Experimental Study on Erosion–Corrosion of TP140 Casing Steel and 13Cr Tubing Steel in Gas–Solid and Liquid–Solid Jet Flows Containing 2 wt % NaCl
Abstract
:1. Introduction
2. Experimental Method
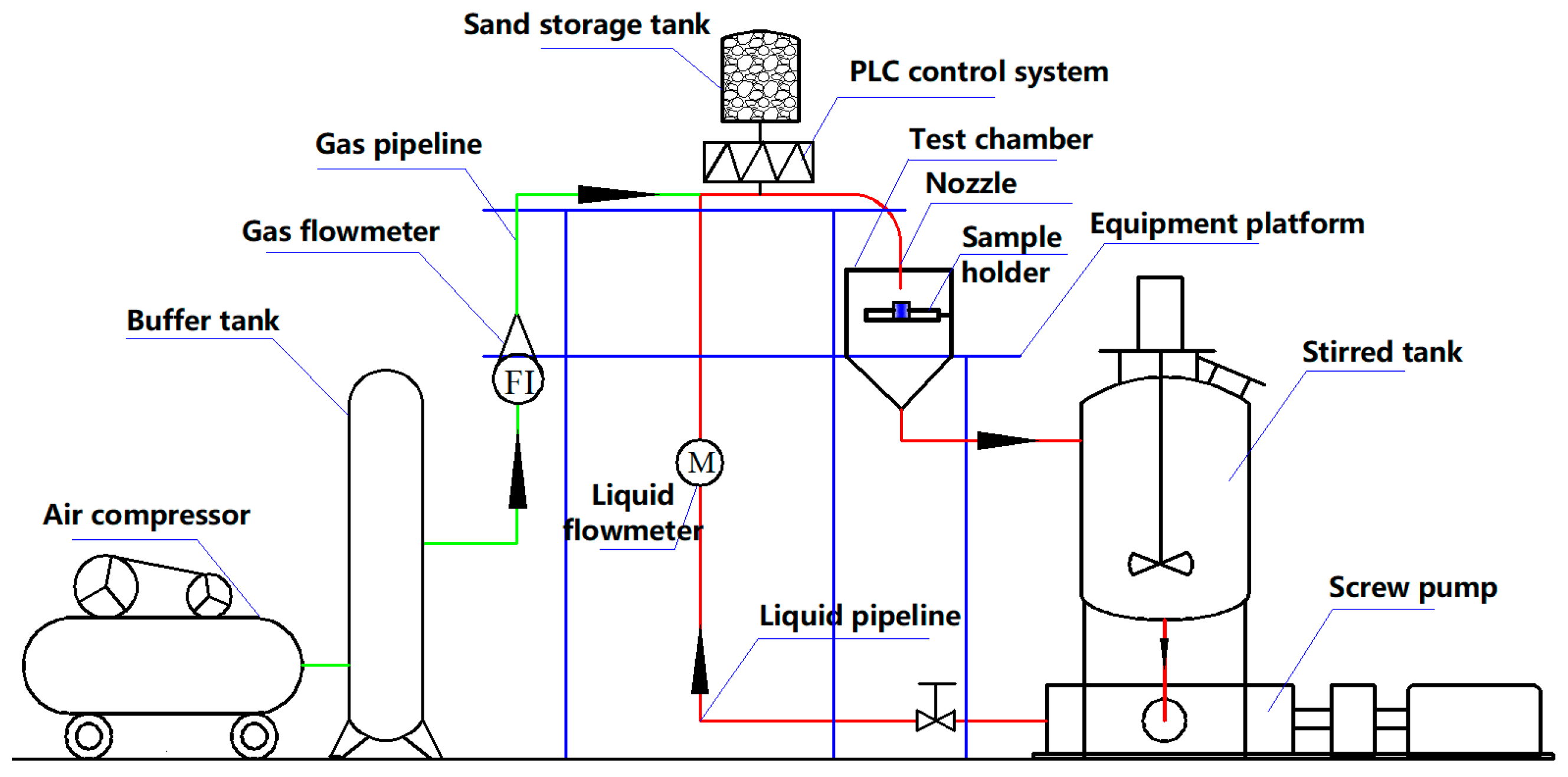
2.1. Pipe Flow System
2.2. Test Section and Experimental Medium
3. Results
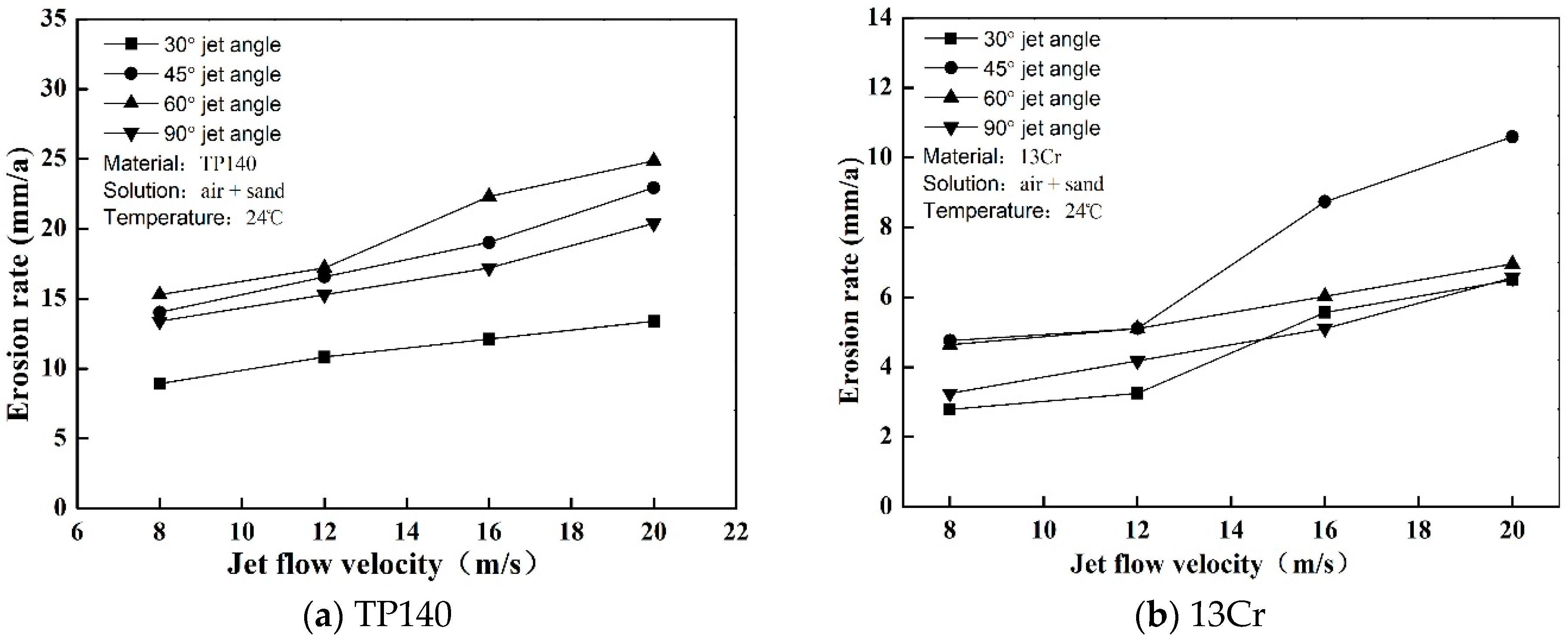
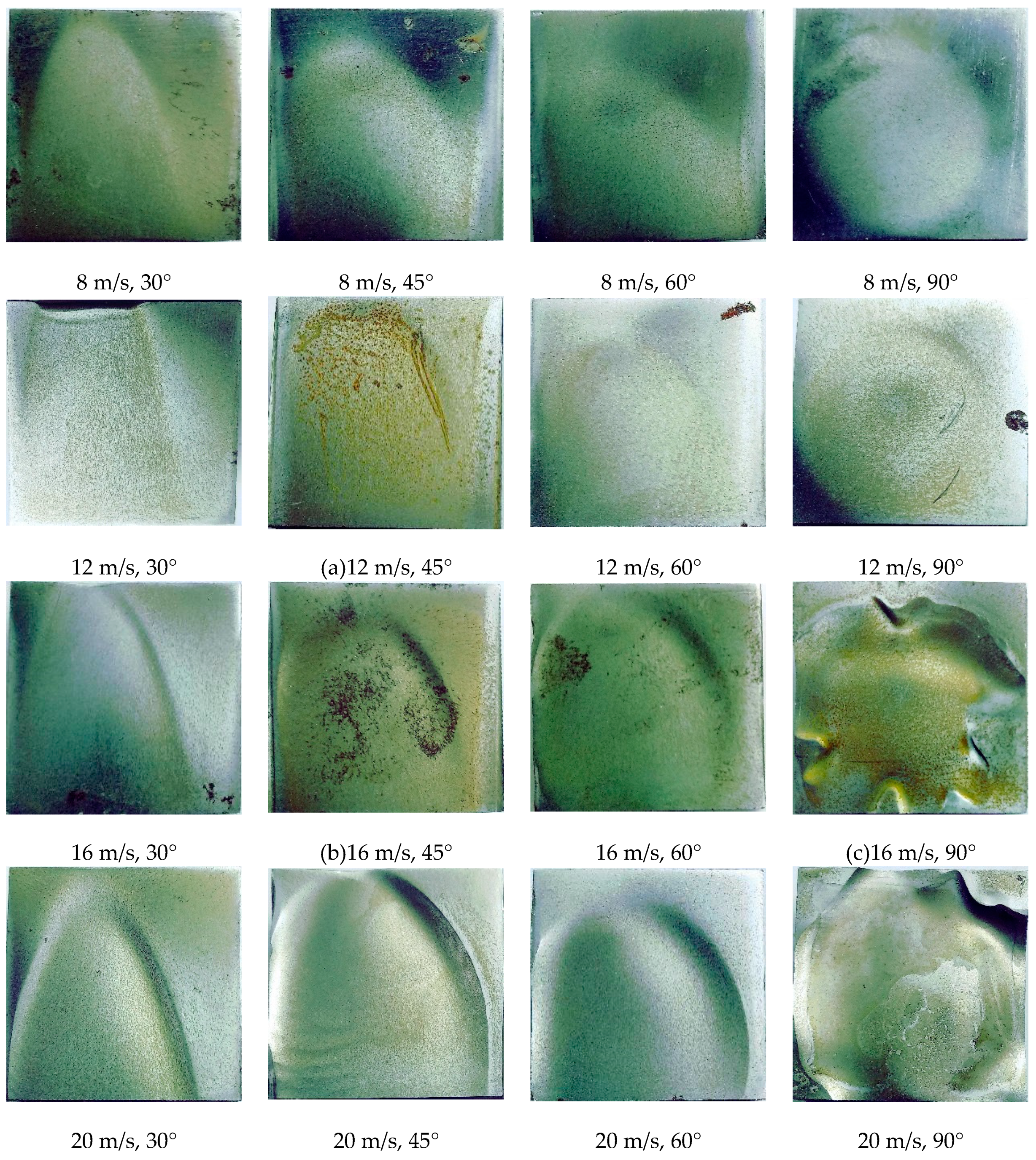
3.1. Gas-Solid Erosion (Pure Erosion)
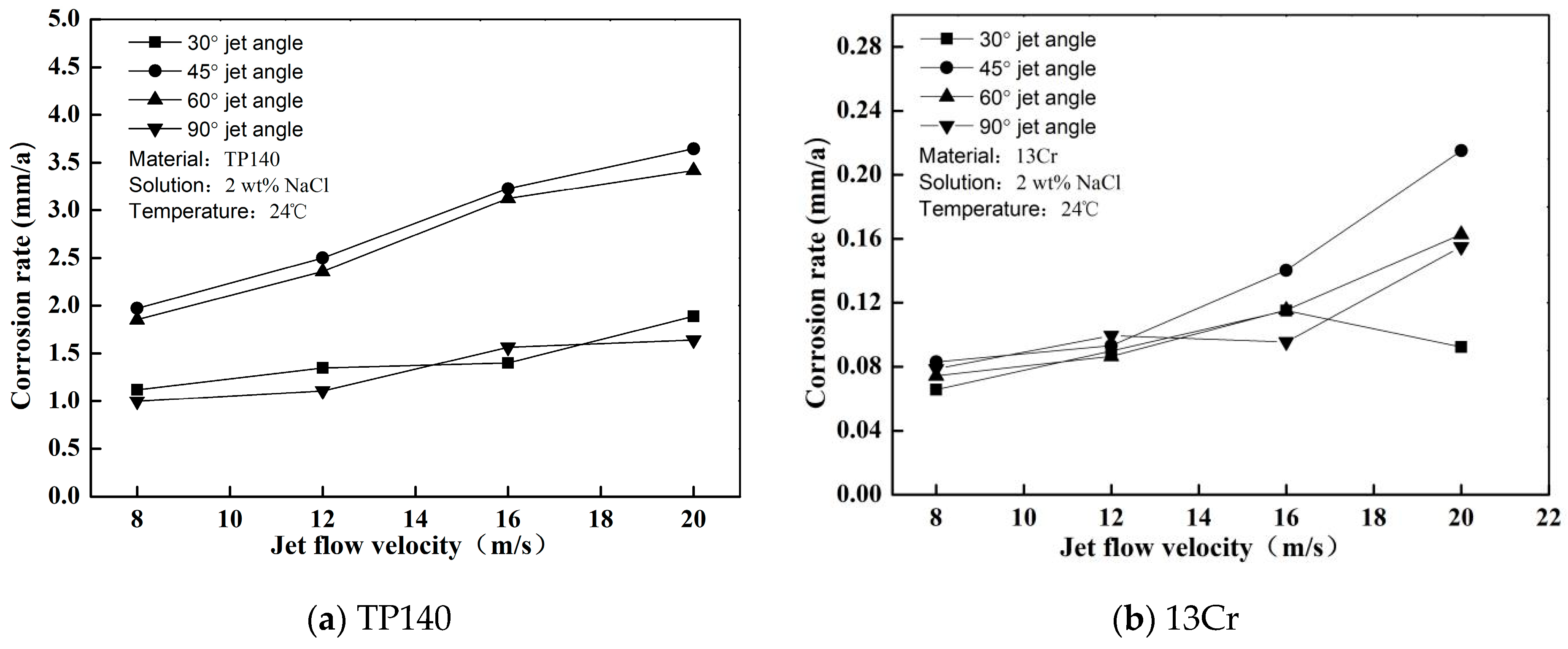
3.2. Flow Induced Corrosion (Pure Corrosion)
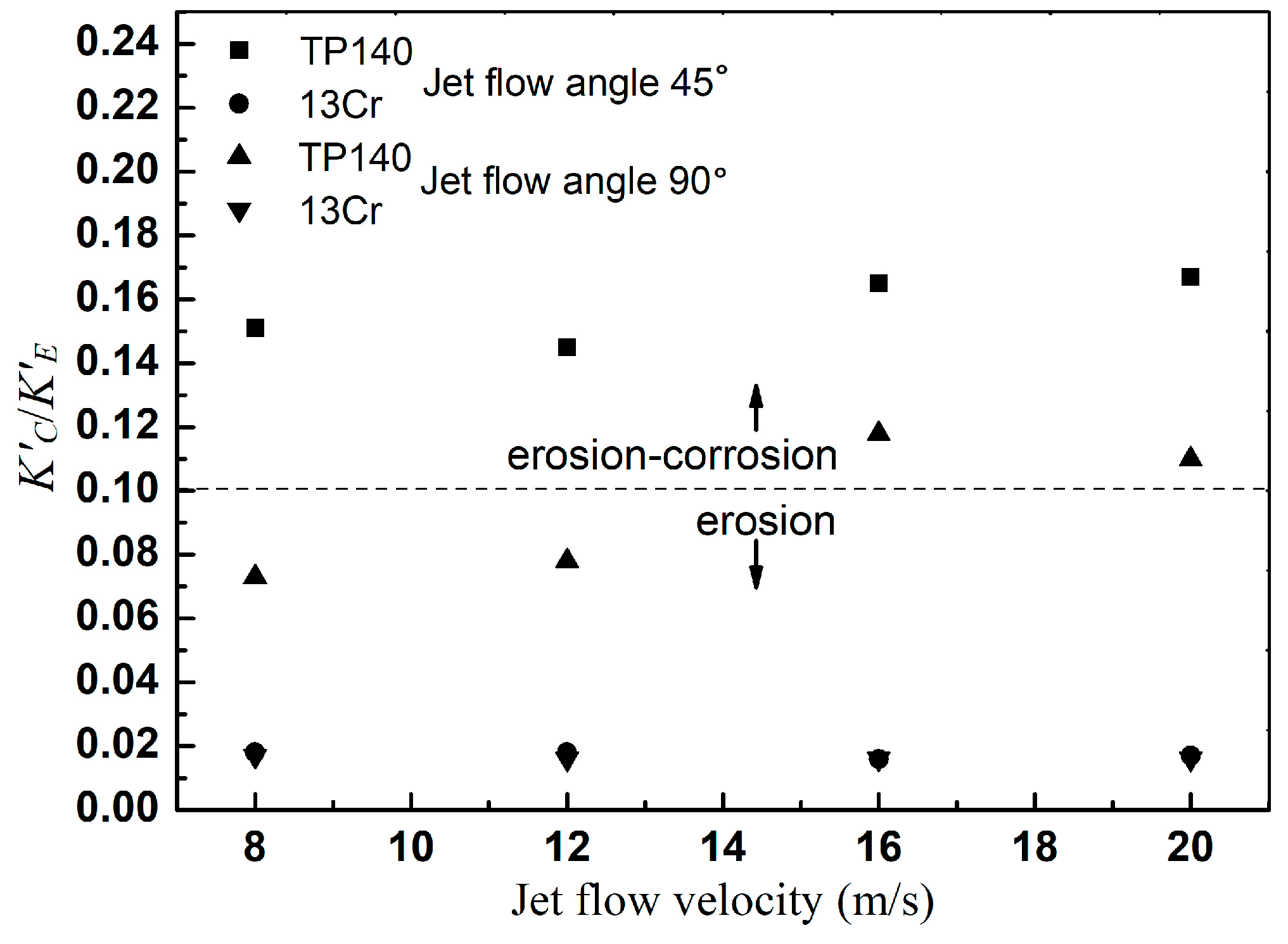
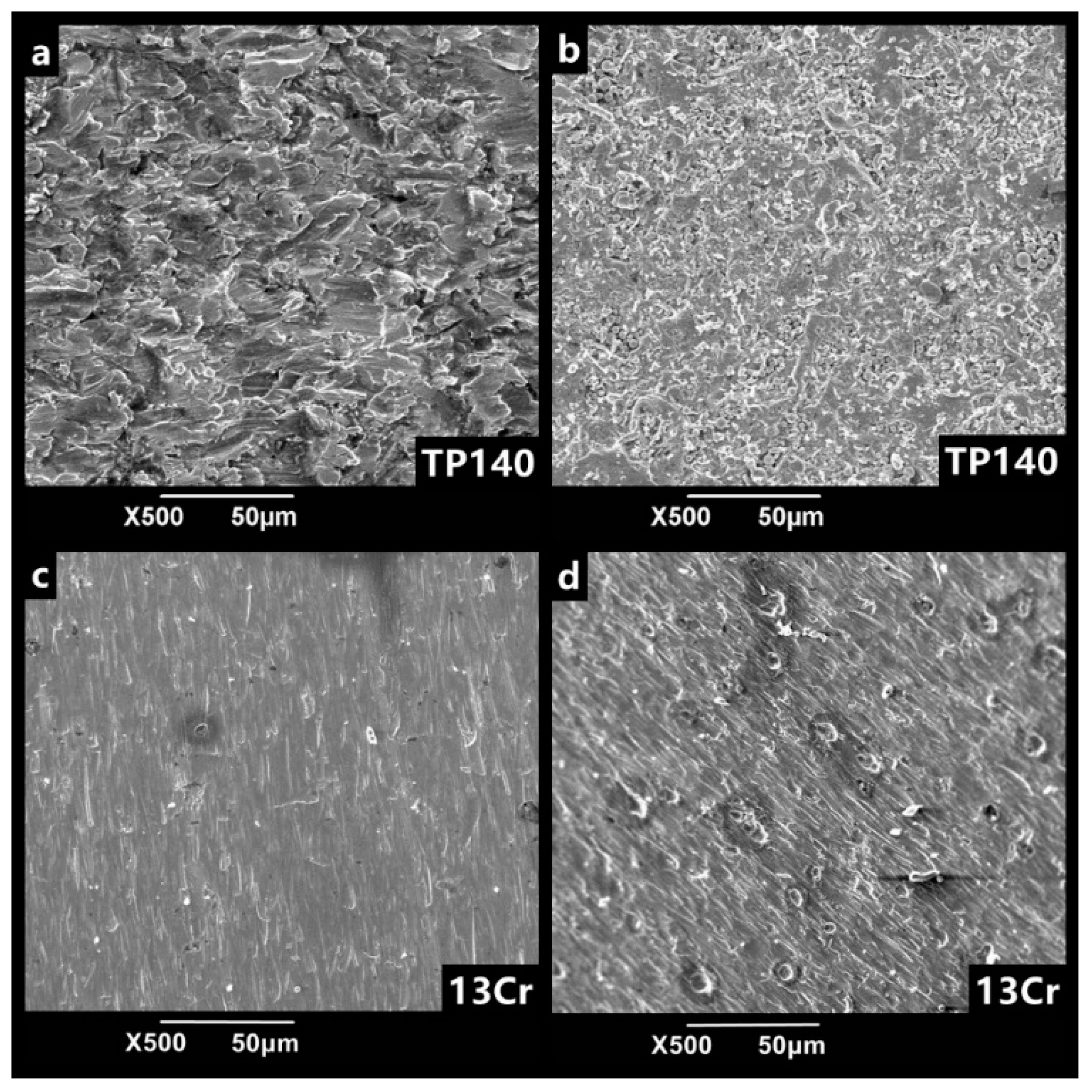
3.3. Synergism of Erosion and Corrosion
4. Discussion
5. Conclusions
- The erosion and corrosion rates of 13Cr and TP140 increase with the flow rate, and a maximum critical angle of erosion exists with the changes in jet angle. The critical angles of TP140 and 13Cr are near 60° and 45°, respectively. In the liquid flow condition, a flow velocity growth increases the current density on the TP140 surface and reduces the corrosion potential of 13Cr, but the effect of the angle on the two parameters is relatively small.
