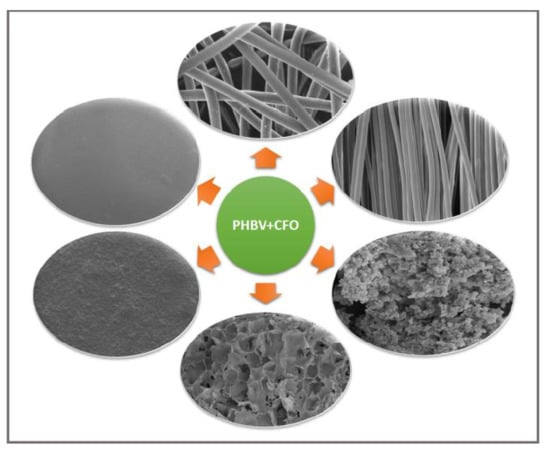
Tailored Biodegradable and Electroactive Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Based Morphologies for Tissue Engineering Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphological Characterization
2.2. Physico-Chemical Properties
2.3. Thermal Analysis
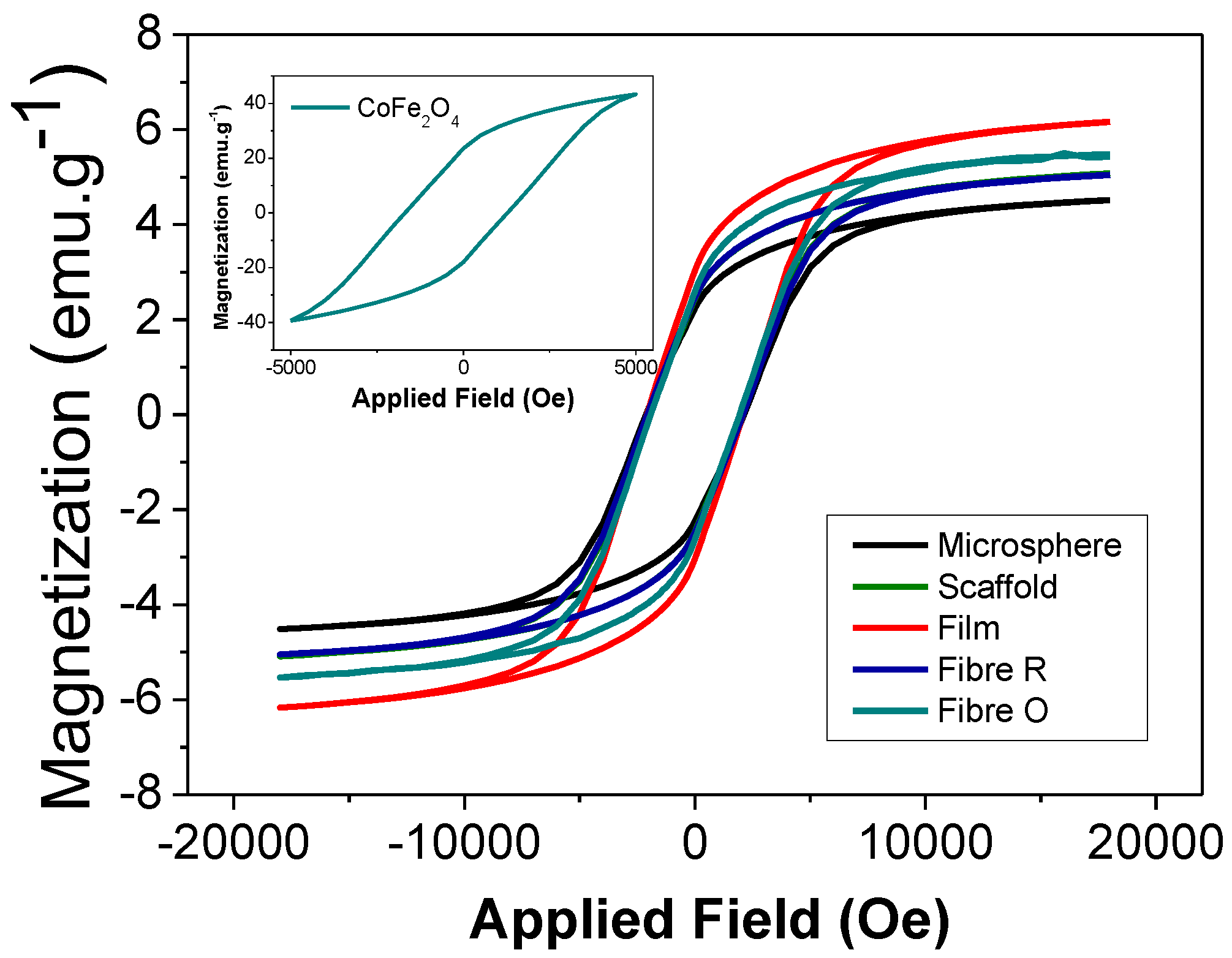
2.4. Magnetic Properties of the Composites
2.5. Contact Angle Measurements
2.6. Mechanical Properties
2.7. Cytotoxicity Evaluation
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Polymer Solution
3.3. Processing of PHBV into Different Morphologies
3.3.1. Films
3.3.2. Electrospun Fibers
3.3.3. Microspheres
3.3.4. Scaffolds
3.4. Characterization
3.5. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Rahman, M.S.; Tsuchiya, T. Enhancement of chondrogenic differentiation of human articular chondrocytes by biodegradable polymers. Tissue Eng. 2001, 7, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Parssinen, J.; Hammaren, H.; Rahikainen, R.; Sencadas, V.; Ribeiro, C.; Vanhatupa, S.; Miettinen, S.; Lanceros-Mendez, S.; Hytonen, V.P. Enhancement of adhesion and promotion of osteogenic differentiation of human adipose stem cells by poled electroactive poly(vinylidene fluoride). J. Biomed. Mater. Res. A 2015, 103, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.; Tanner, E.T.; Bonfield, W. In vitro and in vivo evaluation of polyhydroxybutyrate and of polyhydroxybutyrate reinforced with hydroxyapatite. Biomaterials 1991, 12, 841–847. [Google Scholar] [CrossRef]
- Kose, G.T.; Korkusuz, F.; Korkusuz, P.; Hasirci, V. In vivo tissue engineering of bone using poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) and collagen scaffolds. Tissue Eng. 2004, 10, 1234–1250. [Google Scholar] [CrossRef] [PubMed]
- Fukada, E.; Ando, Y. Piezoelectric properties of poly-β-hydroxybutyrate and copolymers of β-hydroxybutyrate and β-hydroxyvalerate. Int. J. Biol. Macromol. 1986, 8, 361–366. [Google Scholar] [CrossRef]
- Ohigashi, H. Piezoelectric polymers–materials and manufacture. Jpn. J. Appl. Phys. 1985, 24, 23. [Google Scholar] [CrossRef]
- Ando, M.; Kawamura, H.; Kageyama, K.; Tajitsu, Y. Film sensor device fabricated by a piezoelectric poly(l-lactic acid) film. Jpn. J. Appl. Phys. 2012, 51, 09LD14. [Google Scholar] [CrossRef]
- Ribeiro, C.; Moreira, S.; Correia, V.; Sencadas, V.; Rocha, J.G.; Gama, F.M.; Ribelles, J.L.G.; Lanceros-Mendez, S. Enhanced proliferation of pre-osteoblastic cells by dynamic piezoelectric stimulation. RSC Adv. 2012, 2, 11504–11509. [Google Scholar] [CrossRef]
