Extinction Resilience of Island Species: An Amphibian Case and a Predictive Model
Abstract
:1. Introduction

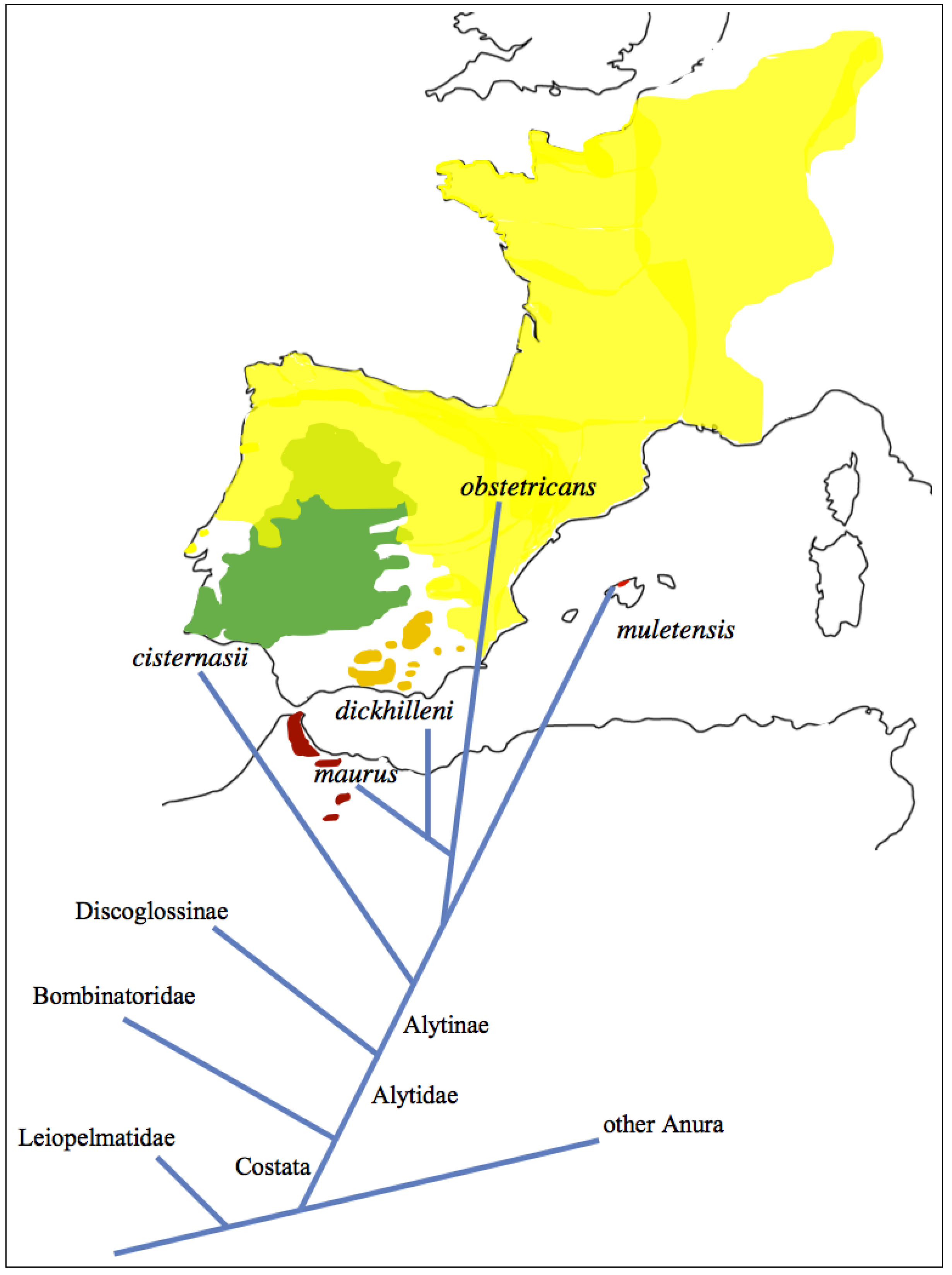
2. Experimental Section
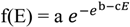
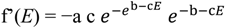
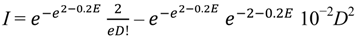
3. Results
3.1. Overall Threats Recognized
| species | threats | Δ− | status | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||
| obstetricans | 1 | 0 | 2 | 1 | 2 | 0 | 2 | 1 | 1 | 2 | VU A2ace + 3ce |
| (2) | (1) | (1) | (2) | (2) | (0) | (3) | (1) | (2) | (2) | ||
| cisternasii | 1 | 0 | 2 | 2 | 2 | 1 | 0 | 1 | 0 | 2 | VU A4ace |
| (2) | (1) | (3) | (2) | (2) | (1) | (3) | (1) | (2) | (2) | ||
| dickhilleni | 2 | 2 | 3 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | EN A4ace; B1ab(i,ii,iii,iv) + 2ab(i,ii,iii,iv) |
| (3) | (3) | (2) | (3) | (2) | (1) | (3) | (1) | (2) | (3) | ||
| maurus | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 2 | EN A2abce; B1ab(i,ii,iii,iv,v) + 2ab(i,ii,iii,iv,v) |
| (3) | (3) | (2) | (3) | (2) | (1) | (3) | (2) | (2) | (3) | ||
| muletensis | 3 | 3 | 2 | 2 | 1 | 3 | 2 | 1 | 1 | 2 | CR B1ab(i,ii,iii,iv) + 2ab(i,ii,iii,iv) |
| (3) | (3) | (3) | (3) | (1) | (3) | (3) | (1) | (2) | (3) | ||
3.2. EDGE Analysis and Model Predictions
| species | ED | GE | EDGE |
|---|---|---|---|
| muletensis | 47.83 | 4 | 6.66 |
| dickhilleni | 43.06 | 3 | 5.86 |
| maurus | 43.06 | 3 | 5.86 |
| cisternasii | 50.83 | 2 | 5.33 |
| obstetricans | 45.16 | 2 | 5.22 |
| Species | l | f | φ | E | d | o | δ | D | I |
|---|---|---|---|---|---|---|---|---|---|
| muletensis | 0.4 | 10 | 4 | 19.20 | 70 | 250 | 1 | 0.22 | 0.313 |
| dickhilleni | 1.3 | 60 | 3 | 2.58 | 1,000 | 10,000 | 2 | 1.20 | −0.011 |
| maurus | 1.3 | 150 | 2 | 1.42 | 1,000 | 10,000 | 2 | 1.20 | −0.005 |
| cisternasii | 0.3 | 100 | 2 | 6.66 | 100 | 140,000 | 3 | 1.54 | −0.003 |
| obstetricans | 1.7 | 200 | 1 | 1.02 | 1,500 | 800,000 | 4 | 3.75 | −0.003 |
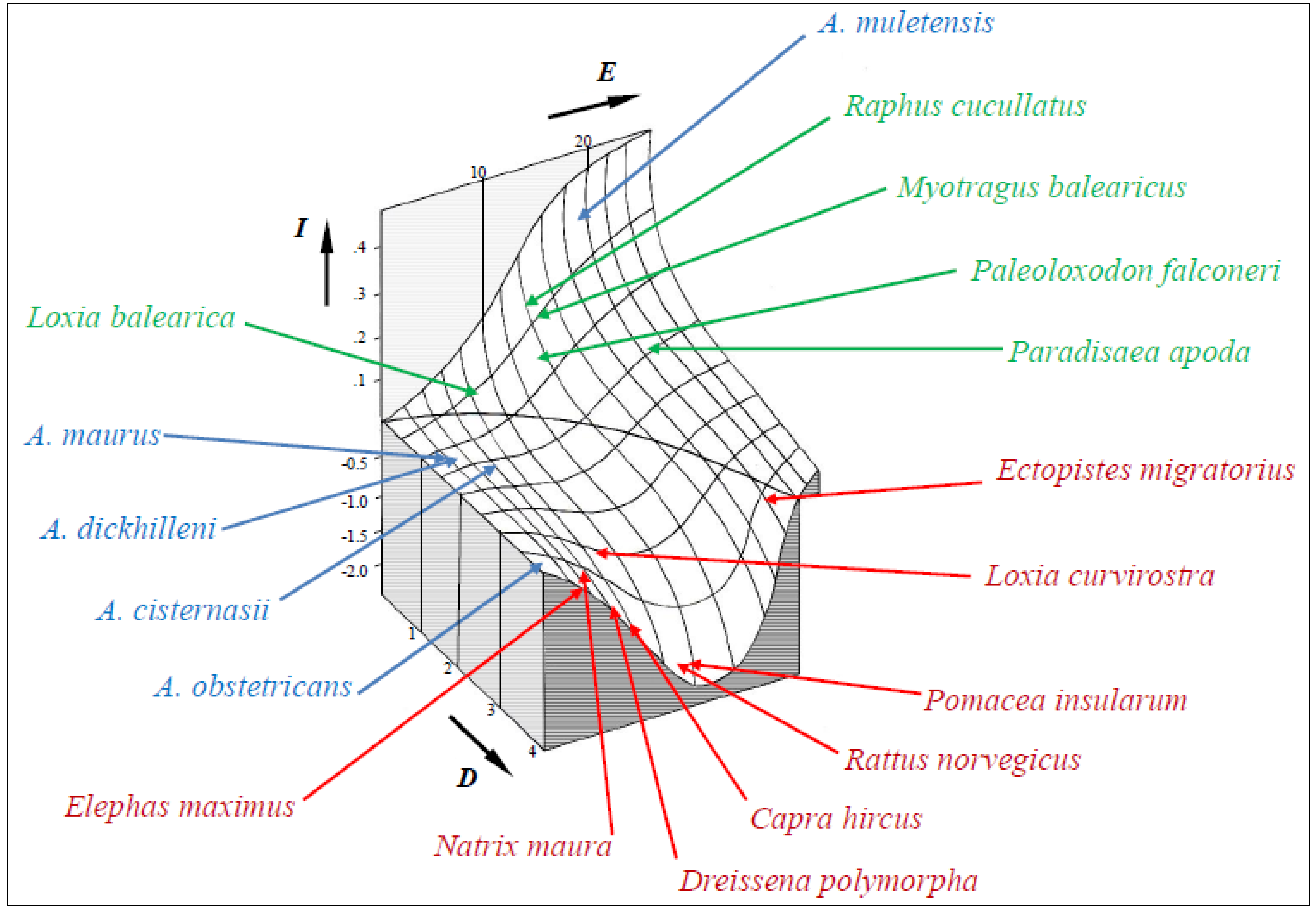
4. Discussion
4.1. Conservation Status
4.2. Limitations in the Existing Studies and Knowledgebase
4.3. Alytine Conservation
4.4. Island Nativeness Matters
4.5. A Predictive Model of Insularity
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Pimm, S.L. The dodo went extinct (and other ecological myths). Ann. Mo. Bot. Gard. 2002, 89, 190–198. [Google Scholar] [CrossRef]
- Altaba, C.R. Biodiversity of the Balearic Islands: A Paradigm for Conservation. In Seminar on Biodiversity and Biological Conservation; Vilà, M., Rodà, F., Ros, J., Eds.; Institut d’Estudis Catalans: Barcelona, Spain, 2004; pp. 371–389. [Google Scholar]
- Steadman, D.W. Extinction and Biogeography of Tropical Pacific Birds; University of Chicago Press: Chicago, IL, USA, 2006. [Google Scholar]
- Stork, N.E. Re-assessing current extinction rates. Biodivers. Conserv. 2010, 19, 357–371. [Google Scholar] [CrossRef]
- Turvey, S.T.; Fritz, S.A. The ghosts of mammals past: Biological and geographical patterns of global mammalian extinction across the Holocene. Philos. Trans. R. Soc. B 2011, 366, 2564–2576. [Google Scholar]
- Sax, D.F.; Gaines, S.D. Species invasions and extinction: The future of native biodiversity on islands. Proc. Natl. Acad. Sci. USA 2008, 105, 11490–11497. [Google Scholar] [CrossRef]
- Davis, M.A. Researching Invasive Species 50 Years after Elton: A Cautionary Tale. In Fifty Years of Invasion Ecology: The Legacy of Charles Elton; Richardson, D.M., Ed.; Wiley-Blackwell: Chichester, UK, 2011; pp. 269–276. [Google Scholar]
- Loehle, C.; Eschenbach, W. Historical bird and terrestrial mammal extinction rates and causes. Divers. Distrib. 2012, 18, 84–91. [Google Scholar] [CrossRef]
- Manne, L.L.; Brooks, T.M.; Pimm, S.L. Relative risk of extinction of passerine birds on continents and islands. Nature 1999, 399, 258–261. [Google Scholar] [CrossRef]
