Building the Resilience of Marginal Rural Areas Using a Complementary Characterization Approach: Possible Beneficial Health Effects and Stress Tolerance of Italian Common Bean (Phaseolus vulgaris L.) Landraces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Collection
2.2. Landrace Diversity Assessment
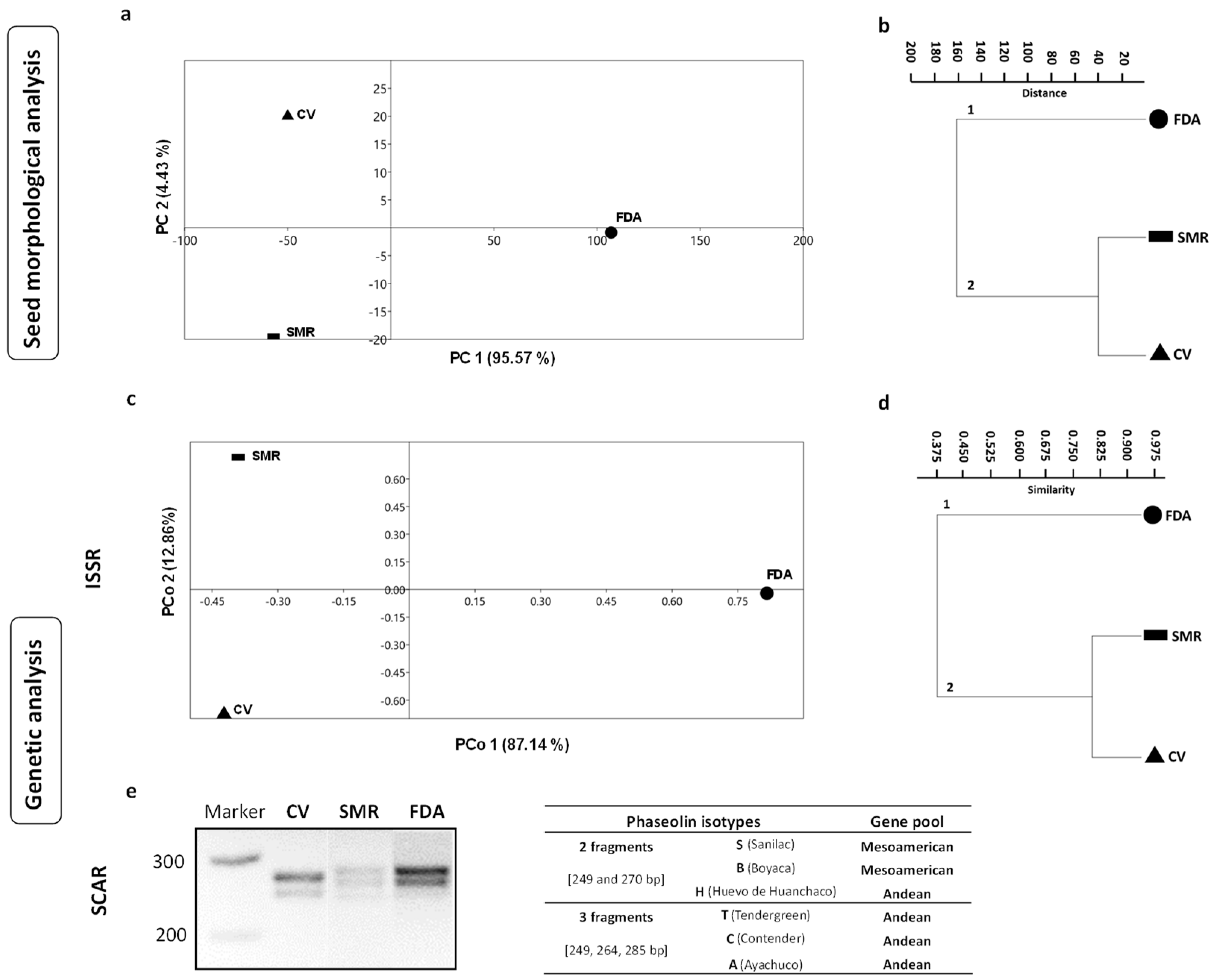
2.2.1. Seed Morphological Analysis
2.2.2. DNA Analysis: ISSR and SCAR Amplification
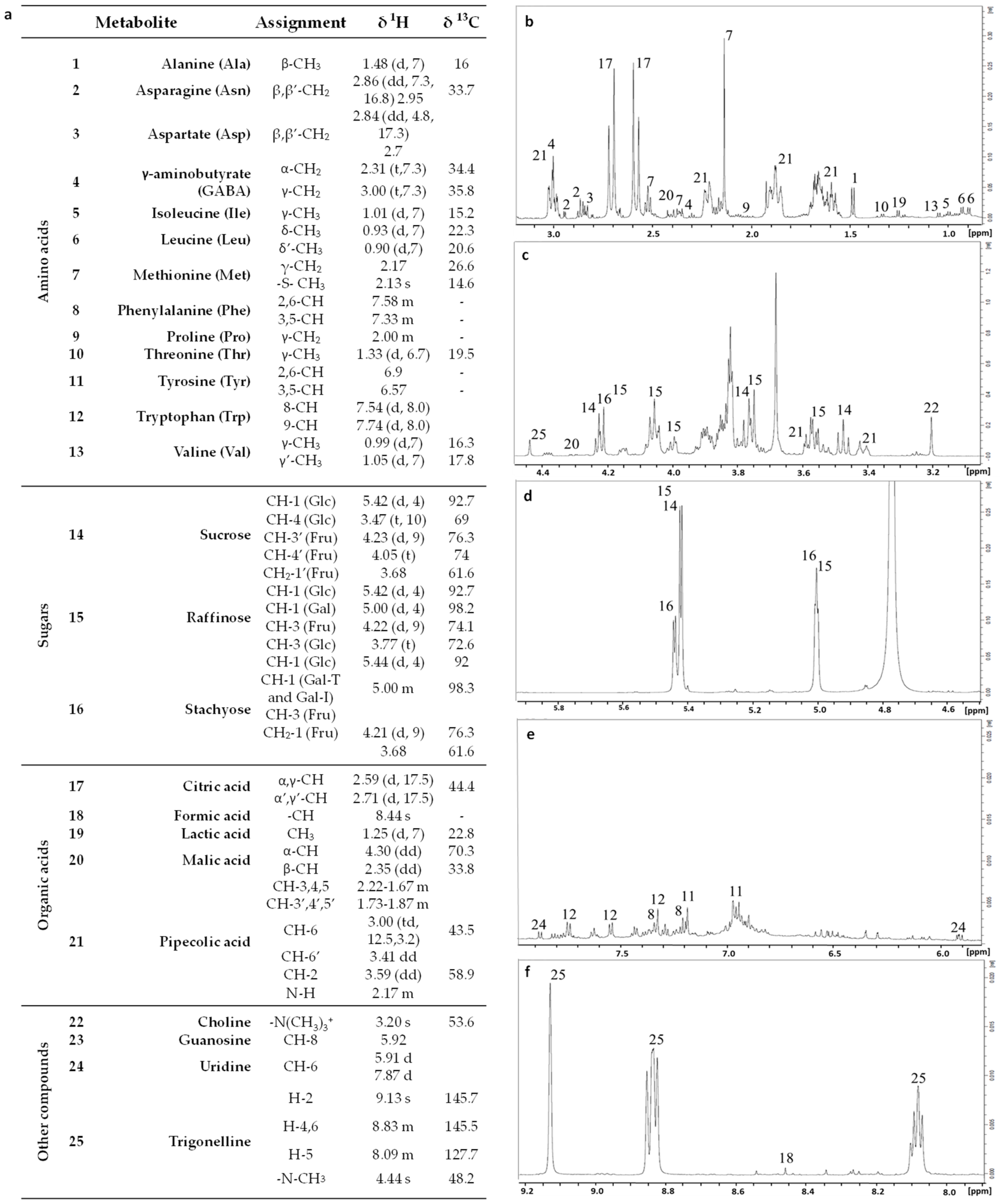
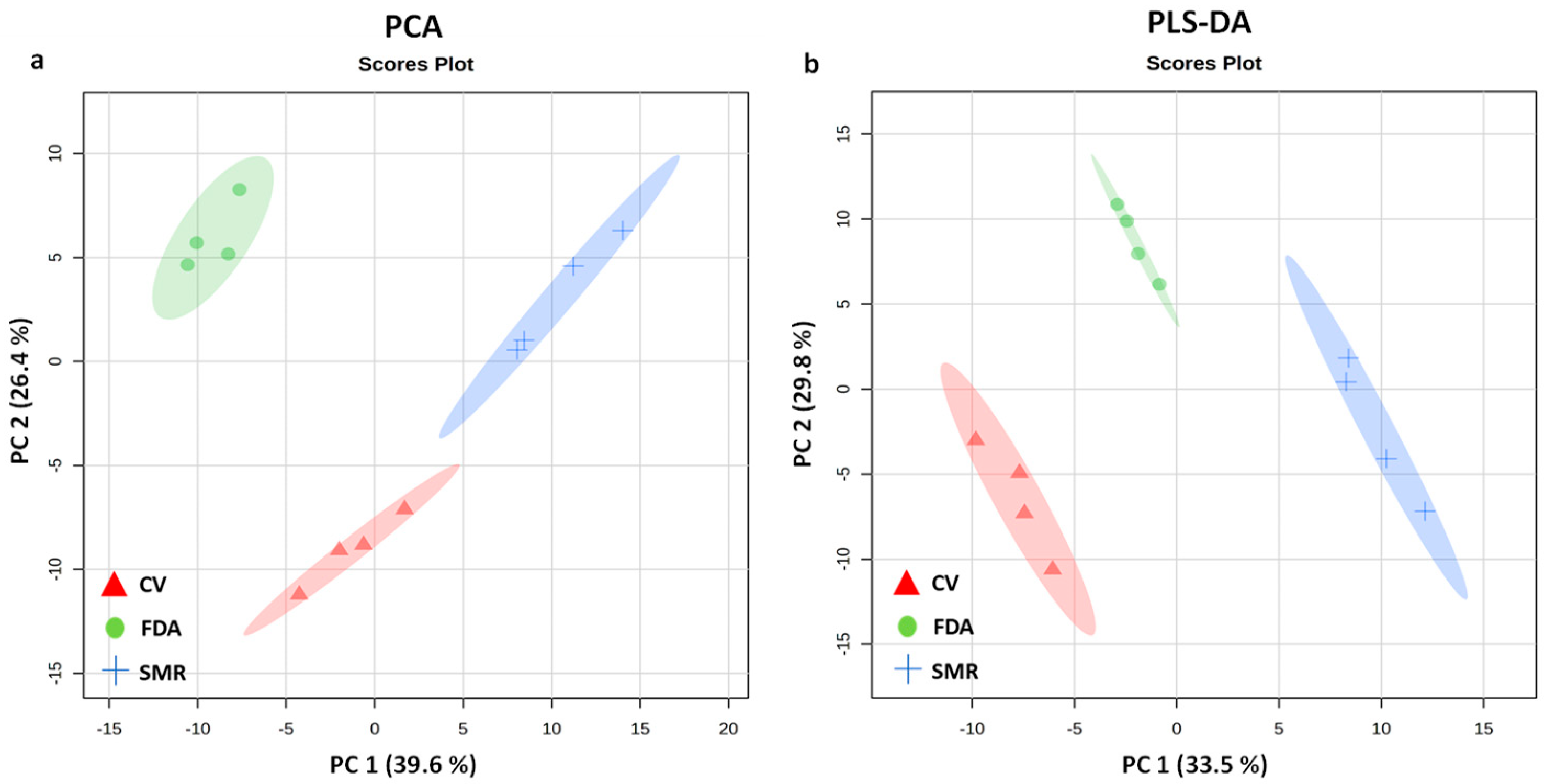
2.2.3. NMR Metabolomic Profiling
2.3. Anti-Proliferative Activity of Seed Aqueous Extracts on Caco-2 Cell Line
2.4. Seed Germination Behavior under Salt Stress
2.5. Plant Performance under Salt Stress
2.6. Statistical Analysis
3. Results
3.1. Seed Morphological Traits and Genetic Data
3.2. NMR Metabolomic Profiling Data
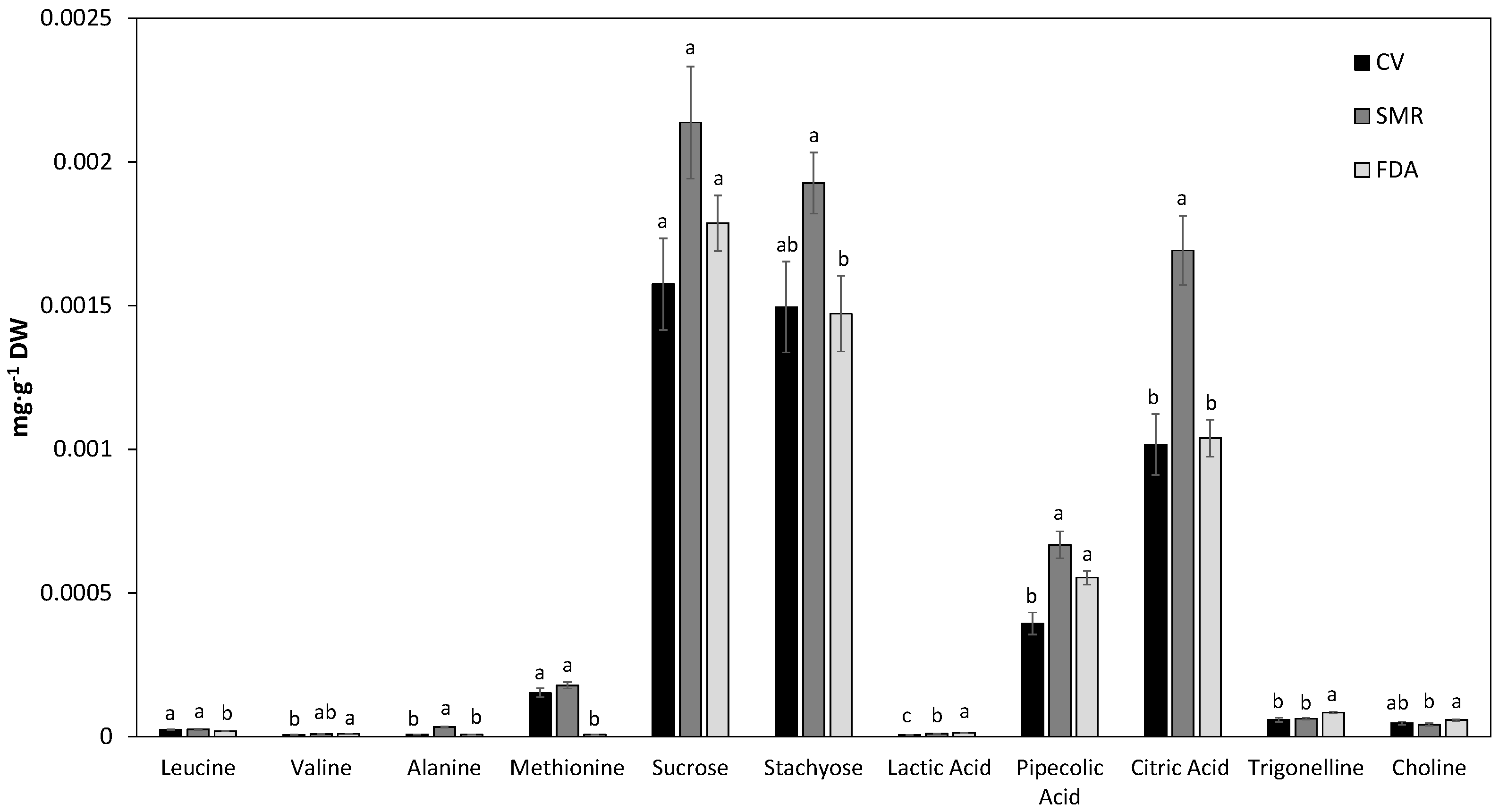
3.3. Quantification of Metabolites in Aqueous Common Bean Seed Extracts
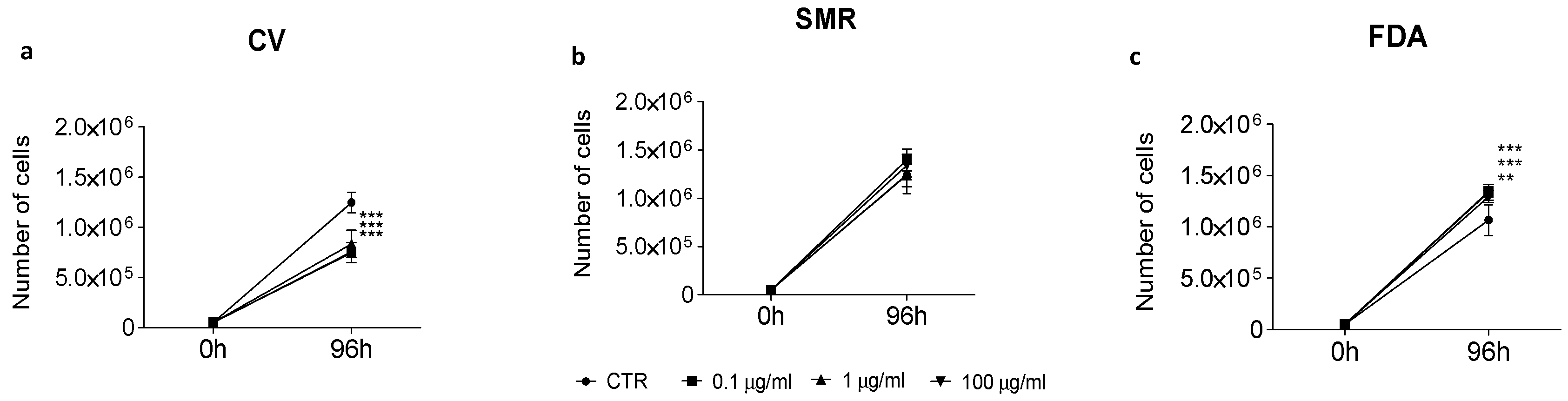
3.4. Anti-Proliferative Activity of Bean Aqueous Extracts on Caco-2 Cell Line
3.5. Effects of Salt Stress on Germination and Plant Morpho-Physiological and Biochemical Responses
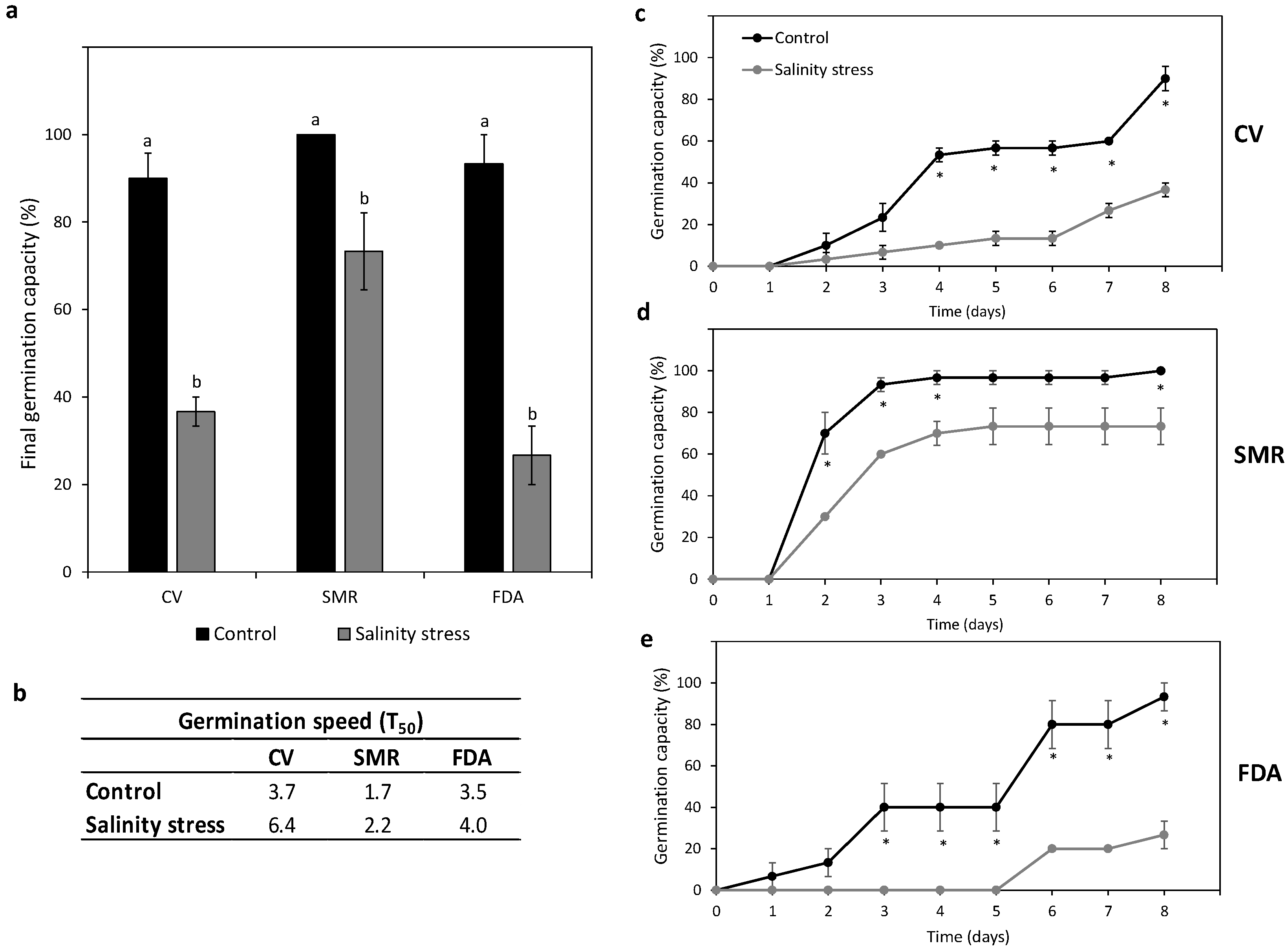
3.5.1. Germination Behavior
3.5.2. Plant Morpho-Physiological Data