- Given the rupture of passive film and the reduced passivation rate in NaCl solution, the increased rates of erosion and corrosion of 13Cr are greater than those of TP140. Meanwhile, uniform corrosion and pitting can change the mechanical properties of the metal surface to increase particle erosion. However, the effect of uniform corrosion on erosion is continuously generated, and the effect of pitting will not occur unless it accumulates to a certain extent.
- Generally, the erosion rate is considerably higher than the corrosion rate in high velocity liquid–solid flow. Therefore, we must pay special attention to corrosion-enhanced erosion, especially for stainless-steel. The experimental results show that the increased erosion rate of TP140 in liquid–solid flow is in the range of 10% to 20%, while that of 13Cr is in the range of 30% to 90% compared to the material erosion rate in the gas–solid phase.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lian, Z.; Zhang, Y.; Zhao, X.; Ding, S.; Lin, T. Mechanical and mathematical models of multi-stage horizontal fracturing strings and their application. Nat. Gas Ind. B 2015, 2, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Tilly, G.P. Two Stage Mechanism of ductile erosion. Wear 1973, 23, 87–96. [Google Scholar] [CrossRef]
- Hutchings, I.M. Particle erosion of ductile metals: A mechanism of material removal. Wear 1974, 27, 121–128. [Google Scholar] [CrossRef]
- Postlethwaite, J.; Nesic, S. Erosion-corrosion in single and multiphase flow. Corrosion 1986, 42, 503–514. [Google Scholar]
- Azarian, N.S.; Ghasemi, H.M.; Monshi, M.R. Synergistic Erosion and Corrosion Behavior of AA5052 Aluminum Alloy in 3.5wt % NaCl Solution under Various Impingement Angles. J. Bio- Tribo-Corros. 2015, 1, 10. [Google Scholar] [CrossRef]
- Fan, A.M.; Long, J.M.; Tao, Z.Y. An investigation of the corrosive wear of stainless steels in aqueous slurries. Wear 1996, 193, 73–77. [Google Scholar]
- Stack, M.M.; Chacon-Nava, J.; Stott, F.H. Relationship between the effects of velocity and alloy corrosion resistance in erosion-corrosion environments at elevated temperatures. Wear 1995, 180, 91–99. [Google Scholar] [CrossRef]
- Stack, M.M.; Corlett, N.; Turgoose, S. Some thoughts on modelling the effects of oxygen and particle concentration on the erosion–corrosion of steels in aqueous slurries. Wear 2003, 255, 225–236. [Google Scholar] [CrossRef]
