Caffeine Inhibits Acetylcholinesterase, But Not Butyrylcholinesterase
Abstract
:1. Introduction
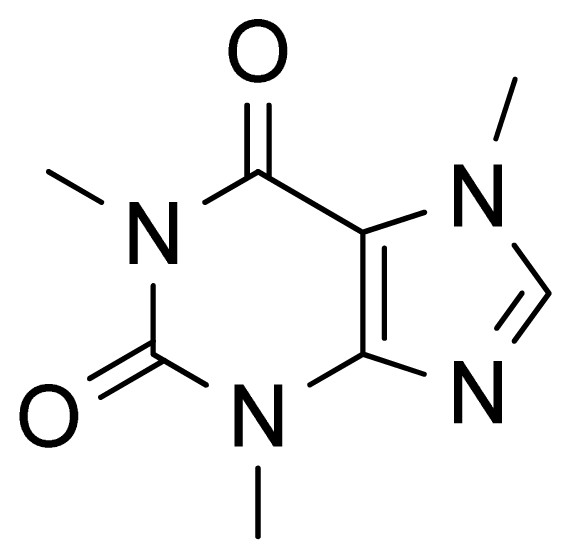
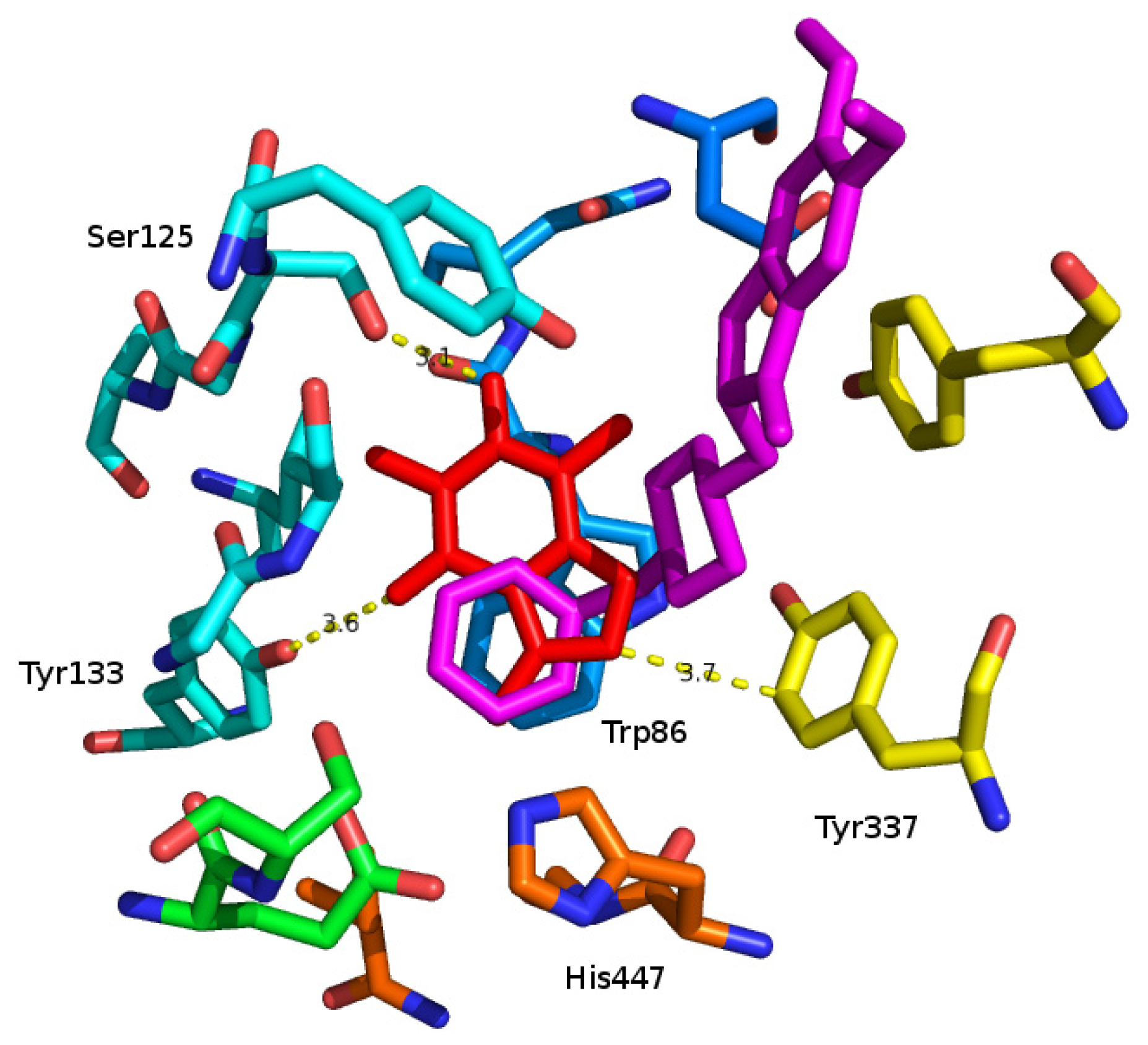
2. Results and Discussion
3. Experimental Section
3.1. Cholinesterases Activity Assay
3.2. Molecular Modeling
3.3. Statistical Processing of Experimental Data
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Metherate, R. Functional connectivity and cholinergic modulation in auditory cortex. Neurosci. Biobehav. Rev 2011, 35, 2058–2063. [Google Scholar]
- Wessler, I.; Kirkpatrick, C.J. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. Br. J. Pharmacol 2008, 154, 1558–1571. [Google Scholar]
- Pohanka, M. Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. Int. J. Mol. Sci 2012, 13, 2219–2238. [Google Scholar]
- Pohanka, M. Cholinesterases, a target of pharmacology and toxicology. Biomed. Pap 2011, 155, 219–229. [Google Scholar]
- Pohanka, M. Acetylcholinesterase inhibitors: A patent review (2008–present). Expert Opin. Ther. Pat 2012, 22, 871–886. [Google Scholar]
- Jokanovic, M. Medical treatment of acute poisoning with organophosphorus and carbamate pesticides. Toxicol. Lett 2009, 190, 107–115. [Google Scholar]
- Marrs, T.C. Organophosphate poisoning. Pharmacol. Ther 1993, 58, 51–66. [Google Scholar]
- Pohanka, M. Alzheimer’s disease and related neurodegenerative disorders: Implication and counteracting of melatonin. J. Appl. Biomed 2011, 9, 185–196. [Google Scholar]
- Holzgrabe, U.; Kapkova, P.; Alptuzun, V.; Scheiber, J.; Kugelmann, E. Targeting acetylcholinesterase to treat neurodegeneration. Expert Opin. Ther. Targets 2007, 11, 161–179. [Google Scholar]
- Krall, W.J.; Sramek, J.J.; Cutler, N.R. Cholinesterase inhibitors: A therapeutic strategy for alzheimer disease. Ann. Pharmacother 1999, 33, 441–450. [Google Scholar]
- Bhat, K.G.; Singhal, V.; Borker, A.S. Successful treatment of vincristine induced ptosis and polyneuropathy with pyridoxine and pyridostigmine in a child with acute lymphoblastic leukemia. Indian J. Med. Paediatr. Oncol 2012, 33, 185–187. [Google Scholar]