3.5.3. Plant Biochemical Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoury, C.K.; Brush, S.; Costich, D.E.; Curry, H.A.; de Haan, S.; Engels, J.M.M.; Guarino, L.; Hoban, S.; Mercer, K.L.; Miller, A.J.; et al. Crop genetic erosion: Understanding and responding to loss of crop diversity. New Phytol. 2022, 233, 84–118. [Google Scholar] [CrossRef]
- Azeez, M.A.; Adubi, A.O.; Durodola, F.A.; Azeez, M.A.; Adubi, A.O.; Durodola, F.A. Landraces and Crop Genetic Improvement. In Rediscovery of Landraces as a Resource for the Future; IntechOpen: London, UK, 2018; pp. 1–19. [Google Scholar]
- Marone, D.; Russo, M.A.; Mores, A.; Ficco, D.B.M.; Laidò, G.; Mastrangelo, A.M.; Borrelli, G.M. Importance of landraces in cereal breeding for stress tolerance. Plants 2021, 10, 1267. [Google Scholar] [CrossRef]
- Berni, R.; Cantini, C.; Romi, M.; Hausman, J.-F.; Guerriero, G.; Cai, G. Agrobiotechnology goes wild: Ancient local varieties as sources of bioactives. Int. J. Mol. Sci. 2018, 19, 2248. [Google Scholar] [CrossRef]
- Idrissi, O.; Piergiovanni, A.R.; Toklu, F.; Houasli, C.; Udupa, S.M.; Keyser, E.D.; Damme, P.V.; Riek, J.D. Molecular variance and population structure of lentil (Lens culinaris Medik.) landraces from Mediterranean countries as revealed by simple sequence repeat DNA markers: Implications for conservation and use. Plant Genet. Resour. 2018, 16, 249–259. [Google Scholar] [CrossRef]
- Pereira-Dias, L.; Vilanova, S.; Fita, A.; Prohens, J.; Rodríguez-Burruezo, A. Genetic diversity, population structure, and relationships in a collection of pepper (Capsicum spp.) landraces from the Spanish centre of diversity revealed by genotyping-by-sequencing (GBS). Hortic. Res. 2019, 6, 54. [Google Scholar] [CrossRef]
