The Mechanism of Melatonin and Its Receptor MT2 Involved in the Development of Bovine Granulosa Cells
Abstract
:1. Introduction
2. Results
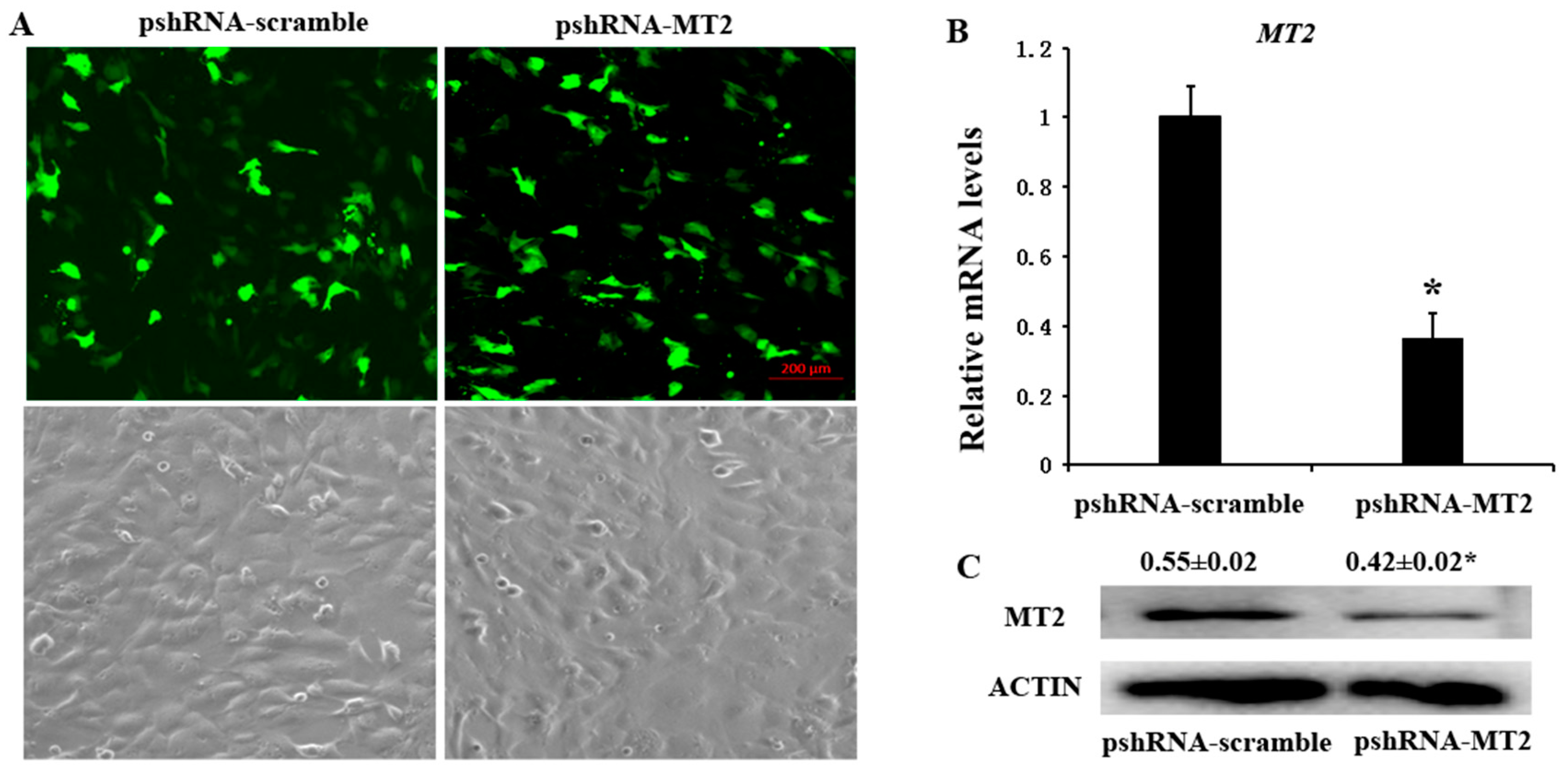
2.1. pshRNA-MT2 Efficiently Silenced MT2 Expression in Bovine GCs
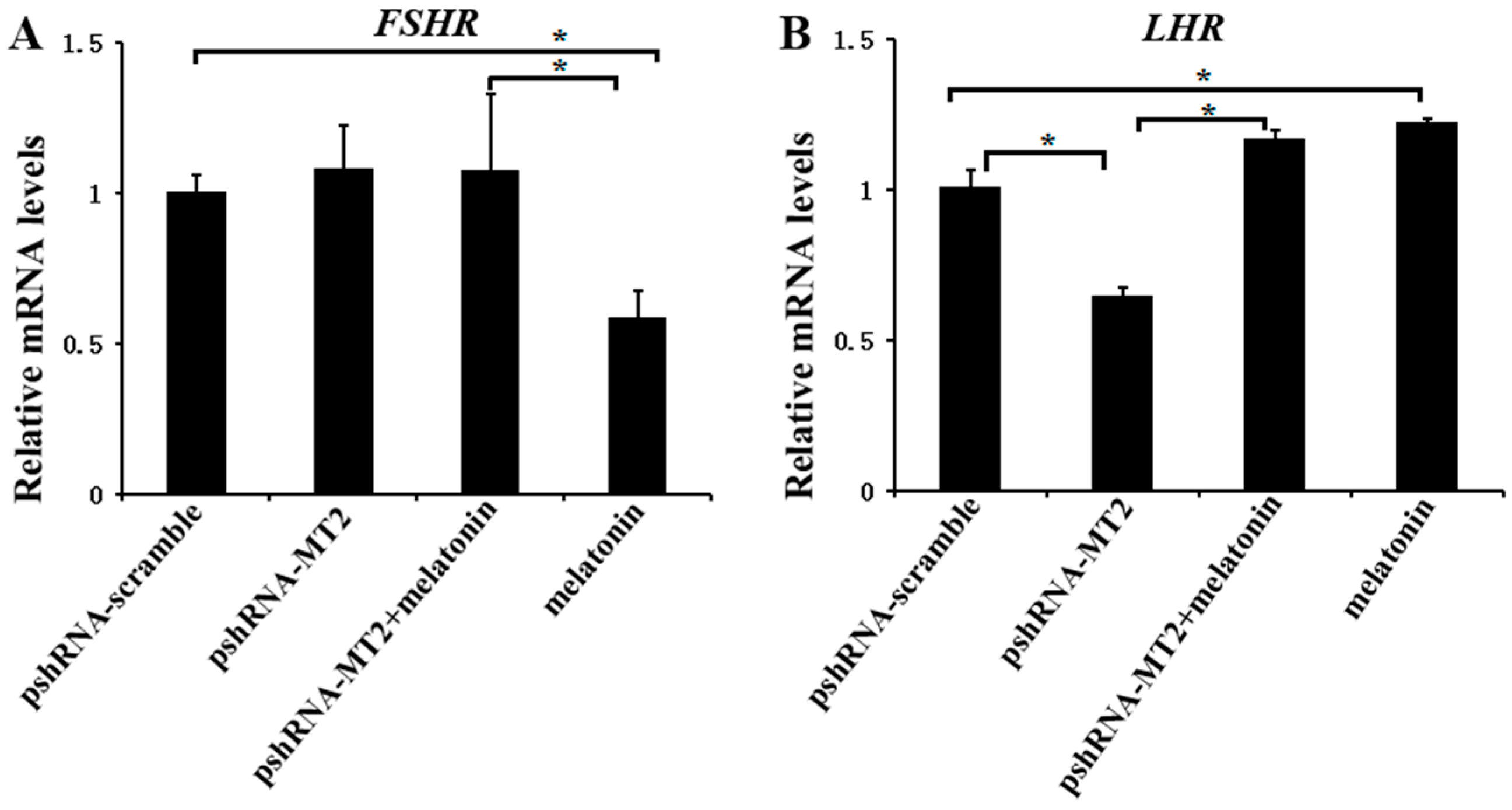
2.2. Effects of MT2 Silencing and Melatonin Treatment on Reproduction Related Genes Expression
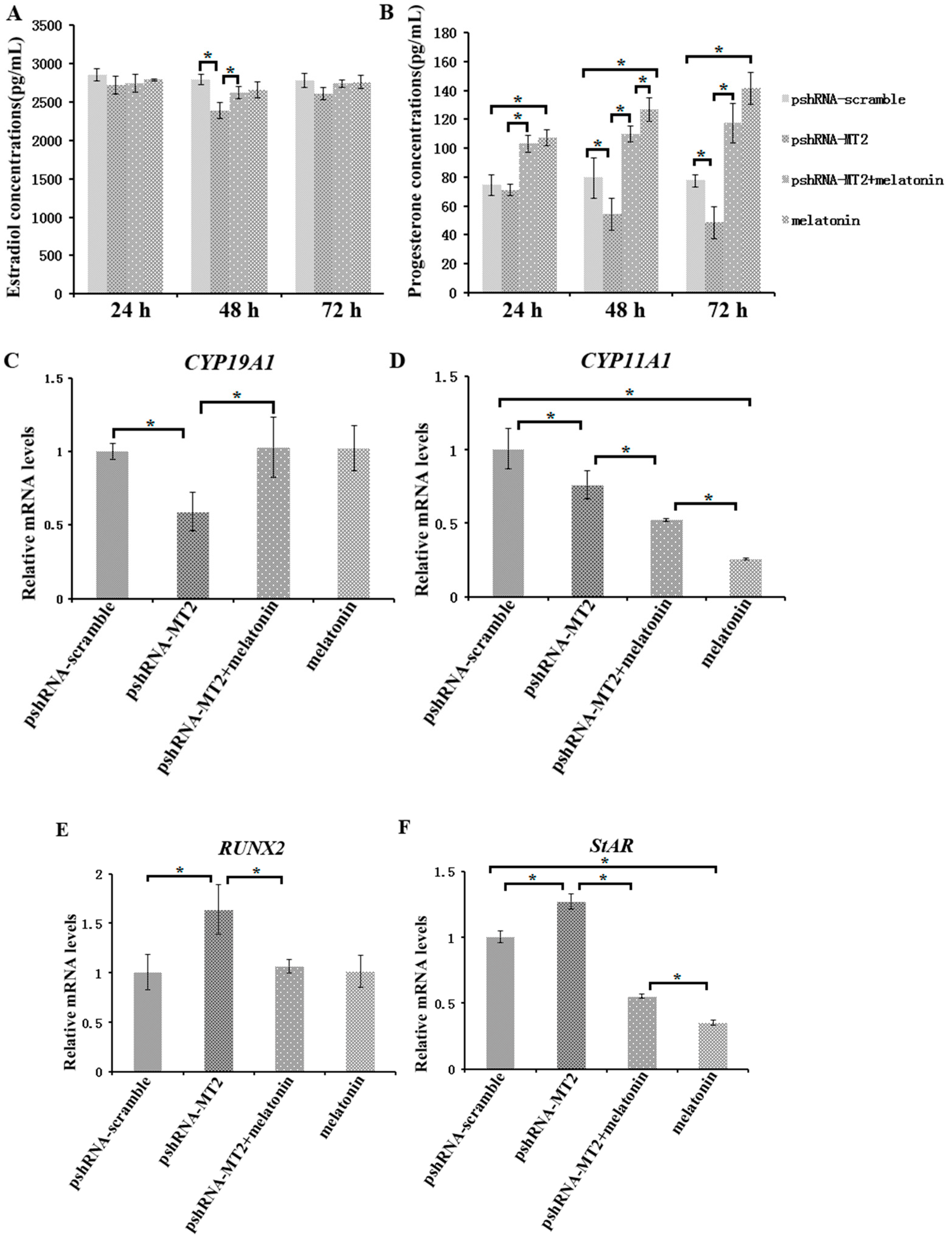
2.3. Effects of MT2 Silencing and Melatonin Treatment on Endocrine Secretions and Endocrine Related Genes Expression
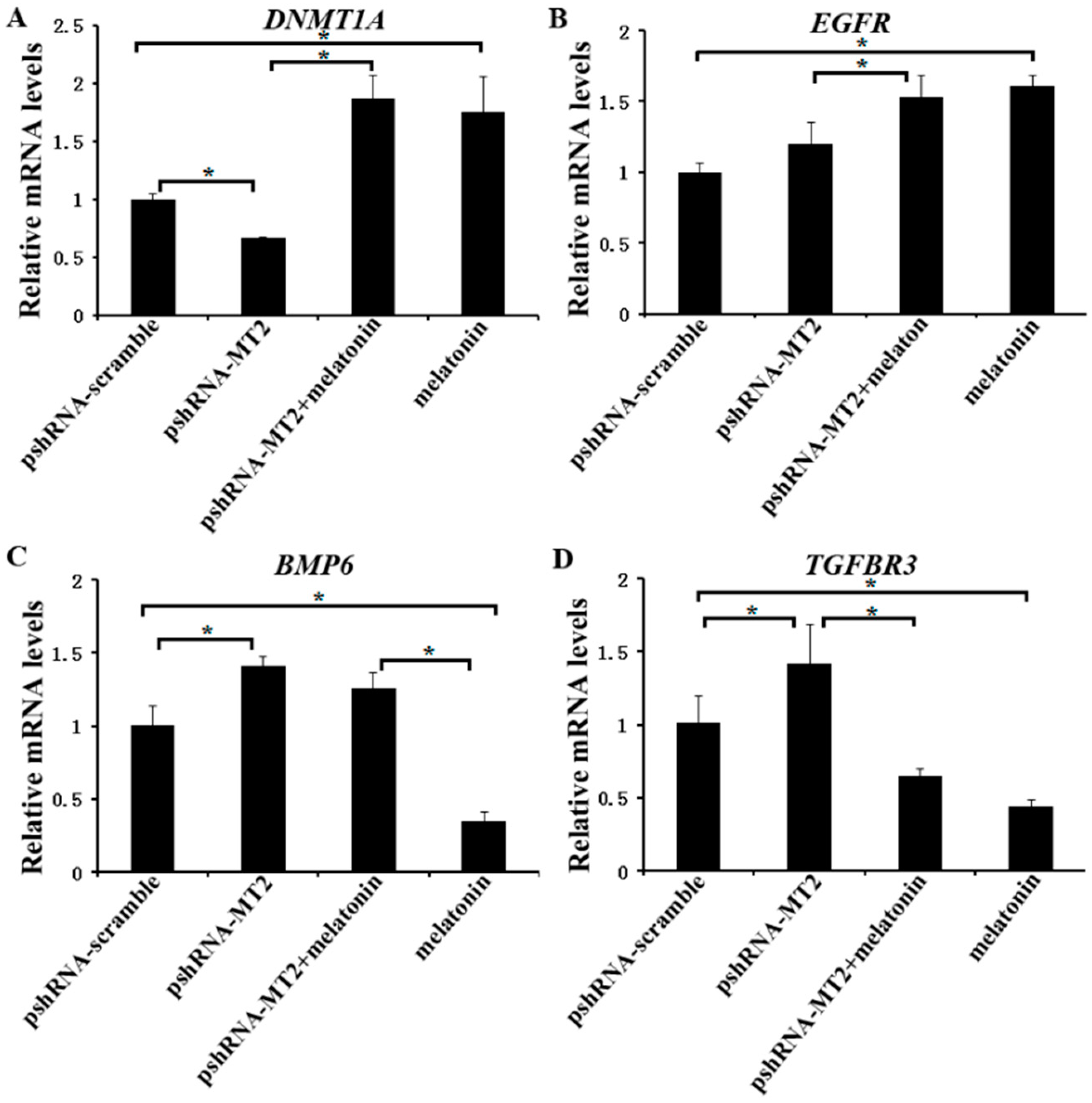
2.4. Effects of MT2 Silencing and Melatonin Treatment on Development Related Genes Expression
3. Discussion
4. Materials and Methods
4.1. Bovine GCs Isolation and Culture
4.2. Transfection of Recombinant Plasmids into GCs
4.3. RNA Extraction and Real-Time PCR
4.4. Western Blot Analysis
4.5. Endocrine Secretions Detection
4.6. Experimental Design
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009, 30, 624–712. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. 2012, 58, 44–50. [Google Scholar] [CrossRef]
- Kaipia, A.; Hsueh, A.J. Regulation of ovarian follicle atresia. Annu. Rev. Physiol. 1997, 59, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Skinner, M.K. Cellular interactions that control primordial follicle development and folliculogenesis. J. Soc. Gynecol. Investig. 2001, 8 (Suppl. 1), S17–S20. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S. Perspective: The ovarian follicle—A perspective in 2001. Endocrinology 2001, 142, 2184–2193. [Google Scholar] [CrossRef] [PubMed]
- Vitt, U.A.; Hsueh, A.J. Stage-dependent role of growth differentiation factor-9 in ovarian follicle development. Mol. Cell. Endocrinol. 2001, 183, 171–177. [Google Scholar] [CrossRef]
- Monget, P.; Fabre, S.; Mulsant, P.; Lecerf, F.; Elsen, J.M.; Mazerbourg, S.; Pisselet, C.; Monniaux, D. Regulation of ovarian folliculogenesis by IGF and BMP system in domestic animals. Domest. Anim. Endocrinol. 2002, 23, 139–154. [Google Scholar] [CrossRef]
- Orisaka, M.; Mizutani, T.; Tajima, K.; Orisaka, S.; Shukunami, K.; Miyamoto, K.; Kotsuji, F. Effects of ovarian theca cells on granulosa cell differentiation during gonadotropin-independent follicular growth in cattle. Mol. Reprod. Dev. 2006, 73, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.H.; Wang, L.; Riaz, H.; Wu, J.B.; Yuan, Y.F.; Han, L.; Wang, Y.L.; Zhao, Y.; Dan, Y.; Huo, LJ. Knockdown of CEBPβ by RNAi in porcine granulosa cells resulted in S phase cell cycle arrest and decreased progesterone and estradiol synthesis. J. Steroid. Biochem. Mol. Biol. 2014, 143, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Y.; Cheung, C.K.; Wang, Y.; Tsang, B.K. Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front. Biosci. 2003, 8, d222–d237. [Google Scholar] [PubMed]
- Choi, J.; Jo, M.; Lee, E.; Choi, D. Induction of apoptotic cell death via accumulation of autophagosomes in rat granulose cells. Fertil. Steril. 2011, 95, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.; Mihm, M.; Canty, M.J.; Zielak, A.E.; Baker, P.J.; Park, S.; Lonergan, P.; Smith, G.W.; Coussens, P.M.; Ireland, J.J.; et al. Differential expression of signal transduction factors in ovarian follicle development: A functional role for betaglycan and FIBP in granulosa cells in cattle. Physiol. Genom. 2008, 33, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Ishikane, S.; Kawabe, S.; Umezawa, A.; Miyamoto, K. Transcriptional regulation of genes related to progesterone production. Endocr. J. 2015, 62, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M. StAR protein and the regulation of steroid hormone biosynthesis. Annu. Rev. Physiol. 2001, 63, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Matti, N.; Irving-Rodgers, H.F.; Hatzirodos, N.; Sullivan, T.R.; Rodgers, R.J. Differential expression of focimatrix and steroidogenic enzymes before size deviation during waves of follicular development in bovine ovarian follicles. Mol. Cell. Endocrinol. 2010, 321, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.; Nguyen, M.N.; Benson, H.A.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In vivo evidence for a novel pathway of vitamin D3metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Chen, J.; Nguyen, M.N.; Li, W.; Yates, C.R.; Sweatman, T.; Janjetovic, Z.; Tuckey, R.C. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int. J. Biochem. Cell Biol. 2012, 44, 2003–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Li, W.; Kim, T.K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015, 151, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ireland, J.J.; Roche, J.F. Development of antral follicles in cattle after prostaglandin-induced luteolysis: Changes in serum hormones, steroids in follicular fluid, and gonadotropin receptors. Endocrinology 