- Yu, Q.S.; Holloway, H.W.; Luo, W.; Lahiri, D.K.; Brossi, A.; Greig, N.H. Long-acting anticholinesterases for myasthenia gravis: Synthesis and activities of quaternary phenylcarbamates of neostigmine, pyridostigmine and physostigmine. Bioorg. Med. Chem 2010, 18, 4687–4693. [Google Scholar]
- Iwasaki, T.; Yoneda, M.; Nakajima, A.; Terauchi, Y. Serum butyrylcholinesterase is strongly associated with adiposity, the serum lipid profile and insulin resistance. Intern. Med 2007, 46, 1633–1639. [Google Scholar]
- Ostergaard, D.; Viby-Moogensen, J.; Hanel, H.K.; Skovgaard, L.T. Half-life of plasma cholinesterase. Acta Anaesthesiol. Scand 1988, 32, 266–269. [Google Scholar]
- Guilbeau, J.R. Health risks of energy drinks: What nurses and consumers need to know. Nurs. Women’s Health 2012, 16, 423–428. [Google Scholar]
- Sepkowitz, K.A. Energy drinks and caffeine-related adverse effects. JAMA 2013, 309, 243–244. [Google Scholar]
- Potenza, R.L.; Armida, M.; Rerrante, A.; Pezzola, A.; Matteucci, A.; Puopolo, M.; Popoli, P. Effects of chronic caffeine intake in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. Res 2013, 91, 585–592. [Google Scholar]
- Szadujkis-Szadurska, K.; Grzesk, G.; Szadujkis-Szadurski, L.; Gajdus, M.; Matusiak, G. Role of acetylcholine and calcium ions in three vascular contraction models: Angiotensin II, phenylephrine and caffeine. Exp. Ther. Med 2012, 4, 329–333. [Google Scholar]
- Glatter, K.A.; Myers, R.; Chiamvimonvat, N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: What do you tell your patients to do or not to do? Curr. Threat. Opt. Cardiovasc. Med 2012, 14, 529–535. [Google Scholar]
- Cummings, K.J.; Commons, K.G.; Trachtenberg, F.L.; Li, A.; Kinney, H.C.; Nattie, E.E. Caffeine improves the ability of serotonin-deficient (pet-1−/−) mice to survive episodic asphyxia. Pediatr. Res 2013, 73, 38–45. [Google Scholar]
