Types and Fecundity of Neotenic Reproductives Produced in 5-Year-Old Orphaned Colonies of the Drywood Termite, Cryptotermes domesticus (Blattodea: Kalotermitidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Termite Collection
2.2. Colony Building by the Primary Pairs
2.3. Orphaning Experiment
2.4. Observation of the Development of Orphaned Colonies
3. Results
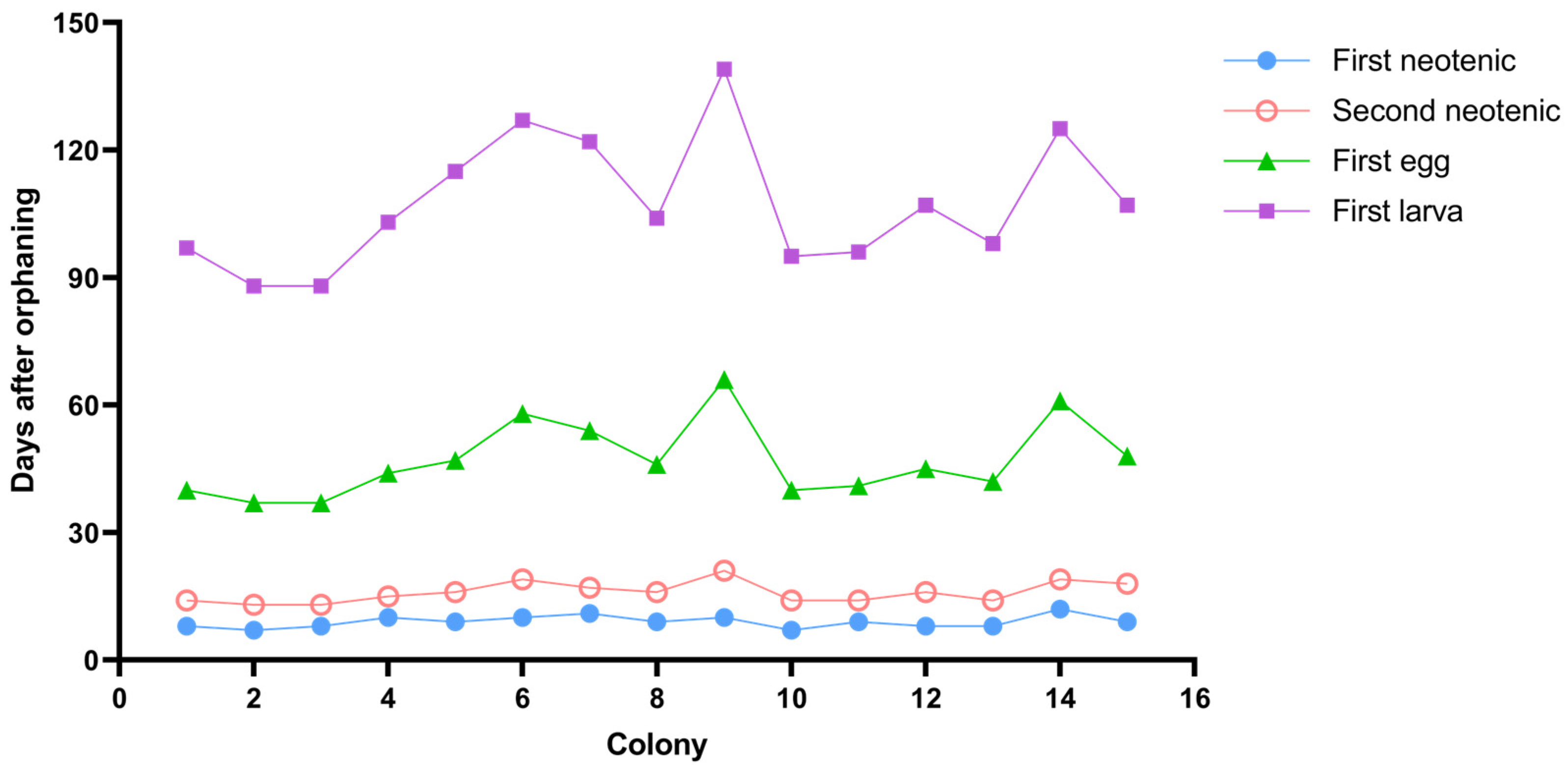
3.1. Emergence of Neotenic Reproductives
3.2. Morphological Observation
3.3. Types and Composition of Neotenic Reproductives
3.4. Fecundity of Neotenic Reproductives
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Colony | First Neotenic | Second Neotenic | Preoviposition Period (Day) | Egg Period (Day) | After One Year | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Emergence Time (Day) | Wing-Bud Length (mm) | Number of Antennal Articles | Emergence Time (Day) | Wing-Bud Length (mm) | Number of Antennal Articles | Egg Number | Number of Larvae | |||
| 1 | 8 | 0.42 | 14 | 14 | 0.13 | 13 | 26 | 57 | 2 | 2 |
| 2 | 7 | 0.35 | 14 | 13 | 0.23 | 13 | 24 | 51 | 0 | 4 |
| 3 | 8 | 0.74 | 15 | 13 | 0.3 | 14 | 24 | 51 | 1 | 5 |
| 4 | 10 | 0.33 | 13 | 15 | 0.14 | 13 | 29 | 59 | 0 | 4 |
| 5 | 9 | 0.38 | 14 | 16 | 0.11 | 12 | 31 | 68 | 1 | 3 |
| 6 | 10 | 0.4 | 14 | 19 | 0.09 | 13 | 39 | 69 | 1 | 3 |
| 7 | 11 | 0.31 | 13 | 17 | 0.07 | 12 | 37 | 68 | 2 | 2 |
| 8 | 9 | 0.36 | 14 | 16 | 0.25 | 13 | 30 | 58 | 1 | 3 |
| 9 | 10 | 0.37 | 13 | 21 | 0.09 | 12 | 45 | 73 | 2 | 2 |
| 10 | 7 | 0.83 | 14 | 14 | 0.15 | 13 | 26 | 55 | 1 | 4 |
| 11 | 9 | 0.38 | 14 | 14 | 0.26 | 14 | 27 | 55 | 0 | 4 |
| 12 | 8 | 0.33 | 13 | 16 | 0.1 | 12 | 29 | 62 | 1 | 3 |
| 13 | 8 | 0.5 | 14 | 14 | 0.11 | 12 | 28 | 56 | 3 | 2 |
| 14 | 12 | 0.3 | 13 | 19 | 0.25 | 12 | 42 | 64 | 2 | 2 |
| 15 | 9 | 0.35 | 14 | 18 | 0.1 | 13 | 30 | 59 | 1 | 3 |
References