1982, 111, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.J.; Roche, J.F. Development of nonovulatory antral follicles inheifers: Changes in steroids in follicular fluid and receptors for gonadotropins. Endocrinology 1983, 112, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y.; et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr. J. 2013, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, F.; He, C.; Zhang, L.; Tan, D.; Reiter, R.J.; Xu, J.; Ji, P.; Liu, G. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J. Pineal Res. 2014, 57, 239–2347. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Liu, W.J.; Wu, C.J.; Ma, F.H.; Ahmad, S.; Liu, B.R.; Han, L.; Jiang, X.P.; Zhang, S.J.; Yang, L.G. Melatonin suppresses apoptosis and stimulates progesterone production by bovine granulosa cells via its receptors (MT1 and MT2). Theriogenology 2012, 78, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, B.; Liu, W.; Xiao, Y.; Zhang, H.; Yang, L. The effects of melatonin on bovine uniparental embryos development in vitro and the hormone secretion of COCs. Peer J. 2017, 5, e3485. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Liu, W.J.; Wang, L.K.; Pang, X.S.; Yang, L.G. The role of Melatonin receptor MTNR1A in the action of Melatonin on bovine granulosa cells. Mol. Reprod. 2017, 84, 1140–1154. [Google Scholar]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Tamura, H.; Takayama, H.; Kato, H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil. Steril. 2003, 80, 1012–1016. [Google Scholar] [CrossRef]
- Liu, W.J.; Wang, S.J.; Zhou, J.X.; Pang, X.S.; Wang, L.K. RNAi-mediated knockdown of MTNR1B without disrupting the effects of melatonin on apoptosis and cell cycle in bovine granulose cells. Peer J. 2018, 6, e4463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, M.H.; Lima-Verde, I.B.; Luque, M.C.; Maia, J.E., Jr.; Silva, J.R.; Celestino, J.J.; Martins, F.S.; Bao, S.N.; Lucci, C.M.; Figueiredo, J.R. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote 2007, 15, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.V.; Rossetto, R.; Brito, I.R.; Celestino, J.J.; Silva, C.M.; Faustino, L.R.; Almeida, A.P.; Bruno, J.B.; Magalhães, D.M.; Matos, M.H.; et al. Dynamic medium produces caprine embryo from preantral follicles grown in vitro. Reprod. Sci. 2010, 17, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Misztal, T.; Romanowicz, K. Effective stimulation of daily LH secretion by the combined treatment with melatonin and naloxone in luteal-phase ewes. Acta Neurobiol. Exp. (Wars) 2005, 65, 1–9. [Google Scholar] [PubMed]