- Martins, P.M.; Ribeiro, S.; Ribeiro, C.; Sencadas, V.; Gomes, A.C.; Gama, F.M.; Lanceros-Mendez, S. Effect of poling state and morphology of piezoelectric poly(vinylidene fluoride) membranes for skeletal muscle tissue engineering. RSC Adv. 2013, 3, 17938–17944. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Mendez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Gonçalves, R.; Cardoso, V.F.; Lanceros-Méndez, S. Electroactive poly(vinylidene fluoride)-based structures for advanced applications. Nat. Protoc. 2018, 13, 681–704. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Correia, V.; Martins, P.; Gama, F.M.; Lanceros-Mendez, S. Proving the suitability of magnetoelectric stimuli for tissue engineering applications. Colloids Surf. B Biointerfaces 2016, 140, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Lanceros-Mendez, S. Polymer-based magnetoelectric materials. Adv. Funct. Mater. 2013, 23, 3371–3385. [Google Scholar] [CrossRef]
- Cho, K.H.; Bichurin, M.I.; Petrov, V.M.; Bhalla, A.; Priya, S. Magnetoelectric laminate composite: Effect of piezoelectric layer on magnetoelectric properties. Ferroelectrics 2014, 473, 110–128. [Google Scholar] [CrossRef]
- Wickens, A.; Robinson, J. Magnetoelectric neural modulation. Biophys. J. 2017, 112, 286a. [Google Scholar] [CrossRef]
- Ribeiro, C.; Correia, D.; Ribeiro, S.; Fernandes, M.; Lanceros-Mendez, S. Piezo-and magnetoelectric polymers as biomaterials for novel tissue engineering strategies. MRS Adv. 2018, 3, 1671–1676. [Google Scholar] [CrossRef]
- Hu, J.M.; Nan, T.X.; Sun, N.X.; Chen, L.Q. Multiferroic magnetoelectric nanostructures for novel device applications. MRS Bull. 2015, 40, 728–735. [Google Scholar] [CrossRef]
- Correia, D.M.; Ribeiro, C.; Sencadas, V.; Vikingsson, L.; Oliver Gasch, M.; Gómez Ribelles, J.L.; Botelho, G.; Lanceros-Méndez, S. Strategies for the development of three dimensional scaffolds from piezoelectric poly(vinylidene fluoride). Mater. Des. 2016, 92, 674–681. [Google Scholar] [CrossRef]
- Fei, B.; Chen, C.; Wu, H.; Peng, S.; Wang, X.; Dong, L. Quantitative FTIR study of PHBV/bisphenol a blends. Eur. Polym. J. 2003, 39, 1939–1946. [Google Scholar] [CrossRef]
- Kim, G.M.; Michler, G.H.; Henning, S.; Radusch, H.J.; Wutzler, A. Thermal and spectroscopic characterization of microbial poly(3-hydroxybutyrate) submicrometer fibers prepared by electrospinning. J. Appl. Polym. Sci. 2007, 103, 1860–1867. [Google Scholar] [CrossRef]