- Korb, J.; Hartfelder, K. Life History and Development—A Framework for Understanding Developmental Plasticity in Lower Termites. Biol. Rev. 2008, 83, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Roisin, Y. Diversity and Evolution of Caste Patterns. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 95–119. [Google Scholar]
- Myles, T.G. Review of Secondary Reproduction in Termites (Insecta: Isoptera) with Comments on Its Role in Termite Ecology and Social Evolution. Sociobiology 1999, 33, 1–43. [Google Scholar]
- Roisin, Y. Philopatric Reproduction, a Prime Mover in the Evolution of Termite Sociality? Insectes Sociaux 1999, 46, 297–305. [Google Scholar] [CrossRef]
- Watanabe, H.; Noda, H. Small-Scale Rearing of a Subterranean Termite. Reticulitermes Speratus (Isoptera:Rhinotermitidae). Appl. Entomol. Zool. 1991; 26, 418–420. [Google Scholar]
- Miyata, H.; Furuichi, H.; Kitade, O. Patterns of Neotenic Differentiation in a Subterranean Termite, Reticulitermes Speratus (Isoptera: Rhinotermitidae). Entomol. Sci. 2004, 7, 309–314. [Google Scholar] [CrossRef]
- Neoh, K.B.; Lenz, M.; Lee, C.Y. Impact of Orphaning on Field Colonies of Southeast Asian Macrotermes Gilvus (Hagen) and M. Carbonarius (Hagen) (Termitidae, Macrotermitinae). Insectes Sociaux 2010, 57, 431–439. [Google Scholar] [CrossRef]
- Costa-Leonardo, A.M.; Arab, A.; Casarin, F.E. Neotenic Formation in Laboratory Colonies of the Termite Coptotermes Gestroi after Orphaning. J. Insect Sci. 2004, 4, 10. [Google Scholar] [CrossRef]
- Liu, Y. Research of Reticulitermes chinensis. Chin. J. Hyg. Insectic. Equip. 2003, 9, 8–12. [Google Scholar]
- Tang, G.; Liu, Y. Observations of the production and development of replacement reproductives of Reticulitermes chinensis Snyder. Acta Entomol. Sin. 1990, 33, 43–48. [Google Scholar]
- Haifig, I.; Vargo, E.L.; Labadie, P.; Costa-Leonardo, A.M. Unrelated Secondary Reproductives in the Neotropical Termite Silvestritermes Euamignathus (Isoptera: Termitidae). Sci. Nat. 2016, 103, 9. [Google Scholar] [CrossRef]