- Vermeij, G. Invasion as Expectation: A Historical Fact of Life. In Species Invasions: Insights into Ecology, Evolution and Biogeography; Sax, D., Stachowicz, J., Gaines, S., Eds.; Sinauer: Sunderland, MA, USA, 2005; pp. 315–339. [Google Scholar]
- Chew, M.K.; Hamilton, A.L. The Rise and Fall of Biotic Nativeness: A Historical Perspective. In Fifty Years of Invasion Ecology: The Legacy of Charles Elton; Richardson, D.M., Ed.; Wiley-Blackwell: Chichester, UK, 2011; pp. 121–130. [Google Scholar]
- Szabo, J.K.; Khwaja, N.; Garnett, S.T.; Butchart, S.H.M. Global patterns and drivers of avian extinctions at the species and subspecies level. PLoS One 2012, 7, e47080:1–e47080:9. [Google Scholar]
- Blackburn, T.M.; Cassey, P.; Duncan, R.P.; Evans, K.L.; Gaston, K.J. Avian extinction and mammalian introductions on oceanic islands. Science 2004, 305, 1955–1958. [Google Scholar] [CrossRef]
- Clavero, M.; Garcia-Berthou, E. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 2005, 20, 110. [Google Scholar] [CrossRef]
- Donlan, C.J.; Wilcox, C. Diversity, invasive species and extinctions in insular ecosystems. J. Appl. Ecol. 2008, 45, 1114–1123. [Google Scholar] [CrossRef]
- Corlett, R.I. Invasive aliens on tropical East Asian islands. Biodivers. Conserv. 2010, 19, 411–423. [Google Scholar] [CrossRef]
- Ricciardi, A. Assessing species invasions as a cause of extinction. Trends Ecol. Evol. 2004, 19, 619–619. [Google Scholar] [CrossRef]
- Roy, H.E.; Adriaens, T.; Isaac, N.J.B.; Kenis, M.; Onkelinx, T.; San Martin, G.; Brown, P.M.J.; Hautier, L.; Poland, R.; Roy, D.B.; et al. Invasive alien predator causes rapid declines of native European ladybirds. Divers. Distrib. 2012, 18, 717–725. [Google Scholar] [CrossRef]
- McIntosh, A.R.; Townsend, C.R. Interpopulation variation in mayfly antipredator tactics: Differential effects of contrasting predatory fish. Ecology 1994, 75, 2078–2090. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Lau, J.A.; Carroll, S.P. Evolutionary responses of natives to introduced species: What do introductions tell us about natural communities? Ecol. Lett. 2006, 9, 357–374. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Lovette, I.J. The roles of island area per se and habitat diversity in the species-area relationships of four Lesser Antillean faunal groups. J. Anim. Ecol. 1999, 68, 1142–1160. [Google Scholar] [CrossRef]
- Kadmon, R.; Allouche, O. Integrating the effects of area, isolation, and habitat heterogeneity on species diversity: A unification of island biogeography and niche theory. Am. Nat. 2007, 170, 443–454. [Google Scholar] [CrossRef]
- Finlayson, C.; Monclova, A.; Carrión, J.S.; Fa, D.A.; Finlayson, G.; Rodríguez-Vidal, J.; Fierro, E.; Fernández, S.; Bernal-Gómez, M.; Giles-Pacheco, F. Ecological transitions—but for whom? A perspective from the Pleistocene. Palaeogeogr. Palaeoclimatiol. 2012, 329–330, 1–9. [Google Scholar] [CrossRef]
- Gillespie, R.G.; Baldwin, B.G. Island Biogeography of Remote Archipelagoes. Interplay between Ecological and Evolutionary Processes. In The Theory of Island Biogeography Revisited; Losos, J.B., Ricklefs, R.E., Eds.; Princeton University Press: Princeton, NJ, USA, 2010; pp. 358–387. [Google Scholar]
- Altaba, C.R. Are all mass invasions alike? Trends Ecol. Evol. 2000, 15, 248. [Google Scholar] [CrossRef]
- Richardson, D.M. Invasion Science: The Roads Travelled and the Roads Ahead. In Fifty Years of Invasion Ecology: The Legacy of Charles Elton; Richardson, D.M., Ed.; Wiley-Blackwell: Chichester, UK, 2011; pp. 397–407. [Google Scholar]
- Altaba, C.R. Phylogeny and biogeography of midwife toads (Alytes, Discoglossidae): A reappraisal. Contrib. Zool. 1997, 66, 257–262. [Google Scholar]
- Altaba, C.R. Phylogenetic relationships and biogeography of midwife toads (Anura: Alytinae): vicariance in a dynamic insular setting preceding adaptive radiation. 2014; unpublished work. [Google Scholar]