- Martinelli, F.; Vollheyde, A.-L.; Cebrián-Piqueras, M.A.; von Haaren, C.; Lorenzetti, E.; Barberi, P.; Loreto, F.; Piergiovanni, A.R.; Totev, V.V.; Bedini, A.; et al. LEGU-MED: Developing biodiversity-based agriculture with legume cropping systems in the Mediterranean basin. Agronomy 2022, 12, 13. [Google Scholar] [CrossRef]
- Maphosa, Y.; Jideani, V.A. The Role of Legumes in Human Nutrition. In Functional Food; Hueda, M.C., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 103–121. [Google Scholar]
- Nadeem, M.A.; Yeken, M.Z.; Shahid, M.Q.; Habyarimana, E.; Yılmaz, H.; Alsaleh, A.; Hatipoğlu, R.; Çilesiz, Y.; Khawar, K.M.; Ludidi, N.; et al. Common bean as a potential crop for future food security: An overview of past, current and future contributions in genomics, transcriptomics, transgenics and proteomics. Biotechnol. Biotechnol. Equip. 2021, 35, 759–787. [Google Scholar] [CrossRef]
- Angioi, S.A.; Rau, D.; Attene, G.; Nanni, L.; Bellucci, E.; Logozzo, G.; Negri, V.; Spagnoletti Zeuli, P.L.; Papa, R. Beans in Europe: Origin and structure of the European landraces of Phaseolus vulgaris L. Theor. Appl. Genet. 2010, 121, 829–843. [Google Scholar] [CrossRef]