- Xu, Y.; Zou, L.; Lu, H.; Wei, Y.; Hua, J.; Chen, S. Preparation and characterization of electrospun phbv/peo mats: The role of solvent and peo component. J. Mater. Sci. 2016, 51, 5695–5711. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Qin, Z.-Y.; Zhou, Z. Cellulose nanocrystals as green fillers to improve crystallization and hydrophilic property of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Prog. Nat. Sci. Mater. Int. 2011, 21, 478–484. [Google Scholar] [CrossRef]
- Ribeiro, C.; Pärssinen, J.; Sencadas, V.; Correia, V.; Miettinen, S.; Hytönen Vesa, P.; Lanceros-Méndez, S. Dynamic piezoelectric stimulation enhances osteogenic differentiation of human adipose stem cells. J. Biomed. Mater. Res. Part A 2014, 103, 2172–2175. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.M.; Sencadas, V.; Ribeiro, C.; Martins, P.M.; Martins, P.; Gama, F.M.; Botelho, G.; Lanceros-Méndez, S. Processing and size range separation of pristine and magnetic poly(l-lactic acid) based microspheres for biomedical applications. J. Colloid Interface Sci. 2016, 476, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.T.; Mirmohammadi, S.A.; Irani, S. Polyhydroxybutyrate (phb) scaffolds as a model for nerve tissue engineering application: Fabrication and in vitro assay. Int. J. Polym. Mater. Polym. Biomater. 2011, 60, 562–575. [Google Scholar] [CrossRef]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) phas and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Sencadas, V.; Gregorio, R., Jr.; Lanceros-Méndez, S. A to β phase transformation and microestructural changes of pvdf films induced by uniaxial stretch. J. Macromol. Sci. Part B Phys. 2009, 48, 514–525. [Google Scholar] [CrossRef]
- Brito-Pereira, R.; Correia, D.M.; Ribeiro, C.; Francesko, A.; Etxebarria, I.; Pérez-Álvarez, L.; Vilas, J.L.; Martins, P.; Lanceros-Mendez, S. Silk fibroin-magnetic hybrid composite electrospun fibers for tissue engineering applications. Compos. Part B Eng. 2018, 141, 70–75. [Google Scholar] [CrossRef]
- Goncalves, R.; Martins, P.; Correia, D.M.; Sencadas, V.; Vilas, J.L.; Leon, L.M.; Botelho, G.; Lanceros-Mendez, S. Development of magnetoelectric cofe2o4/poly(vinylidene fluoride) microspheres. RSC Adv. 2015, 5, 35852–35857. [Google Scholar] [CrossRef]
- Maciel, M.M.; Ribeiro, S.; Ribeiro, C.; Francesko, A.; Maceiras, A.; Vilas, J.L.; Lanceros-Méndez, S. Relation between fiber orientation and mechanical properties of nano-engineered poly(vinylidene fluoride) electrospun composite fiber mats. Compos. Part B Eng. 2018, 139, 146–154. [Google Scholar] [CrossRef]