- Zangari, F.; Cimmaruta, F.; Nascetti, G. Genetic relationships of the western Mediterranean painted frogs based on allozymes and mitochondrial markers: Evolutionary and taxonomic inferences (Amphibia, Anura, Discoglossidae). Biol. J. Linn. Soc. 2006, 87, 515–536. [Google Scholar] [CrossRef]
- Blackburn, D.C.; Bickford, D.P.; Diesmos, A.C.; Iskandar, D.T.; Brown, R.M. An ancient origin for the enigmatic flat-headed frogs (Bombinatoridae: Barbourula) from the islands of Southeast Asia. PLoS One 2010, 5, e12090. [Google Scholar]
- Pabijan, M.; Crottini, A.; Reckwell, D.; Irisarri, I.; Hauswaldt, J.S.; Vences, M. A multigene species tree for Western Mediterranean painted frogs (Discoglossus). Mol. Phylogenet. Evol. 2012, 64, 690–696. [Google Scholar] [CrossRef]
- Biton, R.; Geffen, E.; Vences, M.; Cohen, O.; Bailon, S.; Rabinovich, R.; Malka, Y.; Oron, T.; Boistel, R.; Brumfeld, V.; et al. The rediscovered Hula painted frog is a living fossil. Nat. Commun. 2013, 4, e1959:1–e1959:6. [Google Scholar]
- Roelants, K.; Bossuyt, F. Archaeobatrachian paraphyly and pangaean diversification of crown-group frogs. Syst. Biol. 2005, 54, 111–126. [Google Scholar] [CrossRef]
- San Mauro, D.; Vences, M.; Alcobendas, M.; Zardoya, R.; Meyer, A. Initial diversification of living amphibians predated the breakup of Pangaea. Am. Nat. 2005, 165, 590–599. [Google Scholar] [CrossRef]
- Frost, D.R.; Grant, T.; Faivovich, J.N.; Bain, R.H.; Haas, A.; Haddad, C.F.B.; De Sá, R.O.; Channing, A.; Wilkinson, M.; Donnellan, S.C.; et al. The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 2006, 297, 1–370. [Google Scholar] [CrossRef]
- Roelants, K.; Gower, D.J.; Wilkinson, M.; Loader, S.P.; Biju, S.D.; Guillaume, K.; Moriau, L.; Bossuyt, F. Global patterns of diversification in the history of modern amphibians. Proc. Natl. Acad. Sci. USA 2007, 104, 887–892. [Google Scholar] [CrossRef]
- Irisarri, I.; Vences, M.; San Mauro, D.; Glaw, F.; Zardoya, R. Reversal to air-driven sound production revealed by a molecular phylogeny of tongueless frogs, family Pipidae. BMC Evol. Biol. 2011, 11, 114:1–114:10. [Google Scholar]
- Skerratt, L.F.; Berger, L.; Speare, R.; Cashins, S.; McDonald, K.R.; Phillott, A.D.; Hines, H.B.; Kenyon, N. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 2007, 4, 125–134. [Google Scholar] [CrossRef]
- Rödder, D.; Kielgast, J.; Bielby, J.; Schmidtlein, S.; Bosch, J.; Garner, T.W.J.; Veith, M.; Walker, S.; Fisher, M.C.; Lötters, S. Global amphibian extinction risk assessment for the panzootic chytrid fungus. Diversity 2009, 1, 52–66. [Google Scholar] [CrossRef]
- Rosenblum, E.B.; Voyles, J.; Poorten, T.J.; Stajich, J.E. The deadly chytrid fungus: A story of an emerging pathogen. PLoS Pathog. 2010, 6, e1000550. [Google Scholar] [CrossRef]
- Rosenblum, E.B.; Fisher, M.C.; James, T.Y.; Stajich, J.E.; Longcore, J.E.; Gentry, L.R.; Poorten, T.J. A molecular perspective: Biology of the emerging pathogen Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 2010, 92, 131–147. [Google Scholar]
- Blaustein, A.R.; Walls, S.C.; Bancroft, B.A.; Lawler, J.L.; Searle, C.L.; Gervasi, S.C. Direct and indirect effects of climate change on amphibian populations. Diversity 2010, 2, 281–313. [Google Scholar] [CrossRef]
- Arntzen, J.W.; García-París, M. Morphological and allozyme studies of midwife toads (genus Alytes), including the description of two new taxa from Spain. Contrib. Zool. 1995, 65, 5–34. [Google Scholar]
- García-París, M.; Martínez-Solano, I. Nuevo estatus taxonómico para las poblaciones ibero-mediterráneas de Alytes obstetricans (Anura: Discoglossidae). Rev. Esp. Herpetol. 2001, 15, 99–113. [Google Scholar]
- Bosch, J.; Beebee, T.; Schmidt, B.; Tejedo, M.; Martínez-Solano, I.; Salvador, A.; García-París, M.; Recuero Gil, E.; Arntzen, J.W.; Diaz Paniagua, C.; et al. Alytes Obstetricans . IUCN Red List of Threatened Species. Version 2011. 2. Available online: http://www.iucnredlist.org (accessed on 9 March 2012).