- Gill-Langarica, H.R.; Muruaga-Martínez, J.S.; Vargas-Vázquez, M.L.P.; Rosales-Serna, R.; Mayek-Pérez, N. Genetic diversity analysis of common beans based on molecular markers. Genet. Mol. Biol. 2011, 34, 595–605. [Google Scholar] [CrossRef]
- Bulut, M.; Wendenburg, R.; Bitocchi, E.; Bellucci, E.; Kroc, M.; Gioia, T.; Susek, K.; Papa, R.; Fernie, A.R.; Alseekh, S. A comprehensive metabolomics and lipidomics atlas for the legumes common bean, chickpea, lentil and lupin. Plant J. 2023, 116, 1152–1171. [Google Scholar] [CrossRef]
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S. Dry beans (Phaseolus vulgaris L.) as a vital component of sustainable agriculture and food security—A review. Legume Sci. 2023, 5, e155. [Google Scholar] [CrossRef]
- Everwand, G.; Cass, S.; Dauber, J.; Williams, M.; Stout, J. Legume Crops and Biodiversity. In Legumes in Cropping Systems; Murphy-Bokern, D., Stoddard, F.L., Watson, C.A., Eds.; CABI: Wallingford, UK, 2017; pp. 55–69. [Google Scholar]
- de Koning, R.; Wils, G.E.; Kiekens, R.; De Vuyst, L.; Angenon, G. Impact of drought and salt stress on galactinol and raffinose family oligosaccharides in common bean (Phaseolus vulgaris). AoB PLANTS 2023, 15, plad038. [Google Scholar] [CrossRef]
- Murube, E.; Beleggia, R.; Pacetti, D.; Nartea, A.; Frascarelli, G.; Lanzavecchia, G.; Bellucci, E.; Nanni, L.; Gioia, T.; Marciello, U.; et al. Characterization of nutritional quality traits of a common bean germplasm collection. Foods 2021, 10, 1572. [Google Scholar] [CrossRef]