- Stack, M.M.; Pungwiwat, N. Erosion–corrosion mapping of Fe in aqueous slurries: Some views on a new rationale for defining the erosion–corrosion interaction. Wear 2004, 256, 565–576. [Google Scholar] [CrossRef]
- Stack, M.M.; Jana, B.D. Modelling particulate erosion–corrosion in aqueous slurries: Some views on the construction of erosion–corrosion maps for a range of pure metals. Wear 2004, 256, 986–1004. [Google Scholar] [CrossRef]
- Islam, M.A.; Farhat, Z.N.; Ahmed, E.M.; Alfantazi, A.M. Erosion enhanced corrosion and corrosion enhanced erosion of api X-70 pipeline steel. Wear 2013, 302, 1592–1601. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.F. Parametric effects on the erosion–corrosion rate and mechanism of carbon steel pipes in oil sands slurry. Wear 2012, 276–277, 141–148. [Google Scholar] [CrossRef]
- Tan, K.S.; Wharton, J.A.; Wood, R.J.K. Solid particle erosion-corrosion behaviour of a novel hvof nickel aluminium bronze coating for marine applications-correlation between mass loss and electrochemical measurements. Wear 2005, 258, 629–640. [Google Scholar] [CrossRef]
- Sasaki, K.; Burstein, G.T. Erosion–corrosion of stainless steel under impingement by a fluid jet. Corros. Sci. 2007, 49, 92–102. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Electrochemical characterization and computational fluid dynamics simulation of flow-accelerated corrosion of X65 steel in a CO2-saturated oilfield formation water. Corros. Sci. 2010, 52, 2716–2724. [Google Scholar] [CrossRef]
- Finnie, I. An experimental study of erosion. Wear 1960, 3, 70–76. [Google Scholar]
- Bitter, J.G.A. Study of erosion phenomenon-I. Wear 1963, 6, 169–176. [Google Scholar] [CrossRef]
- Lu, B.T.; Luo, J.L.; Mohammadi, F.; Wang, K.; Wan, X.M. Correlation between repassivation kinetics and corrosion rate over a passive surface in flowing slurry. Electrochim. Acta 2008, 53, 7022–7031. [Google Scholar] [CrossRef]
- Lotz, U.; Schollmaler, M.; Heltz, E. Flow-dependent corrosion–II: Ferrous materials in pure and particulate chloride solutions. Mater. Corros. 1985, 36, 63–173. [Google Scholar] [CrossRef]