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Malkmus, R. Amphibians and Reptiles of Portugal, Madeira and the Azores-Archipelago; Gantner: Ruggell, Liechtenstein, 2004. [Google Scholar]
- Beja, P.; Bosch, J.; Tejedo, M.; Lizana, M.; Martínez-Solano, I.; Salvador, A.; García-París, M.; Recuero Gil, E.; Arntzen, J.W.; Márquez, R.; et al. Alytes Cisternasii . IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2. Available online: http://www.iucnredlist.org (accessed on 9 March 2012).
- París, M.; Martínez-Solano, I.; Izquierdo, E.; García-París, M. Distribución y estado de conservación de los sapos parteros (Anura: Discoglossidae: Alytes) en la provincia de Albacete (Castilla-La Mancha, España). Sabuco: Rev. Estud. Albacet. 2002, 3, 5–22. [Google Scholar]
- Bosch, J.; Tejedo, M.; Lizana, M.; Martínez-Solano, I.; Salvador, A.; García-París, M.; Recuero Gil, E.; Arntzen, J.W.; Marquez, R.; Diaz Paniagua, C.; et al. Alytes dickhilleni . IUCN Red List of Threatened Species. Version 2011.2. Available online: http://www.iucnredlist.org (accessed on 9 March 2012).
- Donaire-Barroso, D.; Bogaerts, S. Datos sobre taxonomía, ecología y biología de Alytes maurus (Pasteur and Bons, 1962) (Anura: Discoglossidae). Butll. Soc. Catalana Herpetol. 2003, 16, 25–41, 139–140. [Google Scholar]
- Fromhage, L.; Vences, M.; Veith, M. Testing alternative vicariance scenarios in Western Mediterranean discoglossid frogs. Mol. Phylogenet. Evol. 2004, 31, 308–322. [Google Scholar] [CrossRef]
- Martínez-Solano, I.; Gonçalves, H.A.; Arntzen, J.W.; García-París, M. Phylogenetic relationships and biogeography of midwife toads (Discoglossidae: Alytes). J. Biogeogr. 2004, 31, 603–618. [Google Scholar] [CrossRef]
- Beukema, W.; De Pous, P.; Donaire-Barroso, D.; Bogaerts, S.; García-Porta, J.; Escoriza, D.; Arribas, O.J.; El Mouden, E.H.; Carranza, S. Review of the systematics, distribution, biogeography and natural history of Moroccan amphibians. Zootaxa 2013, 3661, 1–60. [Google Scholar]
- Donaire-Barroso, D.; Salvador, A.; Slimani, T.; El Mouden, E.H.; Martínez-Solano, I. Alytes maurus . IUCN Red List of Threatened Species. Version 2011.2. Available online: http://www.iucnredlist.org (accessed on 9 March 2012).
- Pleguezuelos, J.M.; Brito, J.C.; Fahd, S.; Feriche, M.F.; Mateo, J.A.; Moreno-Rueda, G.; Reques, R.; Santos, X. Setting conservation priorities for the Moroccan herpetofauna: The utility of regional red listing. Oryx 2010, 44, 501–508. [Google Scholar] [CrossRef]
- Mayol Serra, J.; Griffiths, R.; Bosch, J.; Beebee, T.; Schmidt, B.; Tejedo, M.; Lizana, M.; Martínez-Solano, I.; Salvador, A.; García-París, M.; et al. Alytes muletensis . IUCN Red List of Threatened Species. Version 2011.2. Available online: http://www.iucnredlist.org (accessed on 9 March 2012).
- Sanchiz, F.J.; Alcover, J.A. Un nou discoglòssid (Amphibia: Anura) de l’holocè de Menorca. Butll. Inst. Catalana Hist. Nat. 1982, 48, 99–105. [Google Scholar]
- Mayol, J.; Alcover, J.A. Survival of Baleaphryne Sanchiz and Adrover (Amphibia: Anura: Discoglossidae) on Mallorca. Amphibia-Reptilia 1981, 1, 343–345. [Google Scholar] [CrossRef]
- Alcover, J.A.; Mayol, J. Espèces relictuelles d’amphibiens et de reptiles des îles Baléares et Pityuses: Une extension des résultats. Bull. Soc. Herpéthol. Fr. 1982, 22, 69–74. [Google Scholar]
- Alcover, J.A.; Mayol, J.; Jaume, D.; Alomar, G.; Pomar, G.; Jurado, J. Biologia i ecologia de les poblacions relictes de Baleaphryne muletensis a la muntanya mallorquina. In Història biològica del ferreret; Hemmer, H., Alcover, J.A., Eds.; Moll: Palma de Mallorca, Spain, 1984; pp. 129–151. [Google Scholar]
- Altaba, C.R. La diversitat biològica: Una perspectiva des de Mallorca; Moll: Palma de Mallorca, Spain, 1999. [Google Scholar]
- Griffiths, R.A.; Schley, L.; Sharp, P.E.; Dennis, J.L.; Roman, A. Behavioural responses of Mallorcan midwife toad tadpoles to natural and unnatural snake predators. Anim. Behav. 1998, 55, 207–214. [Google Scholar] [CrossRef]
- Moore, R.D.; Griffiths, R.A.; O’Brien, C.M.; Murphy, A.; Jay, D. Induced defences in an endangered amphibian in response to an introduced snake predator. Oecologia 2004, 141, 139–147. [Google Scholar] [CrossRef]
- Richardson, D.M.; Whittaker, R.J. Conservation biogeography—foundations, concepts and challenges. Divers. Distrib. 2010, 16, 313–320. [Google Scholar] [CrossRef]
- Akçakaya, H.R.; Butchart, S.H.M.; Mace, G.M.; Stuart, S.N.; Hilton-Taylor, C. Use and misuse of the IUCN Red List Criteria in projecting climate change impacts on biodiversity. Glob. Chang. Biol. 2006, 12, 2037–2043. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1; IUCN Species Survival Commission: Gland, Switzerland, Cambridge, UK, 2001. [Google Scholar]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria. Version 9.0. Prepared by the Standards and Petitions Subcommittee.. Available online: http://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 7 June 2012).