- da Silva, I.B.; Haifig, I.; Vargo, E.L.; Casarin, F.E.; da Mota, M.L.; Lima, J.T.; Costa-Leonardo, A.M. Ergatoid Reproductives in the Neotropical Termite Nasutitermes Aquilinus (Holmgren) (Blattaria: Isoptera: Termitidae): Developmental Origin, Fecundity, and Genetics. Insect Sci. 2020, 27, 1322–1333. [Google Scholar] [CrossRef]
- Fujita, A.; Watanabe, H. Inconspicuous Matured Males of Worker Form Are Produced in Orphaned Colonies of Reticulitermes Speratus (Isoptera: Rhinotermitidae) and Participate in Reproduction. J. Insect Physiol. 2010, 56, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Biology of Termites; Krishna, K.; Weesner, F.M. (Eds.) Academic Press: New York, NY, USA; London, UK, 1969; Volume 1. [Google Scholar]
- Himmi, S.K.; Fajar, A.; Wikantyoso, B.; Tjahyono, B.; Nurjanah, N.; Tarmadi, D.; Yusuf, S. Distribution Record of the Invasive Drywood Termite Cryptotermes Domesticus (Haviland) in Riau Province, Sumatra Island, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 918, 012039. [Google Scholar] [CrossRef]
- Eggleton, P.; Tayasu, I. Feeding Groups, Lifetypes and the Global Ecology of Termites. Ecol. Res. 2001, 16, 941–960. [Google Scholar] [CrossRef]
- Evans, T.A.; Forschler, B.T.; Grace, J.K. Biology of Invasive Termites: A Worldwide Review. Annu. Rev. Entomol. 2013, 58, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.S.; Zhu, S.M.; Ping, Z.M.; He, X.S.; Li, G.X.; Gao, D.R. Fauna Sinica. Insecta; Isoptera; Science Press: Beijing, China, 2000; Volume 17. [Google Scholar]
- Gay, F.J. Species Introduced by Man. In Biology of Termites; Krishna, K., Weesner, F.M., Eds.; Academic Press: New York, NY, USA, 1969; pp. 459–494. [Google Scholar]
- Li, G. Termites and Their Control in China; Science Press: Beijing, China, 2002. [Google Scholar]
- Edwards, R.; Mil, A.E. Termites in Buildings. Their Biology and Control; Rentokil Ltd.: East Grinstead, UK, 1986. [Google Scholar]
- Xing, Q.; Huang, Z.; Zhong, J.; Dai, Z.; Liu, B.; Xia, C.; Huang, H.; Xia, F.; Yang, R.; Zhang, R. Studies on formation and development of new colonies of Cryptotermes domesticus (Haviland) (Isoptera: Kalotermitidae). Nat. Enemies Insects 2005, 27, 118–126. [Google Scholar]