- Neville, A.; Hodgkiess, T.; Dallas, J.T. A study of the erosion-corrosion behavior of engineering steels for marine pumping applications. Wear 1995, 37, 497–507. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G.; Zhou, X.; He, S.Y.; Sun, W.H.; Wang, J.Q. Determination of the critical flow velocities for erosion–corrosion of passive materials under impingement by NaCl solution containing sand. Corros. Sci. 2014, 88, 187–196. [Google Scholar] [CrossRef]







| Materials | C | Si | Mn | P | S | Cr | Mo | Ni | Cu |
|---|---|---|---|---|---|---|---|---|---|
| TP140 | 0.26 | 0.25 | 0.92 | 0.007 | 0.001 | 1.027 | 0.423 | 0.041 | 0.078 |
| 13Cr | 0.029 | 0.22 | 0.45 | 0.015 | 0.001 | 13.3 | 1.92 | 4.85 | 1.59 |
| Materials | Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) | Hardness (HV) |
|---|---|---|---|---|
| TP140 | 1090 | 1068 | 16 | 287.2 |
| 13Cr | 970 | 855 | 20 | 323.4 |
| Experiments | Mixed Medium | Jet angle | Jet Flow Velocity |
|---|---|---|---|
| pure erosion | sand + dry air | 30°, 45°, 60°, and 90° | 8, 12, 16, and 20 m/s |
| pure corrosion | 2.0 wt % NaCl + distilled water | 30°, 45°, 60°, and 90° | 8, 12, 16, and 20 m/s |