- He, C.J.; Ma, T.; Shi, J.M.; Zhang, Z.Z.; Wang, J.; Zhu, K.; Li, Y.; Yang, M.; Song, Y.; Liu, G. Melatonin and its receptor MT1 are involved in the downstream reaction to luteinizing hormone and participate in the regulation of luteinization in different species. J. Pineal Res. 2016, 61, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.V.; Celestino, J.J.; Araújo, V.R.; Chaves, R.N.; Almeida, A.P.; Lima-Verde, I.B.; Duarte, A.B.; Silva, G.M.; Martins, F.S.; Bruno, J.B. Expression of follicle-stimulating hormone receptor (FSHR) in goat ovarian follicles and the impact of sequential culture medium on in vitro development of caprine preantral follicles. Zygote 2011, 19, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Barros, V.R.; Cavalcante, A.Y.; Macedo, T.J.; Barberino, R.S.; Lins, T.L.; Gouveia, B.B.; Menezes, V.G.; Queiroz, M.A.; Araújo, V.R.; Palheta, R.C., Jr.; et al. Immunolocalization of melatonin and follicle-stimulating hormone receptors in caprine ovaries and their effects during in vitro development of isolated pre-antral follicles. Reprod. Domest. Anim. 2013, 48, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.M.; Tai, C.J.; Kang, S.K.; Nathwani, P.S.; Pang, S.F.; Leung, P.C. Direct action of melatonin in human granulosa-luteal cells. J. Clin. Endocrinol. Metab. 2001, 86, 4789–4797. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhang, J.; Lin, F.; Ma, X.; Wang, X.; Liu, H. Expression profiles of key candidate genes involved in steroidogenesis during follicular atresia in the pig ovary. Mol. Biol. Rep. 2012, 39, 10823–10832. [Google Scholar] [CrossRef] [PubMed]
- Tanavde, V.S.; Maitra, A. In vitro modulation of steroidogenesis and gene expression by melatonin: A study with porcineantral follicles. Endocr. Res. 2003, 29, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Swan, C.L.; Agostini, M.C.; Bartlewski, P.M.; Feyles, V.; Urban, R.J.; Chedrese, P.J. Effects on progesterone synthesis in a stable porcine granulosa cell line: Control of transcriptional activity of the cytochrome P450Side-chain cleavage gene. Biol. Reprod. 