- Huang, Z.; Qian, X.; Zhong, J.; Hu, J.; Li, Z.; Li, Q.; Liu, B. Influence of Colony Age on Production and Fecundity of Replacement Reproductives in the Dry Wood Termite Cryptotermes Domesticus (Isoptera: Kalotermitidae). Sociobiology 2011, 58, 77–84. [Google Scholar]
- Huang, Z.; Dai, Z.; Xie, X.; Xia, C.C.; Yang, R.; Zhang, R. Influence of different numbers of Cryptotermes domesticus on the formation of the neotenics. Nat. Enemies Insectss 1994, 16, 94–96. [Google Scholar]
- Huang, Z.; Qian, X.; Zhong, J.; Dai, Z.; Liu, B.; Xia, C.; Huang, H.; Xia, F.; Yang, R.; Zhang, R. The cycle of forming primary reproductives in Cryptotermes Domesticus. Chin. Bull. Entomol. 2005, 42, 528–531. [Google Scholar]
- Haifig, I.; Costa-Leonardo, A.M. Caste Differentiation Pathways in the Neotropical Termite Silvestritermes Euamignathus (Isoptera: Termitidae). Entomol. Sci. 2016, 19, 174–179. [Google Scholar] [CrossRef]
- Korb, J.; Katrantzis, S. Influence of Environmental Conditions on the Expression of the Sexual Dispersal Phenotype in a Lower Termite: Implications for the Evolution of Workers in Termites. Evol. Dev. 2004, 6, 342–352. [Google Scholar] [CrossRef]
- Korb, J.; Hoffmann, K.; Hartfelder, K. Endocrine Signatures Underlying Plasticity in Postembryonic Development of a Lower Termite, Cryptotermes Secundus (Kalotermitidae). Evol. Dev. 2009, 11, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Crosland, M.W.J.; Traniello, J.F.A.; Scheffrahn, R.H. Social Organization in the Drywood Termite, Cryptotermes Cavifrons: Is There Polyethism among Instars? Ethol. Ecol. Evol. 2004, 16, 117–132. [Google Scholar] [CrossRef]
- Neoh, K.-B.; Lee, C.-Y. Developmental Stages and Caste Composition of a Mature and Incipient Colony of the Drywood Termite, Cryptotermes Dudleyi (Isoptera: Kalotermitidae). J. Econ. Entomol. 2011, 104, 622–628. [Google Scholar] [CrossRef] [PubMed]

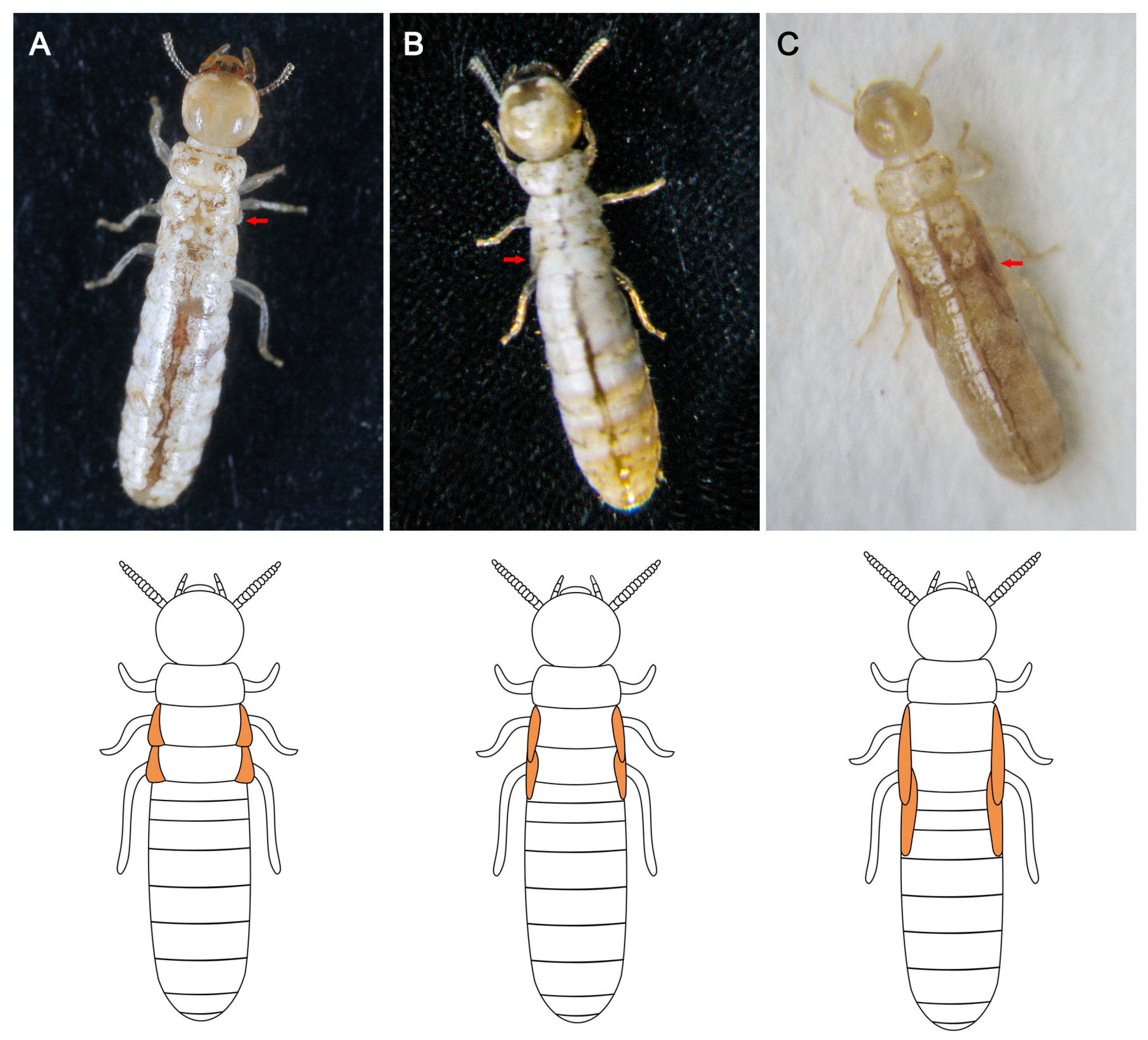