- Golembiowska, K.; Dziubina, A. The effect of adenosine a(2a) receptor antagonists on hydroxyl radical, dopamine, and glutamate in the striatum of rats with altered function of vmat2. Neurotox. Res 2012, 22, 150–157. [Google Scholar]
- Shin, H.J.; Ryu, J.H.; Kim, S.T.; Zuo, Z.; Do, S.H. Caffeine-induced inhibition of the activity of glutamate transporter type 3 expressed in xenopus oocytes. Toxicol. Lett 2013, 217, 143–148. [Google Scholar]
- Daly, J.W. Caffeine analogs: Biomedical impact. Cell. Mol. Life Sci 2007, 64, 2153–2169. [Google Scholar]
- Ribeiro, J.A.; Sebastiao, A.M. Caffeine and adenosine. J. Alzheimers Dis 2010, 20, S3–S15. [Google Scholar]
- Acquas, E.; Tanda, G.; DiChiara, G. Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology 2002, 27, 182–193. [Google Scholar]
- Tomaszewski, M.; Olchowik, G.; Tomaszewska, M.; Burdan, F. Use of X-ray microprobe to diagnose bone tissue demineralization after caffeine administration. Folia Histochem. Cytobiol 2012, 50, 436–443. [Google Scholar]
- Bai, D.L.; Tang, X.C.; He, X.C. Huperzine a, a potential therapeutic agent for treatment of alzheimer’s disease. Curr. Med. Chem 2000, 7, 355–374. [Google Scholar]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem 2012, 55, 10282–10286. [Google Scholar]
- Okello, E.J.; Leylabi, R.; McDougall, G.J. Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. Food Funct 2012, 3, 651–661. [Google Scholar]
- Karadsheh, N.; Kussie, P.; Linthicum, D.C. Inhibition of acetylcholinesterase by caffeine, anabasine, methyl pyrrolidine and their derivatives. Toxicol. Lett 1991, 55, 332–342. [Google Scholar]
- Stoytcheva, M.; Zlatev, R.; Velkova, Z.; Valdez, B.; Ovalle, M. Electrochemical study on the kinetic behavior of the immobilized acetylcholinesterase. ECS Trans 2009, 20, 175–184. [Google Scholar]
- Vukcevic, N.P.; Babic, G.; Segrt, Z.; Ercegovic, G.V.; Jankovic, S.; Acimovic, L. Severe acute caffeine poisoning due to intradermal injections: Mesotherapy hazard. Vojnosanit. Pregl 2012, 69, 707–713. [Google Scholar]
- Pohanka, M. Antioxidants countermeasures against sulfur mustard. Mini Rev. Med. Chem 2012, 12, 742–748. [Google Scholar]