- Giuberti, G.; Tava, A.; Mennella, G.; Pecetti, L.; Masoero, F.; Sparvoli, F.; Lo Fiego, A.; Campion, B. Nutrients’ and antinutrients’ seed content in common bean (Phaseolus vulgaris L.) lines carrying mutations affecting seed composition. Agronomy 2019, 9, 317. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Yang, H.; Gu, J.; Wang, J.; Ren, F. Regular intake of white kidney beans extract (Phaseolus vulgaris L.) induces weight loss compared to placebo in obese human subjects. Food Sci. Nutr. 2020, 8, 1315–1324. [Google Scholar] [CrossRef]
- Ferreira, H.; Vasconcelos, M.; Gil, A.M.; Pinto, E. Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 85–96. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). The State of Food and Agriculture 2016: Climate Change, Agriculture and Food Security—World|ReliefWeb. Available online: https://reliefweb.int/report/world/state-food-and-agriculture-2016-climate-change-agriculture-and-food-security (accessed on 10 October 2023).
- Martínez-Nieto, M.I.; González-Orenga, S.; Soriano, P.; Prieto-Mossi, J.; Larrea, E.; Doménech-Carbó, A.; Tofei, A.M.; Vicente, O.; Mayoral, O. Are traditional Lima Bean (Phaseolus lunatus L.) landraces valuable to cope with climate change? Effects of drought on growth and biochemical stress markers. Agronomy 2022, 12, 1715. [Google Scholar] [CrossRef]
- United Nations. THE 17 GOALS|Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 13 December 2023).
