Thermomechanical Properties of Zeta (Ag3In) Phase
Abstract
:1. Introduction
2. Materials and Methods
3. Results
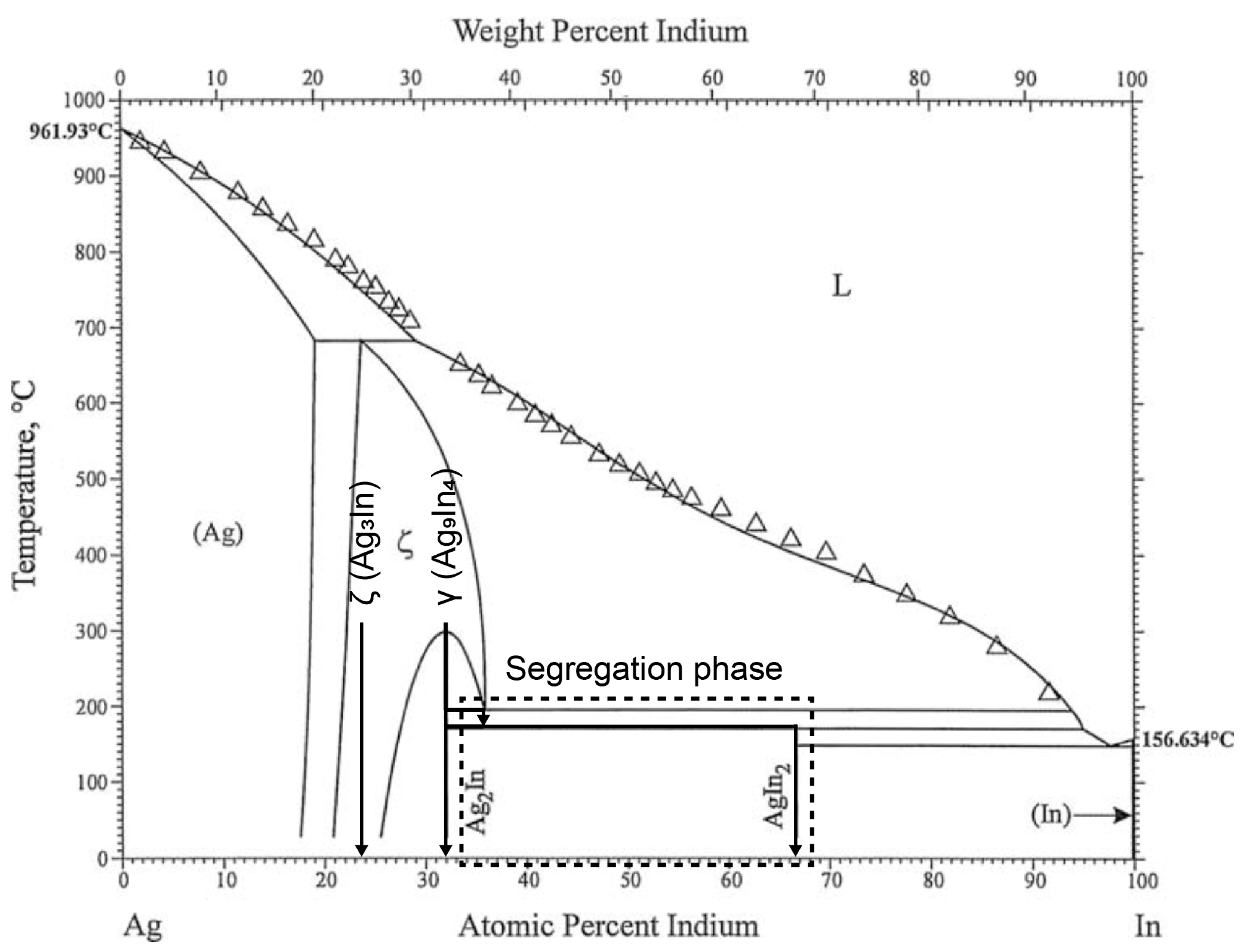