- Yubero-Lahoz, S.; Pardo, R.; Farre, M.; Mathuna, B.O.; Torrens, M.; Mustata, C.; Perez-Mana, C.; Langohr, K.; Carbo, M.L.; de la Torre, R. Changes in cyp1a2 activity in humans after 3,4-methylenedioxymethamphetamine (mdma, ecstasy) administration using caffeine as a probe drug. Drug Metab. Pharmacokineti 2012, 27, 605–613. [Google Scholar]
- Chu, Y.F.; Chang, W.H.; Black, R.M.; Liu, J.R.; Sompol, P.; Chen, Y.M.; Wei, H.L.; Zhao, Q.Y.; Cheng, I.H. Crude caffeine reduces memory impairment and amyloid beta(1–42) levels in an alzheimer’s mouse model. Food Chem 2012, 135, 2095–2102. [Google Scholar]
- Vila-Luna, S.; Cabrera-Isidoro, S.; Vila-Luna, L.; Juarez-Diaz, I.; Bata-Garcia, J.L.; Alvarez-Cervera, F.J.; Zapata-Vazquez, R.E.; Arankowsky-Sandoval, G.; Heredia-Lopez, F.; Flores, G.; et al. Chronic caffeine consumption prevents cognitive decline from young to middle age in rats, and is associated with increased length, branching, and spine density of basal dendrities in ca1 hippocampal neurons. Neuroscience 2012, 202, 384–395. [Google Scholar]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain—. In vitro. Neurochem. Res 2013, 38, 413–419. [Google Scholar]
- Hu, Y.Q.; Zhang, J.; Chandrashankra, O.; Ip, F.C.F.; Ip, N.Y. Design, synthesis and evaluation of novel heterodimers of donepezil and huperzine fragments as acetylcholinesterase inhibitors. Bioorgan. Med. Chem 2013, 21, 676–683. [Google Scholar]
- Karlsson, D.; Fallarero, A.; Brunhofer, G.; Mayer, C.; Prakash, O.; Mohan, C.G.; Vuorela, P.; Erker, T. The exploration of thienothiazines as selective butyrylcholinesterase inhibitors. Eur. J. Pharm. Sci 2012, 47, 190–205. [Google Scholar]
- Catto, M.; Pisani, L.; Leonetti, F.; Nicolotti, O.; Pesce, P.; Stefanachi, A.; Cellamare, S.; Carotti, A. Design, synthesis and biological evaluation of coumarin alkylamines as potent and selective dual binding site inhibitors of acetylcholinesterase. Bioorg. Med. Chem 2013, 21, 146–152. [Google Scholar]