- Martins, P.; Costa, C.M.; Benelmekki, M.; Botelho, G.; Lanceros-Mendez, S. On the origin of the electroactive poly(vinylidene fluoride) β-phase nucleation by ferrite nanoparticles via surface electrostatic interactions. CrystEngComm 2012, 14, 2807–2811. [Google Scholar] [CrossRef]
- Min, M.; Shi, Y.; Ma, H.; Huang, H.; Shi, J.; Chen, X.; Liu, Y.; Wang, L. Polymer-nanoparticle composites composed of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and coated silver nanoparticles. J. Macromol. Sci. Part B 2015, 54, 411–423. [Google Scholar] [CrossRef]
- Thiré, R.M.D.S.M.; Arruda, L.C.; Barreto, L.S. Morphology and thermal properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/attapulgite nanocomposites. Mater. Res. 2011, 14, 340–344. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H.; Kaczmarek-Kędziera, A. Effect of side substituents on thermal stability of the modified chitosan and its nanocomposites with magnetite. J. Therm. Anal. Calorim. 2016, 124, 1267–1280. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact angle and wetting properties. In Springer Series in Surface Sciences; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. [Google Scholar]
- Areias, A.C.; Ribeiro, C.; Sencadas, V.; Garcia-Giralt, N.; Diez-Perez, A.; Gómez Ribelles, J.L.; Lanceros-Méndez, S. Influence of crystallinity and fiber orientation on hydrophobicity and biological response of poly(l-lactide) electrospun mats. Soft Matter 2012, 8, 5818–5825. [Google Scholar] [CrossRef]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Gonçalves, R.; Lanceros-Mendez, S.; Lasheras, A.; Gutiérrez, J.; Barandiarán, J.M. Effect of filler dispersion and dispersion method on the piezoelectric and magnetoelectric response of CoFe2O4/P(VDF-TrFE) nanocomposites. Appl. Surf. Sci. 2014, 313, 215–219. [Google Scholar] [CrossRef]











| Sample | Tm (°C) | ΔHm (J·g−1) | Xc (%) |
|---|---|---|---|
| Film | 174 | 82 | 56 |
| Film/CFO | 177 | 70 | 48 |
| R fibers | 175 | 98 | 67 |
| R/CFO fibers | 177 | 67 | 46 |
| O fibers | 177 | 65 | 45 |
| O/CFO fibers | 179 | 79 | 54 |
| Microsphere | 175 | 57 | 39 |
| Microsphere/CFO | 174 | 57 | 39 |
| Scaffold | 177 | 63 | 43 |
| Scaffold/CFO | 182 | 61 | 42 |
| Film | Film CFO | Fibers O | Fibers O CFO | Fibers R | Fibers R CFO | Scaffold | Scaffold CFO |
|---|---|---|---|---|---|---|---|