- 2004 IUCN Red List of Threatened Species: A Global Species Assessment; Baillie, J.E.M.; Hilton-Taylor, C.; Stuart, S.N. (Eds.) IUCN-SSC: Gland, Switzerland, Cambridge, UK, 2004; p. 191.
- Butchart, S.H.M.; Stattersfield, A.J.; Bennun, L.A.; Shutes, S.M.; Akçakaya, H.R.; Baillie, J.E.M.; Stuart, S.N.; Hilton-Taylor, C.; Mace, G.M. Measuring global trends in the status of biodiversity: Red List Indices for birds. PLoS Biol. 2004, 2, e383:2294–e383:2304. [Google Scholar]
- Butchart, S.H.M.; Stattersfield, A.J.; Baillie, J.; Bennun, L.A.; Stuart, S.N.; Akçakaya, H.R.; Hilton-Taylor, C.; Mace, G.M. Using Red List Indices to measure progress towards the 2010 target and beyond. Philos. Trans. R. Soc. Lond. B 2005, 360, 255–268. [Google Scholar] [CrossRef]
- Butchart, S.H.M.; Akçakaya, H.R.; Chanson, J.; Baillie, J.E.M.; Collen, B.; Quader, S.; Turner, W.R.; Amin, R.; Stuart, S.N.; Hilton-Taylor, C. Improvements to the Red List Index. PLoS One 2007, 2, e140:1–e140:8. [Google Scholar]
- Isaac, N.J.B.; Turvey, S.T.; Collen, B.; Waterman, C.; Baillie, J.E.M. Mammals on the EDGE: Conservation priorities based on threat and phylogeny. PLoS One 2007, 2, e296:1–e296:7. [Google Scholar]
- Haas, A. Phylogeny of frogs as inferred from primarily larval characters (Amphibia: Anura). Cladistics 2003, 19, 23–89. [Google Scholar]
- Waliszewski, P.; Konarski, J. A Mystery of the Gompertz Function. In Fractals in Biology and Medicine; Losa, G.A., Merlini, D., Nonnenmacher, T.F., Weibel, E.R., Eds.; Birkhäuser: Basel, Switzerland, 2005; pp. 277–286. [Google Scholar]
- Gil, M.M.; Miller, F.A.; Brandão, T.R.S.; Silva, C.L.M. On the use of the Gompertz model to predict microbial thermal inactivation under isothermal and non-isothermal conditions. Food Eng. Rev. 2011, 3, 17–25. [Google Scholar] [CrossRef]
- Bosch, J. Alytes obstetricans . In Atlas y libro rojo de los anfibios y reptiles de España; Pleguezuelos, J.M., Márquez, R., Lizana, M., Eds.; Dirección General de Conservación de la Naturaleza & Asociación Herpetológica Española: Madrid, Spain, 2002; pp. 82–84. [Google Scholar]
- Bosch, J.; Martínez-Solano, I.; García-París, M. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol. Conserv. 2001, 97, 331–337. [Google Scholar] [CrossRef]
- Schmidt, B.R.; Zumbach, S. Rote Liste der gefährdeten Amphibien der Schweiz; Bundesamt für Umwelt, Land und Landschaft and Koordinationsstelle Amphibien- und Reptilienschutz in der Schweiz: Bern, Switzerland, 2005. [Google Scholar]
- Tobler, U.; Schmidt, B.R. Within- and among-population variation in chytridiomycosis-induced mortality in the toad Alytes obstetricans. PLoS One 2010, 5, e10927:1–e10927:8. [Google Scholar]
- Ferrer, J.; Filella, E. Sobre la presència dels escurçons Vipera latastei Boscá, 1878 i Vipera aspis (Linnaeus, 1758), i del tòtil Alytes obstetricans (Laurenti, 1768) a la península del Cap de Creus. Butll. Soc. Catalana Herpetol. 2011, 19, 184–194. [Google Scholar]
- Bosch, J.; Carrascal, L.M.; Durán, L.; Walker, S.; Fisher, M.C. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc. R. Soc. B 2007, 274, 253–260. [Google Scholar] [CrossRef]
- Márquez, R.; Crespo, E. Alytes cisternasii . In Atlas y libro rojo de los anfibios y reptiles de España; Pleguezuelos, J.M., Márquez, R., Lizana, M., Eds.; Dirección General de Conservación de la Naturaleza & Asociación Herpetológica Española: Madrid, Spain, 2002; pp. 73–75. [Google Scholar]
- Márquez, R. Sapo partero ibérico –Alytes cisternasii. Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Ed.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2011. Available online: http://www.vertebradosibericos.org/ (accessed on 12 September 2012).
- Sánchez Videgaín, J.; Rubio de Lucas, J.L. Atlas preliminar de los anfibios y reptiles de las sierras prebéticas albaceteñas. Al-Basit 1996, 38, 5–30. [Google Scholar]
- García-París, M.; Arntzen, J.W. Alytes dickhilleni . In Atlas y libro rojo de los anfibios y reptiles de España; Pleguezuelos, J.M., Márquez, R., Lizana, M., Eds.; Dirección General de Conservación de la Naturaleza & Asociación Herpetológica Española: Madrid, Spain, 2002; pp. 76–78. [Google Scholar]
- García-París, M. Anura. In Amphibia Lissamphibia; García-París, M., Montori, A., Herrero, P., Eds.; CSIC: Madrid, Spain, 2004; Fauna Ibérica; Volume 24, pp. 1–639. [Google Scholar]
- Seguimiento de Alytes dickhilleni: Informe final. In Monografías SARE. Asociación Herpetológica Española; Bosch, J.; González-Miras, E. (Eds.) Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2012; p. 84.
- Bosch, J.; García-Alonso, D.; Fernández-Beaskoetxea, S.; Fisher, M.C.; Garner, T.W.J. Evidence for the introduction of lethal chytridiomycosis affecting wild betic midwife toads (Alytes dickhilleni). EcoHealth 2013, 10, 82–89. [Google Scholar] [CrossRef]
- Márquez, R.; Francisco Beltrán, J.; Slimani, T.; Radi, M.; Llusia, D.; El Mouden, H. Description of the advertisement call of the Moroccan midwife toad (Alytes maurus Pasteur and Bons, 1962). Alytes 2011, 27, 142–150. [Google Scholar]
- Román, A. Alytes muletensis . In Atlas y libro rojo de los anfibios y reptiles de España; Pleguezuelos, J.M., Márquez, R., Lizana, M., Eds.; Dirección General de Conservación de la Naturaleza & Asociación Herpetológica Española: Madrid, Spain, 2002; pp. 79–81. [Google Scholar]
- Viada, C. Libro rojo de los vertebrados de las Baleares, 3rd ed.; Conselleria de Medi Ambient: Palma, Spain, 2006. [Google Scholar]
- Moore, R.D.; Griffiths, R.; Román, A. Distribution of the Mallorcan midwife toad (Alytes muletensis) in relation to landscape topography and introduced predators. Biol. Conserv. 2004, 116, 327–332. [Google Scholar] [CrossRef]
- Walker, S.F.; Bosch, J.; James, T.Y.; Litvintseva, A.P.; Valls, J.A.O.; Pinya, S.; García, G.; Rosa, G.A.; Cunningham, A.A.; Hole, S.; et al. Introduced pathogens threaten species recovery programs. Curr. Biol. 2008, 18, R853–R854. [Google Scholar] [CrossRef]
- Alcover, J.A. El sapillo balear, un fósil viviente. Quercus 1989, 39, 14–19. [Google Scholar]
- Mayol, J.; Alcover, J.A. La conservación de Alytes muletensis. In Història biològica del ferreret; Hemmer, H., Alcover, J.A., Eds.; Moll: Palma de Mallorca, Spain, 1984; pp. 245–252. [Google Scholar]
- Climate Change and Water. In Technical Paper of the Intergovernmental Panel on Climate Change; Bates, B.C.; Kundzewicz, Z.W.; Wu, S.; Palutikof, J.P. (Eds.) IPCC Secretariat: Geneva, Switzerland, 2008.