2002, 66, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Steroidogenic acute regulatory protein (StAR), a novel mitochon- drial cholesterol transporter. Biochim. Biophys. Acta 2007, 1771, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.K.; Stouffer, R.L.; Strauss, J.F., 3rd. Quantitative analysis of the hormone-induced hyperacetylation of histoneH3associated with the steroido-genic acute regulatory protein gene promoter. J. Biol. Chem. 2001, 276, 27392–27399. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Lind, A.K.; Dahm-Kahler, P.; Brannstrom, M.; Carletti, M.Z.; Christenson, L.K.; Curry, T.E., Jr.; Jo, M. RUNX2 transcription factor regulates gene expression in luteinizing granulosa cells of rat ovaries. Mol. Endocrinol. 2010, 24, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Papamentzelopoulou, M.; Mavrogianni, D.; Dinopoulou, V.; Theofanakis, H.; Malamas, F.; Marinopoulos, S.; Bletsa, R.; Anagnostou, E.; Kallianidis, K.; Loutradis, D. Detection of RUNX2 gene expression in cumulus cells in women undergoing controlled ovarian stimulation. Reprod. Biol. Endocrinol. 2012, 10, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimasaki, S.; Moore, R.K.; Otsuka, F.; Erickson, G.F. The bone morpho-genetic protein system in mammalian reproduction. Endocr. Rev. 2004, 25, 72–101. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F. Multifunctional bone morphogenetic protein system in endocrinol-ogy. Acta Med. Okayama 2013, 67, 75–86. [Google Scholar] [PubMed]
- Erickson, G.F.; Shimasaki, S. The spatiotemporal expression pattern of the bonemorphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod. Biol. Endocrinol. 2003, 1, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimasaki, S.; Moore, R.K.; Erickson, G.F.; Otsuka, F. The role of bonemorphogenetic proteins in ovarian function. Reprod. Suppl. 2003, 61, 323–337. [Google Scholar] [PubMed]
- Nakamura, E.; Otsuka, F.; Terasaka, T.; Inagaki, K.; Hosoya, T.; Tsukamoto-Yamauchi, N.; Toma, K.; Makino, H. Melatonin counteracts BMP-6 regulation of steroidogenesis by rat granulosa cells. J. Steroid. Biochem. Mol. Biol. 2014, 143, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Moore, R.K.; Shimasaki, S. Biological function and cellular mecha-nism of bone morphogenetic protein-6 in the ovary. J. Biol. Chem. 2001, 276, 32889–32895. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Krassel, F.; Winn, M.E.; Burns, D.; Ireland, J.L.; Ireland, J.J. Evidence for a negative intrafollicular role for inhibin in regulation of estradiol production by granulosa cells. Endocrinology 2003, 144, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Henderson, S.; Souza, C.; Ludlow, H.; Groome, N.; McNeilly, A.S. Activin B is produced early in antral follicular development and suppresses thecal androgen production. Reproduction 2012, 143, 637–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.M.; Cheng, J.C.; Huang, H.F.; Shi, F.T.; Leung, P.C. Activin A, B and AB decrease progesterone production by down-regulating StAR in human granulosa cells. Mol. Cell. Endocrinol. 2015, 412, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Noguchi, J.; Kikuchi, K.; Todoroki, J.; Hasegawa, Y. Alterations in peripheral concentrations of inhibinAin cattle studied using a time-resolved immunofluorometric assay: Relationship with estradiol and follicle-stimulating hormone in various reproductive conditions. Biol. Reprod. 2002, 67, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Thackray, V.G.; Mellon, P.L.; Coss, D. Hormones in synergy: Regulation of the pituitary gonadotropin genes. Mol. Cell. Endocrinol. 2010, 314, 192–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Wu, C.; Riaz, H.; Bai, L.; Chen, J.; Zhen, Y.; Guo, A.; Yang, L. Characterization of the mechanism of inhibin α-subunit gene in mouse anterior pituitary cells by RNA interference. PLoS ONE 2013, 8, E74596. [Google Scholar] [CrossRef] [PubMed]
- Takedomi, T.; Kishi, H.; Medan, M.S.; Aoyagi, Y.; Konishi, M.; Itoh, T.; Yazawa, S.; Watanabe, G.; Taya, K. Active immunization against inhibin improves superovulatory response to exogenous FSH in cattle. J. Reprod. Dev. 2005, 51, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Mao, D.G.; Zhang, D.K.; Liang, A.X.; Fang, M.; Moaeen-ud-Din, M.; Yang, L.G. Development and evaluation of a novel DNA vaccine expressing inhibin alpha (1–32) fragment for improving the fertility in rats and sheep. Anim. Reprod. Sci. 2008, 109, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Dan, X.; Liu, X.; Han, Y.; Liu, Q.; Yang, L. Effect of the novel DNA vaccine fusing inhibin α (1–32) and the RF-amide related peptide-3 genes onimmune response, hormone levels and fertility in Tan sheep. Anim. Reprod. Sci. 2016, 164, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tian, X.; Zhang, L.; Gao, C.; He, C.; Fu, Y.; Ji, P.; Li, Y.; Li, N.; Liu, G. Beneficial effects of melatonin on in vitro bovine embryonic development are mediated by melatonin receptor 1. J. Pineal Res. 2014, 56, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, F.; Zhang, L.; He, C.; Ji, P.; Wang, J.; Zhang, Z.; Lv, D.; Abulizi, W.; Wang, X.; et al. Beneficial Effects of Melatonin on the In Vitro Maturation of Sheep Oocytes and Its Relation to Melatonin Receptors. Int. J. Mol. Sci. 2017, 18, E834. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Grusby, L.; Beard, C.; Possemato, R.; Tudor, M.; Fambrough, D.; Csankovszki, G.; Dausman, J.; Lee, P.; Wilson, C.; Lander, E.; et al. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat. Genet. 2001, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Takeda, Y.; Janjetovic, Z.; Brozyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORα and RORγ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar] [CrossRef] [PubMed]
- Steinhilber, D.; Brungs, M.; Werz, O.; Wiesenberg, I.; Danielsson, C.; Kahlen, J.P.; Nayeri, S.; Schräder, M.; Carlberg, C. The nuclear receptor for melatonin represses 5-lipoxygenase gene expression in human B lymphocytes. J. Biol. Chem. 1995, 270, 7037–7040. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Jetten, A.M. RORα is not a receptor for melatonin (response to DOI 10.1002/bies.201600018). Bioessays 2016, 38, 1193–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prendergast, B.J. MT1 melatonin receptors mediate somatic, behavioral, and reproductive neuroendocrine responses to photoperiod and melatonin in Siberian hamsters (Phodopus sungorus). Endocrinol 2010, 151, 714–721. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Mice Oocyte’s Quality underin Vitro Conditions. Int. J. Mol. Sci. 2016, 17, E939. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yang, M.; Zhu, K.; Wang, L.; Song, Y.; Wang, J.; Qin, W.; Xu, Z.; Chen, Y.; Liu, G. Melatonin implantation improved the egg-laying rate and quality in hens past their peak egg-laying age. Sci. Rep. 2016, 6, 39799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espino, J.; Ortiz, Á.; Bejarano, I.; Lozano, G.M.; Monllor, F.; García, J.F.; Rodríguez, A.B.; Pariente, J.A. Melatonin protects human spermatozoa from apoptosis via melatonin receptor- and extracellular signal-regulated kinase-mediated pathways. Fertil. Steril. 