| 90 ± 12° | 96 ± 4° | 103 ± 11° | 119 ± 5° | 125 ± 2° | 128 ± 2° | 97 ± 13° | 106 ± 9° |
| Morphologies | E (MPa) |
|---|---|
| Film | 17 ± 5 |
| Film CFO | 27 ± 5 |
| Fibers R | 1.1 ± 0.6 |
| Fibers R CFO | 1.7 ± 0.5 |
| Fibers O | 66 ± 41 |
| Fibers O CFO | 83 ± 9 |
| Scaffold | 8.9 × 10−3 ± 1.7 × 10−3 |
| Scaffold CFO | 1.3 × 10−2 ± 6.4 × 10−4 |
| Nomenclature | Composition | Morphology | Processing Technique |
|---|---|---|---|
| Film | PHBV | Film | Solvent-casting |
| Film CFO | PHBV, CoFe2O4 | ||
| Fibers R | PHBV | Randomly oriented fibers | Electrospinning |
| Fibers R CFO | PHBV, CoFe2O4 | ||
| Fibers O | PHBV | Oriented fibers | |
| Fibers O CFO | PHBV, CoFe2O4 | ||
| Microspheres | PHBV | Microspheres | Oil/water emulsion |
| Microspheres CFO | PHBV, CoFe2O4 | ||
| Scaffold | PHBV | 3D Scaffold | Solvent-casting/particulate leaching |
| Scaffold CFO | PHBV, CoFe2O4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaro, L.; Correia, D.M.; Marques-Almeida, T.; Martins, P.M.; Pérez, L.; Vilas, J.L.; Botelho, G.; Lanceros-Mendez, S.; Ribeiro, C. Tailored Biodegradable and Electroactive Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Based Morphologies for Tissue Engineering Applications. Int. J. Mol. Sci. 2018, 19, 2149. https://doi.org/10.3390/ijms19082149
Amaro L, Correia DM, Marques-Almeida T, Martins PM, Pérez L, Vilas JL, Botelho G, Lanceros-Mendez S, Ribeiro C. Tailored Biodegradable and Electroactive Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Based Morphologies for Tissue Engineering Applications. International Journal of Molecular Sciences. 2018; 19(8):2149. https://doi.org/10.3390/ijms19082149
Chicago/Turabian StyleAmaro, Luís, Daniela M. Correia, Teresa Marques-Almeida, Pedro M. Martins, Leyre Pérez, José L. Vilas, Gabriela Botelho, Senentxu Lanceros-Mendez, and Clarisse Ribeiro. 2018. "Tailored Biodegradable and Electroactive Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Based Morphologies for Tissue Engineering Applications" International Journal of Molecular Sciences 19, no. 8: 2149. https://doi.org/10.3390/ijms19082149
APA StyleAmaro, L., Correia, D. M., Marques-Almeida, T., Martins, P. M., Pérez, L., Vilas, J. L., Botelho, G., Lanceros-Mendez, S., & Ribeiro, C. (2018). Tailored Biodegradable and Electroactive Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Based Morphologies for Tissue Engineering Applications. International Journal of Molecular Sciences, 19(8), 2149. https://doi.org/10.3390/ijms19082149