- Homar, V.; Ramis, C.; Romero, R.; Alonso, S. Recent trends in temperature and precipitation over the Balearic Islands (Spain). Clim. Chang. 2010, 98, 199–211. [Google Scholar] [CrossRef]
- Hardiman, N.; Burgin, S. Effects of trampling on in-stream macroinvertebrate communities from canyoning activity in the Greater Blue Mountains World Heritage Area. Wetl. Ecol. Manag. 2011, 19, 61–71. [Google Scholar] [CrossRef]
- Pinya, S.; Pérez-Mellado, V.; Suárez-Fernández, J.J. First records of limb malformations in wild populations of the endangered Balearic Midwife Toad’s, Alytes muletensis. Herpetol. Rev. 2012, 43, 240–243. [Google Scholar]
- Iglesias, A.; Garrote, L.; Flores, F.; Moneo, M. Challenges to manage the risk of water scarcity and climate change in the Mediterranean. Water Resour. Manag. 2007, 21, 775–788. [Google Scholar] [CrossRef]
- Sumner, G.N.; Romero, R.; Homar, V.; Ramis, C.; Alonso, S.; Zorita, E. An estimate of the effects of climate change on the rainfall of Mediterranean Spain by the late twenty first century. Clim. Dyn. 2003, 20, 789–805. [Google Scholar]
- Berger, L.; Speare, R.; Daszak, P.; Green, D.E.; Cunningham, A.A.; Goggin, C.L.; Slocombe, R.; Ragan, M.A.; Hyatt, A.D.; McDonald, K.R.; et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. USA 1998, 95, 9031–9036. [Google Scholar] [CrossRef]
- Lips, K.R.; Brem, F.; Brenes, R.; Reeve, J.D.; Alford, R.A.; Voyles, J.; Carey, C.; Livo, L.; Pessier, A.P.; Collins, J.P. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc. Natl. Acad. Sci. USA 2006, 103, 3165–3170. [Google Scholar] [CrossRef]
- Kriger, K.M.; Hero, J.-M. Chytridiomycosis, amphibian extinctions, and lessons for the prevention of future Panzootics. EcoHealth 2009, 6, 6–10. [Google Scholar] [CrossRef]
- Crawford, A.J.; Lips, K.R.; Bermingham, E. Epidemic disease decimates amphibian abundance, species diversity, and evolutionary history in the highlands of central Panama. Proc. Natl. Acad. Sci. USA 2010, 107, 13777–13782. [Google Scholar] [CrossRef]
- Collins, J.P.; Crump, M.L. Extinction in Our Times: Global Amphibian Decline. Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Fisher, M.C.; Garner, T.W.J.; Walker, S.F. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu. Rev. Microbiol. 2009, 63, 291–310. [Google Scholar] [CrossRef]
- Pounds, A.J.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef]
- El Mouden, E.H.; Slimani, T.; Donaire, D.; Fernández-Beaskoetxea, S.; Fisher, M.C.; Bosch, J. First record of the chytrid fungus Batrachochytrium dendrobatidis in North Africa. Herpetol. Rev. 2011, 42, 71–75. [Google Scholar]
- Bloxam, Q.M.C.; Tonge, S.J. Amphibians: Suitable candidates for breeding-release programmes. Biodiver. Conserv. 1995, 4, 636–644. [Google Scholar] [CrossRef]
- Mayol Serra, J. Rèptils i amfibis de les Balears, edició revisada; Moll: Ciutat de Mallorca, Spain, 2003. [Google Scholar]
- Boisvert, S.P.; Davidson, E.W. Growth of the amphibian pathogen, Batrachochytrium dendrobatidis, in response to chemical properties of the aquatic environment. J. Wildl. Dis. 2011, 47, 694–698. [Google Scholar] [CrossRef]
- Andre, S.E.; Parker, J.; Briggs, C.J. Effect of temperature on host response to Batrachochytrium dendrobatidis infection in the mountain yellow-legged frog (Rana muscosa). J. Wildl. Dis. 2008, 44, 716–720. [Google Scholar] [CrossRef]
- Forrest, M.J.; Schlaepfer, M.A. Nothing a hot bath won’t cure: Infection rates of amphibian chytrid fungus correlate negatively with water temperature under natural field settings. PLoS One 2011, 6, e28444:1–e28444:9. [Google Scholar]
- Altaba, C.R. Un endemisme ornitològic ignorat: el trencapinyons balear (Loxia balearica). Butll. Inst. Catalana Hist. Nat. 2001, 69, 77–90. [Google Scholar]
- EDGE of Existence: Evolutionarily Distinct and Globally Endangered. Available online: http://www.edgeofexistence.org/amphibians/default.php (accessed on 18 January 2013).