- Pohanka, M. Role of oxidative stress in infectious diseases. A review. Folia Microbiol. 2013. [Google Scholar] [CrossRef]
- Sanchez-Lopez, F.; Tasset, I.; Aguera, E.; Feijoo, M.; Fernandez-Bolanos, R.; Sanchez, F.M.; Ruiz, M.C.; Cruz, A.H.; Gascon, F.; Tunez, I. Oxidative stress and inflammation biomarkers in the blood of patients with huntington’s disease. Neurol. Res 2012, 34, 721–724. [Google Scholar]
- Ramalingam, M.; Kim, S.J. Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. J. Neural. Transm 2012, 119, 891–910. [Google Scholar]
- Holmes, C.; Ballard, C.; Lehmann, D.; Smith, A.D.; Beaumont, H.; Day, I.N.; Khan, M.N.; Lovestone, S.; McCulley, M.; Morris, C.M.; et al. Rate of progression of cognitive decline in alzheimer’s disease: Effect of butyrylcholinesterase K gene variation. J. Neurol. Neurosurg. Psychiatr 2005, 76, 640–643. [Google Scholar]
- Pohanka, M. Acetylcholinesterase based dipsticks with indoxylacetate as a substrate for assay of organophosphates and carbamates. Anal. Lett 2012, 45, 367–374. [Google Scholar]
- Pohanka, M. Spectrophotomeric assay of aflatoxin b1 using acetylcholinesterase immobilized on standard microplates. Anal. Lett. 2013. [Google Scholar] [CrossRef]
- Pohanka, M. Cholinesterases in biorecognition and biosensor construction, a review. Anal. Lett. 2013. [Google Scholar] [CrossRef]
- Eyer, P.; Worek, F.; Kiderlen, D.; Sinko, G.; Stuglin, A.; Simeon-Rudolf, V.; Reiner, E. Molar absorption coefficients for the reduced ellman reagent: Reassessment. Anal. Biochem 2003, 312, 224–227. [Google Scholar]
- Trott, O.; Olson, A.J. Software news and update autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem 2010, 31, 455–461. [Google Scholar]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with pymol and autodock/vina. J. Comput. Aid. Mol. Des 2010, 24, 417–422. [Google Scholar]
- Dixon, M. The determination of enzyme inhibitor constants. Biochem. J 1953, 55, 170–171. [Google Scholar]
- Cornish-Bowden, A. A simple graphical method for determinating the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem. J 1973, 137, 143–144. [Google Scholar]