- Lee, C.E.; Downey, K.; Colby, R.S.; Freire, C.A.; Nichols, S.; Burgess, M.N.; Judy, K.J. Recognizing salinity threats in the climate crisis. Integr. Comp. Biol. 2022, 62, 441–460. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Vineeth, T.V.; Krishna, G.K.; Pandesha, P.H.; Sathee, L.; Thomas, S.; James, D.; Ravikiran, K.T.; Taria, S.; John, C.; Vinaykumar, N.M.; et al. Photosynthetic machinery under salinity stress: Trepidations and adaptive mechanisms. Photosynthetica 2023, 61, 73–93. [Google Scholar] [CrossRef]
- Ahmadian, S.; Bayat, F. Morpho-biochemical responses to salinity tolerance in common bean (Phaseolus vulgaris L.). Afr. J. Agric. Res. 2016, 11, 1289–1298. [Google Scholar] [CrossRef]
- Kaymakanova, M. Effect of salinity on germination and seed physiology in bean (Phaseolus vulgaris L.). Biotechnol. Biotechnol. Equip. 2009, 23, 326–329. [Google Scholar] [CrossRef]
- Cokkizgin, A. Salinity stress in common bean (Phaseolus vulgaris L.) seed germination. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 177–182. [Google Scholar] [CrossRef]
- Bourgault, M.; Madramootoo, C.A.; Webber, H.A.; Stulina, G.; Horst, M.G.; Smith, D.L. Effects of deficit irrigation and salinity stress on common bean (Phaseolus vulgaris L.) and mungbean (Vigna radiata (L.) Wilczek) grown in a controlled environment. J. Agron. Crop Sci. 2010, 196, 262–272. [Google Scholar] [CrossRef]
- Çirka, M.; Tunçtürk, R.; Kulaz, H.; Tunçtürk, M. Effects of salt stress on some growth parameters and biochemical changes in bean (Phaseolus vulgaris L.). Acta Sci. Pol. Hortorum Cultus 2022, 3, 53–63. [Google Scholar] [CrossRef]
- Al Hassan, M.; Morosan, M.; López-Gresa, M.D.P.; Prohens, J.; Vicente, O.; Boscaiu, M. Salinity-induced variation in biochemical markers provides insight into the mechanisms of salt tolerance in common (Phaseolus vulgaris) and runner (P. coccineus) beans. Int. J. Mol. Sci. 2016, 17, 1582. [Google Scholar] [CrossRef]
- Assimakopoulou, A.; Salmas, I.; Nifakos, K.; Kalogeropoulos, P. Effect of salt stress on three green bean (Phaseolus vulgaris L.) cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 113–118. [Google Scholar] [CrossRef]
- Dasgan, H.; Koc, S. Evaluation of salt tolerance in common bean genotypes by ion regulation and searching for screening parameters. J. Food Agric. Environ. 2009, 77, 363–372. [Google Scholar]
- De Luca, D.; Cennamo, P.; Del Guacchio, E.; Di Novella, R.; Caputo, P. Conservation and genetic characterisation of common bean landraces from Cilento region (southern Italy): High differentiation in spite of low genetic diversity. Genetica 2018, 146, 29–44. [Google Scholar] [CrossRef]
- Catarcione, G.; Paolacci, A.R.; Alicandri, E.; Gramiccia, E.; Taviani, P.; Rea, R.; Costanza, M.T.; De Lorenzis, G.; Puccio, G.; Mercati, F.; et al. Genetic diversity and population structure of common bean (Phaseolus vulgaris L.) landraces in the Lazio Region of Italy. Plants 2023, 12, 744. [Google Scholar] [CrossRef]
- Agenzia per la Coesione Territoriale Strategia Nazionale Aree Interne. Available online: https://www.agenziacoesione.gov.it/strategia-nazionale-aree-interne/?lang=en (accessed on 7 October 2023).
- Bertini, A.; Caruso, I.; Vitolo, T. Inland areas, protected natural areas and sustainable development. Eng. Proc. 2022, 18, 20. [Google Scholar] [CrossRef]
- Stagnati, L.; Soffritti, G.; Martino, M.; Lanubile, A.; Desiderio, F.; Ravasio, A.; Marocco, A.; Rossi, G.; Busconi, M. Morphological and genetic characterization of local maize accessions from Emilia Romagna Region, Italy. Sustainability 2022, 14, 91. [Google Scholar] [CrossRef]
- Yıldız Madakbaş, S.; Halima, A.; Kelly, J. Determination of phaseolin types in common bean (Phaseolus vulgaris) varieties from Turkey. Greener J. Agric. Sci. 2014, 4, 39–45. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Bernal, J.; Franco, D.; Zapata, C. Evaluating two-dimensional electrophoresis profiles of the protein phaseolin as markers of genetic differentiation and seed protein quality in common bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2014, 62, 7200–7208. [Google Scholar] [CrossRef]
- Longobardi, F.; Innamorato, V.; Di Gioia, A.; Ventrella, A.; Lippolis, V.; Logrieco, A.F.; Catucci, L.; Agostiano, A. Geographical origin discrimination of lentils (Lens culinaris Medik.) using 1H NMR fingerprinting and multivariate statistical analyses. Food Chem. 2017, 237, 743–748. [Google Scholar] [CrossRef]
- Llorach, R.; Favari, C.; Alonso, D.; Garcia-Aloy, M.; Andres-Lacueva, C.; Urpi-Sarda, M. Comparative metabolite fingerprinting of legumes using LC-MS-based untargeted metabolomics. Food Res. Int. 2019, 126, 108666. [Google Scholar] [CrossRef]
- Hernández-Guerrero, C.J.; Villa-Ruano, N.; Zepeda-Vallejo, L.G.; Hernández-Fuentes, A.D.; Ramirez-Estrada, K.; Zamudio-Lucero, S.; Hidalgo-Martínez, D.; Becerra-Martínez, E. Bean cultivars (Phaseolus vulgaris L.) under the spotlight of NMR metabolomics. Food Res. Int. 2021, 150, 110805. [Google Scholar] [CrossRef]
- Mecha, E.; Erny, G.L.; Guerreiro, A.C.L.; Feliciano, R.P.; Barbosa, I.; Bento da Silva, A.; Leitão, S.T.; Veloso, M.M.; Rubiales, D.; Rodriguez-Mateos, A.; et al. Metabolomics profile responses to changing environments in a common bean (Phaseolus vulgaris L.) germplasm collection. Food Chem. 2022, 370, 131003. [Google Scholar] [CrossRef]
- Agenzia per la Coesione Territoriale Alto Medio Sannio. Available online: https://www.agenziacoesione.gov.it/strategia-nazionale-aree-interne/regione-molise-aree-interne/alto-medio-sannio/ (accessed on 7 November 2023).