- Rachmayuningtyas, B.A.; Bickford, D.P.; Kamsi, M.; Kutty, S.N.; Meier, R.; Arifin, U.; Rachmansah, A.; Iskandar, D.T. Conservation status of the only lungless frog Barbourula kalimantanensis Iskandar, 1978 (Amphibia: Anura: Bombinatoridae). J. Threat. Taxa 2011, 3, 1981–1989. [Google Scholar] [CrossRef]
- Galán, P. Redescubierto el sapillo pintojo de vientre negro o sapillo de Hula. Quercus 2012, 313, 46–48. [Google Scholar]
- McElduff, F.; Cortina-Borja, M.; Chan, S.-K.; Wade, A. When t-tests or Wilcoxon-Mann-Whitney tests won’t do. Adv. Physiol. Educ. 2010, 34, 128–133. [Google Scholar] [CrossRef]
- Lea, J.; Dyson, M.; Halliday, T. The effects of cohort structure and density on larval growth and development in Alytes muletensis: Implications for conservation. Herpetol. J. 2002, 12, 155–164. [Google Scholar]
- Carfagno, G.L.F.; Carithers, J.M.; Mycoff, L.J.; Lehtinen, R.M. How the cricket frog lost its spot: The inducible defense hypothesis. Herpetologica 2011, 67, 386–396. [Google Scholar] [CrossRef]
- Marquis, O.; Saglio, P.; Neveu, A. Effects of predators and conspecific chemical cues on the swimming activity of Rana temporaria and Bufo bufo tadpoles. Arch. Hydrobiol. 2004, 160, 153–170. [Google Scholar] [CrossRef]
- Mandrillon, A.L.; Saglio, P. Prior exposure to conspecific chemical cues affects predator recognition in larval Common Toad (Bufo bufo). Arch. Hydrobiol. 2005, 164, 1–12. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Messier, F.; Chivers, D.P. First documentation of cultural transmission of predator recognition by larval amphibians. Ethology 2007, 113, 621–627. [Google Scholar] [CrossRef]
- Gonzalo, A.; López, P.; Martin, J. Iberian Green Frog tadpoles may learn to recognize novel predators from chemical alarm cues of conspecifics. Anim. Behav. 2007, 74, 447–453. [Google Scholar] [CrossRef]
- Jara, F.B.; Perotti, M.G. Risk of predation and behavioural response in three anuran species: Influence of tadpole size and predator type. Hydrobiologia 2010, 644, 313–324. [Google Scholar] [CrossRef]
- Shah, A.A.; Ryan, M.J.; Bevilacqua, E.; Schlaepfer, M.A. Prior experience alters the behavioral response of prey to a nonnative predator. J. Herpetol. 2010, 44, 185–192. [Google Scholar] [CrossRef]
- Scoville, A.G.; Pfrender, M.E. Phenotypic plasticity facilitates recurrent rapid adaptation to introduced predators. Proc. Natl. Acad. Sci. USA 2010, 107, 4260–4263. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Díaz-Paniagua, C. Invasive predatory crayfish do not trigger inducible defences in tadpoles. Proc. R. Soc. B 2011, 278, 3364–3370. [Google Scholar] [CrossRef] [Green Version]
- Cromsigt, J.P.G.M.; Kerley, G.I.H.; Kowalczyk, R. The difficulty of using species distribution modelling for the conservation of refugee species –the example of European bison. Divers. Distrib. 2012, 18, 1253–1257. [Google Scholar] [CrossRef]
- Fisher, M.C.; Bosch, J.; Yin, Z.K.; Stead, D.; Walker, J.; Selway, L.; Brown, A.J.P.; Walker, L.A.; Gow, N.A.R.; Stajich, J.E.; et al. Proteomic and phenotypic profiling of an emerging pathogen of amphibians, Batrachochytrium dendrobatidis, shows that genotype is linked to virulence. Mol. Ecol. 2009, 18, 415–429. [Google Scholar] [CrossRef]
- Gonçalves, H.; Martínez-Solano, I.; Pereira, R.J.; Carvalho, B.; García-París, M.; Ferrand, N. High levels of population subdivision in a morphologically conserved Mediterranean toad (Alytes cisternasii) result from recent, multiple refugia: Evidence from mtDNA, microsatellites and nuclear genealogies. Mol. Ecol. 2009, 18, 5143–5160. [Google Scholar]
- Chen, X.; Jiao, J.; Tong, X. A generalized model of island biogeography. Sci. China Life Sci. 2011, 54, 1055–1061. [Google Scholar] [CrossRef]
- Gewin, V. Riders of a modern-day ark. PLoS Biol. 2008, 6, e24:18–e24:21. [Google Scholar] [CrossRef]
- Scott, J.M.; Davis, F.; Csuti, B.; Noss, R.; Butterfield, B.; Groves, C.; Anderson, H.; Caicco, S.; D'Erchia, F.; Edwards, T.C., Jr.; et al. Gap analysis: A geographic approach to protection of biological diversity. Wildl. Monogr. 1993, 123, 3–41. [Google Scholar]
- Rodrigues, A.S.L.; Akçakaya, H.R.; Andelman, S.J.; Bakkarr, M.I.; Boitani, L.; Brooks, T.M.; Chanson, J.S.; Fishpool, L.D.C.; da Fonseca, G.A.B.; Gaston, K.J.; et al. Global gap analysis: Priority regions for expanding the global protected-area network. Bioscience 2004, 54, 1092–1100. [Google Scholar] [CrossRef]
- Lowe, W.H.; Nislow, K.H.; Likens, G.E. Forest structure and stream salamander diets: Implications for terrestrial-aquatic connectivity. Verh. Int. Verein Limnol. 2005, 29, 279–286. [Google Scholar]
- Baldwin, R.F.; deMaynadier, P.G. Assessing threats to pool-breeding amphibian habitat in an urbanizing landscape. Biol. Conserv. 2009, 142, 1628–1638. [Google Scholar] [CrossRef]
- Beier, P.; Noss, R.F. Do habitat corridors provide connectivity? Conserv. Biol. 1998, 12, 1241–1252. [Google Scholar] [CrossRef]
- Surasinghe, T.D. Major issues in threat analysis and resolving such problems: An addendum to the GAP analysis. J. Threat. Taxa 2012, 4, 2545–2550. [Google Scholar] [CrossRef]
- Crooks, K.R.; Sanjayan, M. Connectivity Conservation; Cambridge University Press: Cambridge, UK, New York, NY, USA, 2006; p. 732. [Google Scholar]
- Stevens, V.M.; Baguette, M. Importance of habitat quality and landscape connectivity for the persistence of endangered natterjack toads. Conserv. Biol. 2008, 22, 1194–1204. [Google Scholar] [CrossRef]
- Smith, K.F.; Sax, D.F.; Lafferty, K.D. Evidence for the role of infectious disease in species extinction and endangerment. Conserv. Biol. 2006, 20, 1349–1357. [Google Scholar] [CrossRef]
- Garner, T.W.J.; Garcia, G.; Carroll, B.; Fisher, M.C. Using itraconazole to clear Batrachochytrium dendrobatidis infection, and subsequent depigmentation of Alytes muletensis tadpoles. Dis. Aquat. Organ. 2009, 83, 257–260. [Google Scholar] [CrossRef]
- Kriger, K.M.; Hero, J.-M. After the horse has bolted: A reply to Garner et al. EcoHealth 2009, 6, 152. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Han, B.A.; Relyea, R.A.; Johnson, P.T.J.; Buck, J.C.; Gervasi, S.S.; Kats, L.B. The complexity of amphibian population declines: Understanding the role of cofactors in driving amphibian losses. Ann. N. Y. Acad. Sci. 2011, 1223, 108–119. [Google Scholar] [CrossRef]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Contreras Balderas, S.; Bussing, W.; et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. Bioscience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Bass, M.S.; Finer, M.; Jenkins, C.N.; Kreft, H.; Cisneros-Heredia, D.F.; McCracken, S.F.; Pitman, N.C.A.; English, P.H.; Swing, K.; Villa, G.; et al. Global conservation significance of Ecuador’s Yasuní National Park. PLoS One 2010, 5, e8767. [Google Scholar] [CrossRef]