| Types | Number of Neotenic Reproductives | Wing-Bud Length (mm) | Number of Antennal Articles | ||||
|---|---|---|---|---|---|---|---|
| Total | First Neotenic | Second Neotenic | Range | Mean ± SD * | Range | Mean ± SD * | |
| I Micro wing bud | 10 | 0 | 10 | 0.07–0.15 | 0.11 ± 0.02 c | 12–13 | 12.50 ± 0.82 c |
| II Short wing bud | 18 | 13 | 5 | 0.23–0.50 | 0.34 ± 0.07 b | 12–14 | 13.50 ± 0.62 b |
| III Long wing bud | 2 | 2 | 0 | 0.74–0.83 | 0.79 ± 0.06 a | 14–15 | 14.50 ± 0.71 a |
| Combination Pattern | Total Number | Preoviposition Period (day) (Mean ± SD) | Egg Period (Day) (Mean ± SD) | After One Year | |||
|---|---|---|---|---|---|---|---|
| Egg Number | Number of Larvae | ||||||
| Total | Mean ± SD | Total | Mean ± SD | ||||
| I + II | 9 | 32.67 ± 6.26 | 63.44 ± 6.15 | 13 | 1.44 ± 0.88 | 24 | 2.67 ± 0.71 |
| I + III | 1 | 26 | 55 | 1 | 1 ± 0 * | 4 | 4 ± 0 |
| II + II | 4 | 30.75 ± 7.89 | 57.00 ± 5.48 | 3 | 0.75 ± 0.96 | 13 | 3.25 ± 0.96 |
| II + III | 1 | 24 | 51 | 1 | 1 ± 0 | 5 | 5 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Huang, Z.; Zhang, S.; Li, Z.; Liu, B.; Zeng, W.; Xia, C. Types and Fecundity of Neotenic Reproductives Produced in 5-Year-Old Orphaned Colonies of the Drywood Termite, Cryptotermes domesticus (Blattodea: Kalotermitidae). Diversity 2024, 16, 250. https://doi.org/10.3390/d16040250
Wu W, Huang Z, Zhang S, Li Z, Liu B, Zeng W, Xia C. Types and Fecundity of Neotenic Reproductives Produced in 5-Year-Old Orphaned Colonies of the Drywood Termite, Cryptotermes domesticus (Blattodea: Kalotermitidae). Diversity. 2024; 16(4):250. https://doi.org/10.3390/d16040250
Chicago/Turabian StyleWu, Wenjing, Zhenyou Huang, Shijun Zhang, Zhiqiang Li, Bingrong Liu, Wenhui Zeng, and Chuanguo Xia. 2024. "Types and Fecundity of Neotenic Reproductives Produced in 5-Year-Old Orphaned Colonies of the Drywood Termite, Cryptotermes domesticus (Blattodea: Kalotermitidae)" Diversity 16, no. 4: 250. https://doi.org/10.3390/d16040250