- Loddo, A.; Di Ruberto, C.; Vale, A.M.P.G.; Ucchesu, M.; Soares, J.M.; Bacchetta, G. An effective and friendly tool for seed image analysis. Vis. Comput. 2023, 39, 335–352. [Google Scholar] [CrossRef]
- Falcione, M.; Simiele, M.; Renella, A.; Scippa, G.S.; Di Martino, P.; Trupiano, D. A multi-level approach as a powerful tool to identify and characterize some Italian autochthonous common bean (Phaseolus vulgaris L.) landraces under a changing environment. Plants 2022, 11, 2790. [Google Scholar] [CrossRef] [PubMed]
- Samukha, V.; Fantasma, F.; D’Urso, G.; Caprari, C.; De Felice, V.; Saviano, G.; Lauro, G.; Casapullo, A.; Chini, M.G.; Bifulco, G.; et al. NMR metabolomics and chemometrics of commercial varieties of Phaseolus vulgaris L. seeds from Italy and in vitro antioxidant and antifungal activity. Plants 2024, 13, 227. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Ramírez-Meraz, M.; Méndez-Aguilar, R.; Zepeda-Vallejo, L.G.; Álvarez-Bravo, A.; Pérez-Hernández, N.; Becerra-Martínez, E. 1H NMR-based metabolomics profiling of ten new races from Capsicum annuum cv. serrano produced in Mexico. Food Res. Int. 2019, 119, 785–792. [Google Scholar] [CrossRef]
- Sosa, L.; Llanes, A.; Reinoso, H.; Reginato, M.; Luna, V. Osmotic and specific ion effects on the germination of Prosopis strombulifera. Ann. Bot. 2005, 96, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Kouam, E.B.; Ndo, S.M.; Mandou, M.S.; Chotangui, A.H.; Tankou, C.M. Genotypic variation in tolerance to salinity of common beans cultivated in Western Cameroon as assessed at germination and during early seedling growth. Open Agric. 2017, 2, 600–610. [Google Scholar] [CrossRef]
- Smart, R.E. Rapid estimates of relative water content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef]
- Polzella, A.; De Zio, E.; Arena, S.; Scippa, G.; Scaloni, A.; Montagnoli, A.; Chiatante, D.; Trupiano, D. Toward an understanding of mechanisms regulating plant response to biochar application. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2018, 153, 163–172. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y.; Prometheus Wiki Contributors. PROTOCOL: Extraction and determination of proline. PrometheusWiki 2011, 2011, 1–5. [Google Scholar]
- Abdallah, M.B.; Trupiano, D.; Polzella, A.; Zio, E.D.; Sassi, M.; Scaloni, A.; Zarrouk, M.; Youssef, N.B.; Scippa, G.S. Unraveling physiological, biochemical and molecular mechanisms involved in olive (Olea europaea L. cv. Chétoui) tolerance to drought and salt stresses. J. Plant Physiol. 2018, 220, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Pagnotta, M.A.; Fernández, J.A.; Sonnante, G.; Egea-Gilabert, C. Genetic diversity and accession structure in European Cynara cardunculus collections. PLoS ONE 2017, 12, e0178770. [Google Scholar] [CrossRef]
- Raggi, L.; Pacicco, L.C.; Caproni, L.; Álvarez-Muñiz, C.; Annamaa, K.; Barata, A.M.; Batir-Rusu, D.; Díez, M.J.; Heinonen, M.; Holubec, V.; et al. Analysis of landrace cultivation in Europe: A means to support in situ conservation of crop diversity. Biol. Conserv. 2022, 267, 109460. [Google Scholar] [CrossRef]
- De La Fuente, M.; López-Pedrouso, M.; Alonso, J.; Santalla, M.; AM, D.; Alvarez, G.; Zapata, C. In-depth characterization of the phaseolin protein diversity of common bean (Phaseolus vulgaris L.) based on two-dimensional electrophoresis and mass spectrometry. Food Technol. Biotechnol. 2012, 50, 315–325. [Google Scholar]
- Beancyclopedia True Red Cranberry. Available online: https://beancyclopedia.com/index.php/True_Red_Cranberry (accessed on 13 November 2023).
- Slow Food True Red Cranberry Bean—Arca Del Gusto. Available online: https://www.fondazioneslowfood.com/en/ark-of-taste-slow-food/true-red-cranberry-bean/ (accessed on 1 December 2023).
- Chávez-Mendoza, C.; Sánchez, E. Bioactive compounds from Mexican varieties of the common bean (Phaseolus vulgaris): Implications for health. Molecules 2017, 22, 1360. [Google Scholar] [CrossRef] [PubMed]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The nutritional content of common bean (Phaseolus vulgaris L.) landraces in comparison to modern varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Doria, E.; Campion, B.; Sparvoli, F.; Tava, A.; Nielsen, E. Anti-nutrient components and metabolites with health implications in seeds of 10 common bean (Phaseolus vulgaris L. and Phaseolus lunatus L.) landraces cultivated in southern Italy. J. Food Compos. Anal. 2012, 26, 72–80. [Google Scholar] [CrossRef]
- Sławińska, N.; Olas, B. Selected seeds as sources of bioactive compounds with diverse biological activities. Nutrients 2023, 15, 187. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, H.; Liu, G.; Wu, J.; Yuan, Y.; Shang, A. Tumor microenvironment: Lactic acid promotes tumor development. J. Immunol. Res. 2022, 2022, 3119375. [Google Scholar] [CrossRef]
- Allred, K.F.; Yackley, K.M.; Vanamala, J.; Allred, C.D. Trigonelline is a novel phytoestrogen in coffee beans. J. Nutr. 2009, 139, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Shaw, I.C. Dietary isoflavone-induced, estrogen receptor-β-mediated proliferation of Caco-2 cells is modulated by gallic acid. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 145, 111743. [Google Scholar] [CrossRef]
- Saito, R.d.F.; Andrade, L.N.d.S.; Bustos, S.O.; Chammas, R. Phosphatidylcholine-derived lipid mediators: The crosstalk between cancer cells and immune cells. Front. Immunol. 2022, 13, 768606. [Google Scholar] [CrossRef]
- Zeng, T.; Zhu, L.; Liao, M.; Zhuo, W.; Yang, S.; Wu, W.; Wang, D. Knockdown of PYCR1 inhibits cell proliferation and colony formation via cell cycle arrest and apoptosis in prostate cancer. Med. Oncol. Northwood Lond. Engl. 2017, 34, 27. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Farha, A.K.; Cheng, L.; Kim, G.; Zhang, T.; Corke, H. Phenolic content and in vitro antioxidant activity in common beans (Phaseolus vulgaris L.) are not directly related to anti-proliferative activity. Food Biosci. 2020, 36, 100662. [Google Scholar] [CrossRef]