- He, F.; Hubbell, S.P. Species-area relationships always overestimate extinction rates from habitat loss. Nature 2011, 473, 368–371. [Google Scholar] [CrossRef]
- Fisher, D.O.; Blomberg, S.P. Correlates of rediscovery and the detectability of extinction in mammals. Philos. Trans. R. Soc. B 2011, 278, 1090–1097. [Google Scholar]
- Pereira, S.L.; Johnson, K.P.; Clayton, D.H.; Baker, A.J. Mitochondrial and nuclear DNA sequences support a Cretaceous origin of Columbiformes and a dispersal-driven radiation in the Paleogene. Syst. Biol. 2007, 56, 656–672. [Google Scholar] [CrossRef]
- Eastwood, A.; Gibby, M.; Cronk, Q.C.B. Evolution of St Helena arborescent Astereae (Asteraceae): Relationships of the genera Commidendrum and Melanodendron. Bot. J. Linn. Soc. 2004, 144, 69–83. [Google Scholar] [CrossRef]
- Altaba, C.R. Documenting biodiversity: The need for species identifications. Trends Ecol. Evol. 1997, 12, 358–359. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Brown, J.H. The reticulating phylogeny of island biogeography theory. Q. Rev. Biol. 2009, 84, 357–390. [Google Scholar] [CrossRef]
- Catford, J.A.; Jansson, R.; Nilsson, C. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers. Distrib. 2009, 15, 22–40. [Google Scholar] [CrossRef]
- Kalmar, A.; Currie, D.J. A global model of island biogeography. Glob. Ecol. Biogeogr. 2006, 15, 72–81. [Google Scholar] [CrossRef]
- Lomolino, M.V. A call for a new paradigm of island biogeography. Glob. Ecol. Biogeogr. 2000, 9, 1–6. [Google Scholar] [CrossRef]
- Lomolino, M.V. A species-based theory of insular zoogeography. Glob. Ecol. Biogeogr. 2000, 9, 39–58. [Google Scholar] [CrossRef]
- Lomolino, M.V. Body size evolution in insular vertebrates: Generality of the island rule. J. Biogeogr. 2005, 32, 1683–1699. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Brown, J.H.; Sax, D.F. Island Biogeography Theory. Reticulations of “a Biogeography of the Species”. In The Theory of Island Biogeography Revisited; Losos, J.B., Ricklefs, R.E., Eds.; Princeton University Press: Princeton, NJ, USA, 2010; pp. 13–51. [Google Scholar]
- Cox, J.G.; Lima, S.L. Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol. Evol. 2006, 21, 674–680. [Google Scholar] [CrossRef]
- Margalef, R. Limnología; Omega: Barcelona, Spain, 1983. [Google Scholar]
- Margalef, R.; Alcover, J.A.; Altaba, C.R.; Camarasa, J.M. De l’aïllament a la insularitat (in Catalan). In Biosfera; Folch, R., Camarasa, J.M., Eds.; Enciclopèdia Catalana: Barcelona, Spain, 1995; Volume 9, pp. 328–337, [English version: Gale Group, Madison, WI, USA, 2000.]. [Google Scholar]
- Altaba, C.R. Testing vicariance: Melanopsid snails and Neogene tectonics in the Western Mediterranean. J. Biogeogr. 1998, 25, 541–551. [Google Scholar]
- Ricciardi, A.; MacIsaac, H.J. Biodiversity as a Bulwark against Invasion: Conceptual Threads since Elton. In Fifty Years of Invasion Ecology: The Legacy of Charles Elton; Richardson, D.M., Ed.; Wiley-Blackwell: Chichester, UK, 2011; pp. 211–224. [Google Scholar]
- Fridley, J.D. Biodiversity as a Bulwark against Invasion: Conceptual Threads since Elton. In Fifty Years of Invasion Ecology: The Legacy of Charles Elton; Richardson, D.M., Ed.; Wiley-Blackwell: Chichester, UK, 2011; pp. 121–130. [Google Scholar]
- Buckley, R. The habitat-unit model of island biogeography. J. Biogeogr. 1982, 9, 339–344. [Google Scholar] [CrossRef]
- Losos, J.B.; Parent, C.E. The Speciation-Area Relationship. In The Theory of Island Biogeography Revisited; Losos, J.B., Ricklefs, R.E., Eds.; Princeton University Press: Princeton, NJ, USA, 2010; pp. 415–438. [Google Scholar]
- Rosindell, J.; Phillimore, A.B. A unified model of island biogeography sheds light on the zone of radiation. Ecol. Lett. 2011, 14, 552–560. [Google Scholar] [CrossRef]
- Vermeij, G. An agenda for invasion biology. Biol. Conserv. 1996, 78, 3–9. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Tilman, D. What Species Invasions Tell Us about the Relationship between Community Saturation, Species Diversity and Ecosystem Functioning. In Species Invasions: Insights into Ecology, Evolution and Biogeography; Sax, D., Stachowicz, J., Gaines, S., Eds.; Sinauer: Sunderland, MA, USA, 2005; pp. 41–64. [Google Scholar]
- Van Kleunen, M.; Dawson, W.; Schlaepfer, D.; Jeschke, J.M.; Fischer, M. Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol. Lett. 2010, 13, 947–958. [Google Scholar]
- Gould, S.J. The Structure of Evolutionary Theory; Belknap Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Whittaker, R.J.; Triantis, K.A.; Ladle, R.J. A general dynamic theory of oceanic island biogeography. J. Biogeogr. 2008, 35, 977–994. [Google Scholar] [CrossRef]
- Morrone, J.J. Evolutionary Biogeography: An Integrative Approach with Case Studies; Columbia University Press: New York, NY, USA, 2009; p. 301. [Google Scholar]
- Raia, P.; Barbera, C.; Conte, M. The fast life of a dwarfed giant. Evol. Ecol. 2003, 17, 293–312. [Google Scholar] [CrossRef]
- Marín-Moratalla, N.; Jordana, X.; García-Martínez, R.; Köhler, M. Tracing the evolution of fitness components in fossil bovids under different selective regimes. C. R. Palevol 2011, 10, 469–478. [Google Scholar] [CrossRef]
- Irestedt, M.; Jønsson, K.A.; Fjeldså, J.; Christidis, L.; Ericson, P.G.P. An unexpectedly long history of sexual selection in birds-of-paradise. BMC Evol. Biol. 2009, 9, e235. [Google Scholar] [CrossRef]
- Johnson, K.P.; Clayton, D.H.; Dumbacher, J.P.; Fleischer, R.C. The flight of the passenger pigeon: Phylogenetics and biogeographic history of an extinct species. Mol. Phylogenet. Evol. 2010, 57, 455–458. [Google Scholar] [CrossRef]
- Bucher, E.H. The Causes of Extinction of the Passenger Pigeon. In Current Onrnithology; Power, D.M., Ed.; Plenum Press: New York, NY, USA, 1992; Volume 9, pp. 1–36. [Google Scholar]
- Van Kleunen, M.; Richardson, D.M. Invasion biology and conservation biology: Time to join forces to explore the links between species traits and extinction risk and invasiveness. Prog. Phys. Geogr. 2007, 31, 447–450. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Altaba, C.R. Extinction Resilience of Island Species: An Amphibian Case and a Predictive Model. Diversity 2014, 6, 43-71. https://doi.org/10.3390/d6010043
Altaba CR. Extinction Resilience of Island Species: An Amphibian Case and a Predictive Model. Diversity. 2014; 6(1):43-71. https://doi.org/10.3390/d6010043
Chicago/Turabian StyleAltaba, Cristian R. 2014. "Extinction Resilience of Island Species: An Amphibian Case and a Predictive Model" Diversity 6, no. 1: 43-71. https://doi.org/10.3390/d6010043




