N-28), 3.89 (s, 1H), 3.67 (s, 2H), 3.41 (d, J = 10.2 Hz, 1H, H-2), 3.34 (s, 3H, OCH3-2), 2.55–2.33 (m, 4H), 2.28 (d, J = 1.8 Hz, 3H), 2.22–2.09 (m, 1H), 2.02 (s, 3H, CH3COO-3), 1.09 (s, 3H), 1.01 (s, 3H), 0.85 (d, J = 6.3 Hz, 3H), 0.68 (d, J = 2.2 Hz, 3H). 13C-NMR (101 MHz, CDCl3) δ 177.56 (C-28), 172.21 (C-23), 170.17 (COO-3), 139.82, 138.35, 128.63, 127.89, 127.38, 125.05 (C-12), 77.30, 60.36, 57.65 (OCH3-2), 54.94, 53.86, 53.57, 50.23, 48.53, 47.69, 46.25, 45.77, 43.66, 43.41, 42.54, 39.68, 39.01, 37.44, 37.21, 32.50, 30.83, 27.79, 24.75, 23.43, 23.32, 21.19, 21.02, 20.41, 17.18, 17.04, 16.95, 16.00, 14.20. HRMS (ESI) observed C45H68N3O5 730.5144 (MH+) requires 730.5153.