| erosion-corrosion | 2.0 wt % NaCl solution + sand | 30°, 45°, 60°, and 90° | 8, 12, 16, and 20 m/s |
| Jet Flow Angle | Materials | Flow Velocity | K′E | KE | ΔKE | K′C | KC | ΔKC | ΔK |
|---|---|---|---|---|---|---|---|---|---|
| 45° | TP140 | 8 m/s | 15.93 | 14.02 | 1.91 | 2.4 | 1.97 | 0.43 | 2.34 |
| 12 m/s | 18.79 | 16.57 | 2.22 | 2.73 | 2.5 | 0.23 | 2.45 | ||
| 16 m/s | 21.40 | 19.03 | 2.37 | 3.53 | 3.23 | 0.3 | 2.67 | ||
| 20 m/s | 25.73 | 22.94 | 2.79 | 4.29 | 3.65 | 0.64 | 3.43 | ||
| 13Cr | 8 m/s | 6.22 | 4.75 | 1.47 | 0.12 | 0.08 | 0.04 | 1.51 | |
| 12 m/s | 8.42 | 5.1 | 3.32 | 0.15 | 0.09 | 0.06 | 3.38 | ||
| 16 m/s | 13.25 | 8.74 | 4.51 | 0.22 | 0.14 | 0.08 | 4.59 | ||
| 20 m/s | 15.07 | 10.59 | 4.48 | 0.38 | 0.22 | 0.16 | 4.64 | ||
| 90° | TP140 | 8 m/s | 15.13 | 13.38 | 1.75 | 1.11 | 0.99 | 0.12 | 1.87 |
| 12 m/s | 17.28 | 15.3 | 1.98 | 1.35 | 1.11 | 0.24 | 2.22 | ||
| 16 m/s | 19.09 | 17.21 | 1.88 | 2.26 | 1.57 | 0.69 | 2.57 | ||
| 20 m/s | 22.82 | 20.39 | 2.43 | 2.51 | 1.64 | 0.87 | 3.30 | ||
| 13Cr | 8 m/s | 4.76 | 3.25 | 1.51 | 0.19 | 0.08 | 0.11 | 1.62 | |
| 12 m/s | 6.90 | 4.17 | 2.73 | 0.23 | 0.1 | 0.13 | 2.86 | ||