- Martínez-Alonso, C.; Taroncher, M.; Castaldo, L.; Izzo, L.; Rodríguez-Carrasco, Y.; Ritieni, A.; Ruiz, M.-J. Effect of phenolic extract from red beans (Phaseolus vulgaris L.) on T-2 toxin-induced cytotoxicity in HepG2 cells. Foods 2022, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Borsai, O.; Al Hassan, M.; Boscaiu, M.; Sestras, R.; Vicente, O. Effects of salt and drought stress on seed germination and seedling growth in Portulaca. Rom. Biotechnol. Lett. 2018, 23, 13340–13349. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247–260. [Google Scholar] [CrossRef]
- Mallhi, Z.I.; Rizwan, M.; Mansha, A.; Ali, Q.; Asim, S.; Ali, S.; Hussain, A.; Alrokayan, S.H.; Khan, H.A.; Alam, P.; et al. Citric acid enhances plant growth, photosynthesis, and phytoextraction of lead by alleviating the oxidative stress in Castor Beans. Plants 2019, 8, 525. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, K.K.; Chaudhary, N.; Gupta, S.; Sahu, M.; Tejaswini, B.; Sarkar, S. Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front. Plant Sci. 2022, 13, 1006617. [Google Scholar] [CrossRef]
- Shahid, S.; Kausar, A.; Zahra, N.; Hafeez, M.B.; Raza, A.; Ashraf, M.Y. Methionine-induced regulation of secondary metabolites and antioxidants in maize (Zea mays L.) subjected to salinity stress. Gesunde Pflanz. 2023, 75, 1143–1155. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Lim, G.-H.; de Lorenzo, L.; Yu, K.; Zhang, K.; Hunt, A.G.; Kachroo, A.; Kachroo, P. Pipecolic acid confers systemic immunity by regulating free radicals. Sci. Adv. 2018, 4, 4509. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Baset Mia, M.A.; Quddus, M.A.; Sarker, K.K.; Rahman, M.; Skalicky, M.; Brestic, M.; Gaber, A.; Alsuhaibani, A.M.; Hossain, A. Salinity-induced physiological changes in pea (Pisum sativum L.): Germination rate, biomass accumulation, relative water content, seedling vigor and salt tolerance index. Plants 2022, 11, 3493. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Taïbi, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Sanwal, S.K.; Kumar, P.; Kesh, H.; Gupta, V.K.; Kumar, A.; Kumar, A.; Meena, B.L.; Colla, G.; Cardarelli, M.; Kumar, P. Salinity stress tolerance in potato cultivars: Evidence from physiological and biochemical traits. Plants 2022, 11, 1842. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Zhang, J.; Luo, T.; Liu, J.; Ni, F.; Rizwan, M.; Fahad, S.; Hu, L. Morpho-physiological and biochemical responses of tolerant and sensitive rapeseed cultivars to drought stress during early seedling growth stage. Acta Physiol. Plant. 2019, 41, 25. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Ampuero, A.; Agurto, M.; Matus, J.T.; Hoppe, G.; Huidobro, C.; Inostroza-Blancheteau, C.; Reyes-Díaz, M.; Stange, C.; Canessa, P.; Vega, A. Salinity impairs photosynthetic capacity and enhances carotenoid-related gene expression and biosynthesis in tomato (Solanum lycopersicum L. cv. Micro-Tom). PeerJ 2020, 8, e9742. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zeng, W.; Li, Q.; Yang, X.; Wu, J.; Huang, J. Shoot and root biomass allocation of sunflower varying with soil salinity and nitrogen applications. Agron. J. 2017, 109, 2545–2555. [Google Scholar] [CrossRef]
- Kumar, R.; Sagar, V.; Verma, V.C.; Kumari, M.; Gujjar, R.S.; Goswami, S.K.; Kumar Jha, S.; Pandey, H.; Dubey, A.K.; Srivastava, S.; et al. Drought and salinity stresses induced physio-biochemical changes in sugarcane: An overview of tolerance mechanism and mitigating approaches. Front. Plant Sci. 2023, 14, 1225234. [Google Scholar] [CrossRef] [PubMed]
- Moles, T.M.; Pompeiano, A.; Huarancca Reyes, T.; Scartazza, A.; Guglielminetti, L. The efficient physiological strategy of a tomato landrace in response to short-term salinity stress. Plant Physiol. Biochem. 2016, 109, 262–272. [Google Scholar] [CrossRef]
- Stefanov, M.A.; Rashkov, G.D.; Yotsova, E.K.; Dobrikova, A.G.; Apostolova, E.L. Impact of salinity on the energy transfer between pigment–protein complexes in photosynthetic apparatus, functions of the oxygen-evolving complex and photochemical activities of photosystem II and photosystem I in two Paulownia lines. Int. J. Mol. Sci. 2023, 24, 3108. [Google Scholar] [CrossRef]
- Kavas, M.; Akça, O.E.; Akçay, U.C.; Peksel, B.; Eroğlu, S.; Öktem, H.A.; Yücel, M. Antioxidant responses of peanut (Arachis hypogaea L.) seedlings to prolonged salt-induced stress. Arch. Biol. Sci. 2015, 67, 1303–1312. [Google Scholar] [CrossRef]
- Bandeoğlu, E.; Eyidoğan, F.; Yücel, M.; Avni Öktem, H. Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regul. 2004, 42, 69–77. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcione, M.; Renella, A.; Samukha, V.; Colardo, M.; Simiele, M.; Scippa, G.S.; Segatto, M.; Trupiano, D. Building the Resilience of Marginal Rural Areas Using a Complementary Characterization Approach: Possible Beneficial Health Effects and Stress Tolerance of Italian Common Bean (Phaseolus vulgaris L.) Landraces. Diversity 2024, 16, 240. https://doi.org/10.3390/d16040240
Falcione M, Renella A, Samukha V, Colardo M, Simiele M, Scippa GS, Segatto M, Trupiano D. Building the Resilience of Marginal Rural Areas Using a Complementary Characterization Approach: Possible Beneficial Health Effects and Stress Tolerance of Italian Common Bean (Phaseolus vulgaris L.) Landraces. Diversity. 2024; 16(4):240. https://doi.org/10.3390/d16040240
Chicago/Turabian StyleFalcione, Martina, Alessandra Renella, Vadym Samukha, Mayra Colardo, Melissa Simiele, Gabriella Stefania Scippa, Marco Segatto, and Dalila Trupiano. 2024. "Building the Resilience of Marginal Rural Areas Using a Complementary Characterization Approach: Possible Beneficial Health Effects and Stress Tolerance of Italian Common Bean (Phaseolus vulgaris L.) Landraces" Diversity 16, no. 4: 240. https://doi.org/10.3390/d16040240