- Cortes, A.; Cascante, M.; Cardenas, M.L.; Cornish-Bowden, A. Relationships between inhibition constants, inhibitor concentrations for 50% inhibition and types of inhibition: New ways of analysing data. Biochem. J 2001, 357, 263–268. [Google Scholar]
- Cer, R.Z.; Mudunuri, U.; Stephens, R.; Lebeda, F.J. Ic50-to-ki: A web-based tool for converting ic50 to ki values for inhibitors of enzyme activity and ligand binding. Nucleic Acids Res 2009, 37, W441–W445. [Google Scholar]
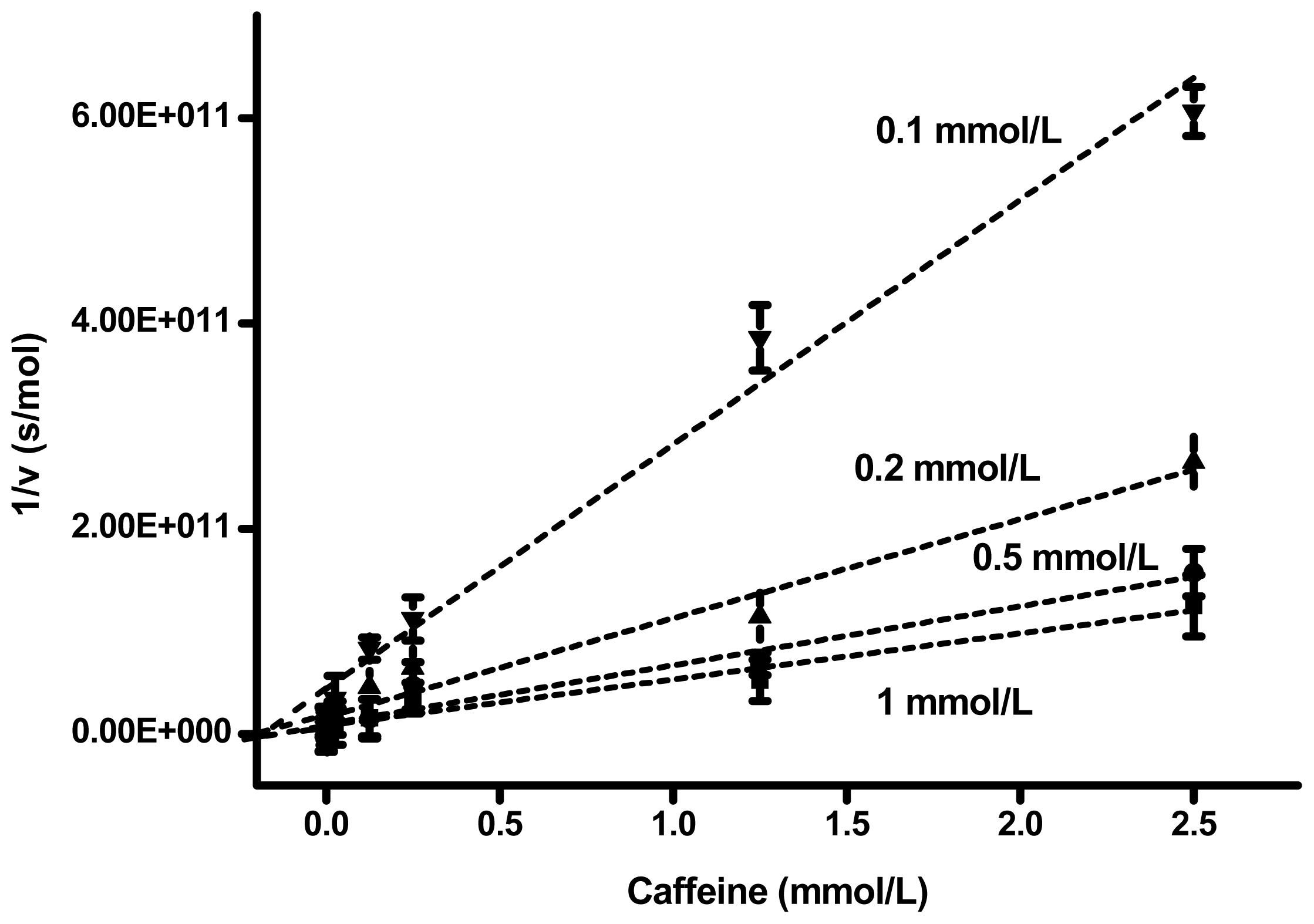
| Substrate (mmol/L) | Slope (s × L/mol 2) | Interception (s/mol) | Correlation coefficient | Ki (mmol/L) |
|---|---|---|---|---|
| 1 | 4.49 × 10 13 | 8.23 × 10 9 | 0.978 | 0.183 |
| 0.5 | 5.75 × 10 13 | 9.43 × 10 9 | 0.976 | 0.164 |
| 0.2 | 9.64 × 10 13 | 1.64 × 10 10 | 0.981 | 0.170 |
| 0.1 | 2.38 × 10 13 | 4.41 × 10 10 | 0.966 | 0.185 |
| Substrate (mmol/L) | Slope (s × L/mol 2) | Interception (s/mol) | Correlation coefficient | Ki (mol/L) |
|---|---|---|---|---|
| 5 | 2.76 × 10 8 | 6.60 × 10 9 | 0.277 | 24.0 |
| 1 | 8.75 × 10 8 | 5.80 × 10 9 | 0.609 | 6.64 |
| 0.2 | 4.62 × 10 8 | 5.07 × 10 9 | 0.400 | 11.0 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pohanka, M.; Dobes, P. Caffeine Inhibits Acetylcholinesterase, But Not Butyrylcholinesterase. Int. J. Mol. Sci. 2013, 14, 9873-9882. https://doi.org/10.3390/ijms14059873
Pohanka M, Dobes P. Caffeine Inhibits Acetylcholinesterase, But Not Butyrylcholinesterase. International Journal of Molecular Sciences. 2013; 14(5):9873-9882. https://doi.org/10.3390/ijms14059873
Chicago/Turabian StylePohanka, Miroslav, and Petr Dobes. 2013. "Caffeine Inhibits Acetylcholinesterase, But Not Butyrylcholinesterase" International Journal of Molecular Sciences 14, no. 5: 9873-9882. https://doi.org/10.3390/ijms14059873
APA StylePohanka, M., & Dobes, P. (2013). Caffeine Inhibits Acetylcholinesterase, But Not Butyrylcholinesterase. International Journal of Molecular Sciences, 14(5), 9873-9882. https://doi.org/10.3390/ijms14059873