| 16 m/s | 9.53 | 5.1 | 4.43 | 0.35 | 0.12 | 0.23 | 4.66 | ||
| 20 m/s | 11.24 | 6.56 | 4.68 | 0.12 | 0.16 | −0.04 | 4.64 |
| Dominant Factor | Value |
|---|---|
| erosion | K′C/K′E < 0.1 |
| erosion-corrosion | 0.1 ≤ K′C/K′E < 1 |
| corrosion-erosion | 1 ≤ K′C/K′E < 10 |
| corrosion | K′C/K′E ≥ 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Li, Z.; Zhang, N.; Dou, Y.; Cui, L. Experimental Study on Erosion–Corrosion of TP140 Casing Steel and 13Cr Tubing Steel in Gas–Solid and Liquid–Solid Jet Flows Containing 2 wt % NaCl. Materials 2019, 12, 358. https://doi.org/10.3390/ma12030358
Cheng J, Li Z, Zhang N, Dou Y, Cui L. Experimental Study on Erosion–Corrosion of TP140 Casing Steel and 13Cr Tubing Steel in Gas–Solid and Liquid–Solid Jet Flows Containing 2 wt % NaCl. Materials. 2019; 12(3):358. https://doi.org/10.3390/ma12030358
Chicago/Turabian StyleCheng, Jiarui, Zhen Li, Ningsheng Zhang, Yihua Dou, and Lu Cui. 2019. "Experimental Study on Erosion–Corrosion of TP140 Casing Steel and 13Cr Tubing Steel in Gas–Solid and Liquid–Solid Jet Flows Containing 2 wt % NaCl" Materials 12, no. 3: 358. https://doi.org/10.3390/ma12030358