2011, 95, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Rodríguez, A.B.; Pariente, J.A. The inhibition of TNF-α-induced leucocyte apoptosis by melatonin involves membrane receptor MT1/MT2 interaction. J. Pineal Res. 2013, 54, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Radogna, F.; Cristofanon, S.; Paternoster, L.; D’Alessio, M.; De Nicola, M.; Cerella, C.; Dicato, M.; Diederich, M.; Ghibelli, L. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J. Pineal Res. 2008, 44, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Name | Target sequence (5’→3’) | Position on CDs |
|---|---|---|
| pshRNA-MT2 * | GCTACTTCCTGGCCTATTTCA | 878 |
| pshRNA-scramble * | CTTCATAAGGCGCATAGC |
| Gene | Forward Primer Sequence (5′→3′) | Reverse Primer Sequence (5′→3′) | Length |
|---|---|---|---|
| MTNR1B | GGAGCTTTCTGAGCATGTTTG | CCCTGCGGAAGTTCTGGTT | 210 |
| CYP11A1 | ATGCTGGAGGAGACAGTGAACC | GCAGTAGAGGATGCCTGGGTAA | 249 |
| CYP19A1 | CACCCATCTTTGCCAGGTAGTC | ACCCACAGGAGGTAAGCCTATAAA | 78 |
| StAR | GTG GAT TTT GCC AAT CAC CT | TTATTG AAA ACG TGC CAC CA | 203 |
| RUNX2 | AAGGCAAGGCTAGGTGGAAT | AGAGGGGCACAGACTTTGAA | 189 |
| DNMT1A | ACGAATGGTGGATTGCTGGT | CACGTCTTCGTAGGTGGAGTC | 197 |
| EGFR | CACTCATGCTCTATGACCCTACC | CTCACCGATTCCTATTCCGTTAC | 176 |
| BMP6 | TACGCTGCCAACTACTGTGAC | GATGGCGTTCAGTTTCGTG | 153 |
| INHA | GCACCCTCCCAGTTTCATCT | GGTTGGGCACCATCTCATACT | 230 |
| INHBA | GCAGTCGCACAGACCTTTCCT | CTCACAGTAGTTGGCGTGGTAGC | 196 |
| INHBB | CCTCATCGGCTGGAACGACTGG | TGGACATGGTGCTCAGCTTGGTG | 114 |
| FSHR | GAAGAAAGCAGGTGGATGGA | GGCAGAGGAAAACTCCGTTA | 126 |
| LHR | GACACTAATTGCCACATCATCCT | GTGTCTTGGGTAAGCAGAAACC | 203 |
| TGFBR3 | ACTGTTGCCCCACCATAGAG | CCTGGAAATCTTAGCCCTCA | 103 |
| ACTB | CATCGGCAATGAGCGGTTCC | CCGTGTTGGCGTAGAGGTCC | 145 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liu, W.; Pang, X.; Dai, S.; Liu, G. The Mechanism of Melatonin and Its Receptor MT2 Involved in the Development of Bovine Granulosa Cells. Int. J. Mol. Sci. 2018, 19, 2028. https://doi.org/10.3390/ijms19072028
Wang S, Liu W, Pang X, Dai S, Liu G. The Mechanism of Melatonin and Its Receptor MT2 Involved in the Development of Bovine Granulosa Cells. International Journal of Molecular Sciences. 2018; 19(7):2028. https://doi.org/10.3390/ijms19072028
Chicago/Turabian StyleWang, Shujuan, Wenju Liu, Xunsheng Pang, Sifa Dai, and Guodong Liu. 2018. "The Mechanism of Melatonin and Its Receptor MT2 Involved in the Development of Bovine Granulosa Cells" International Journal of Molecular Sciences 19, no. 7: 2028. https://doi.org/10.3390/ijms19072028