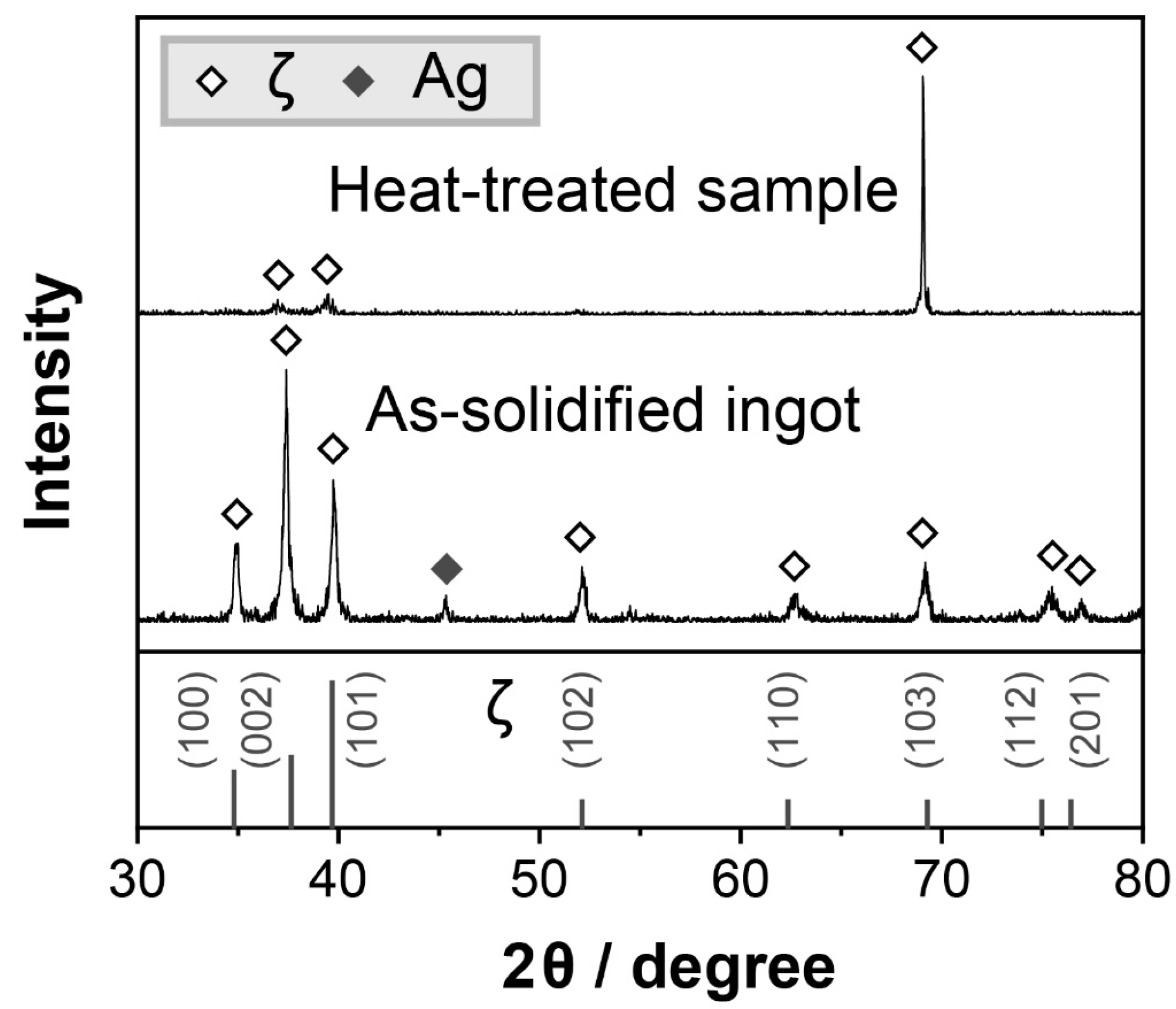
3.1. Characterization of Bulk ζ (Ag3In) Samples
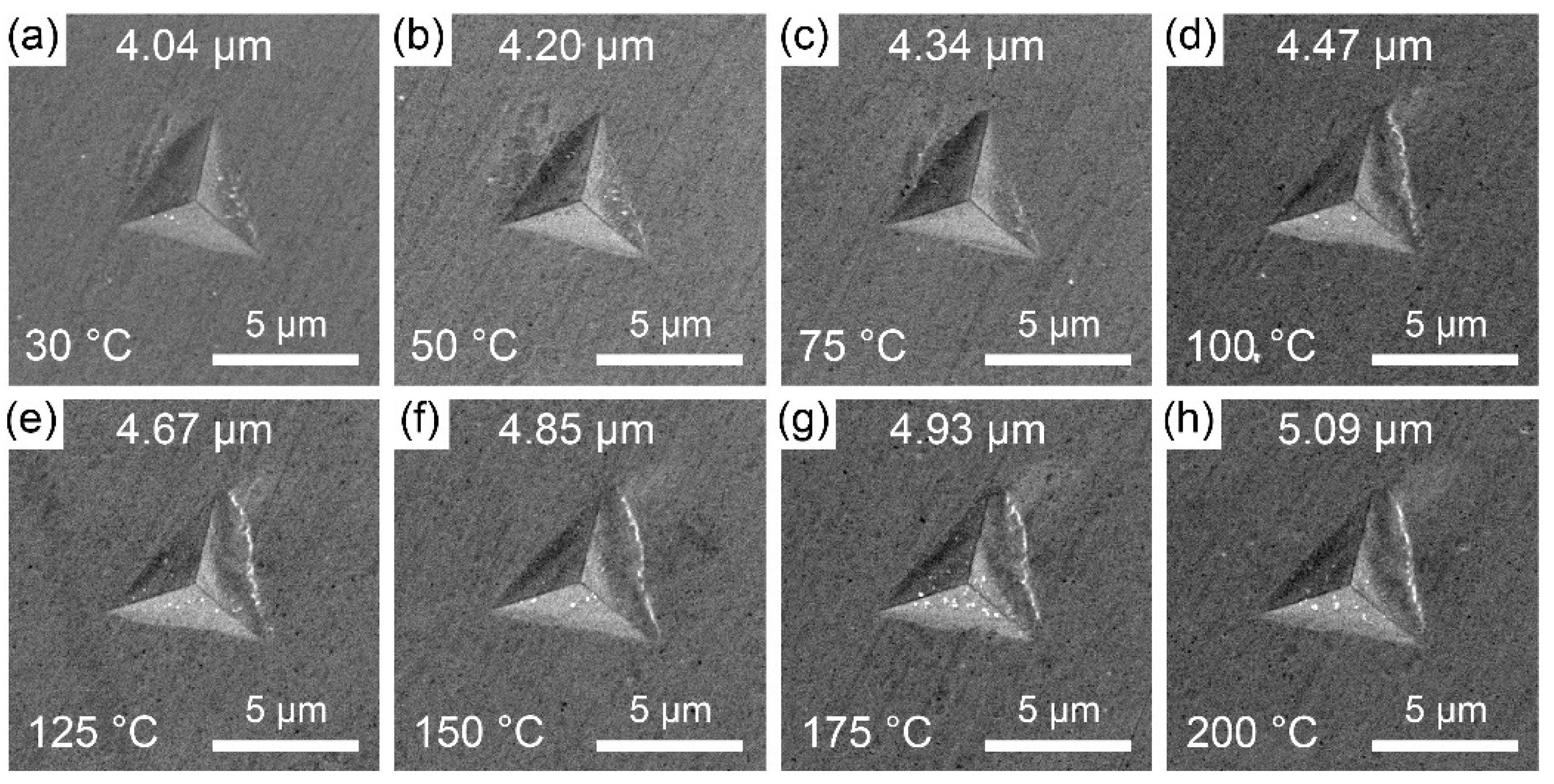
3.2. Hardness of ζ (Ag3In) Phase from 30 °C to 200 °C
3.3. Young’s Modulus of ζ (Ag3In) Phase from 30 °C to 200 °C
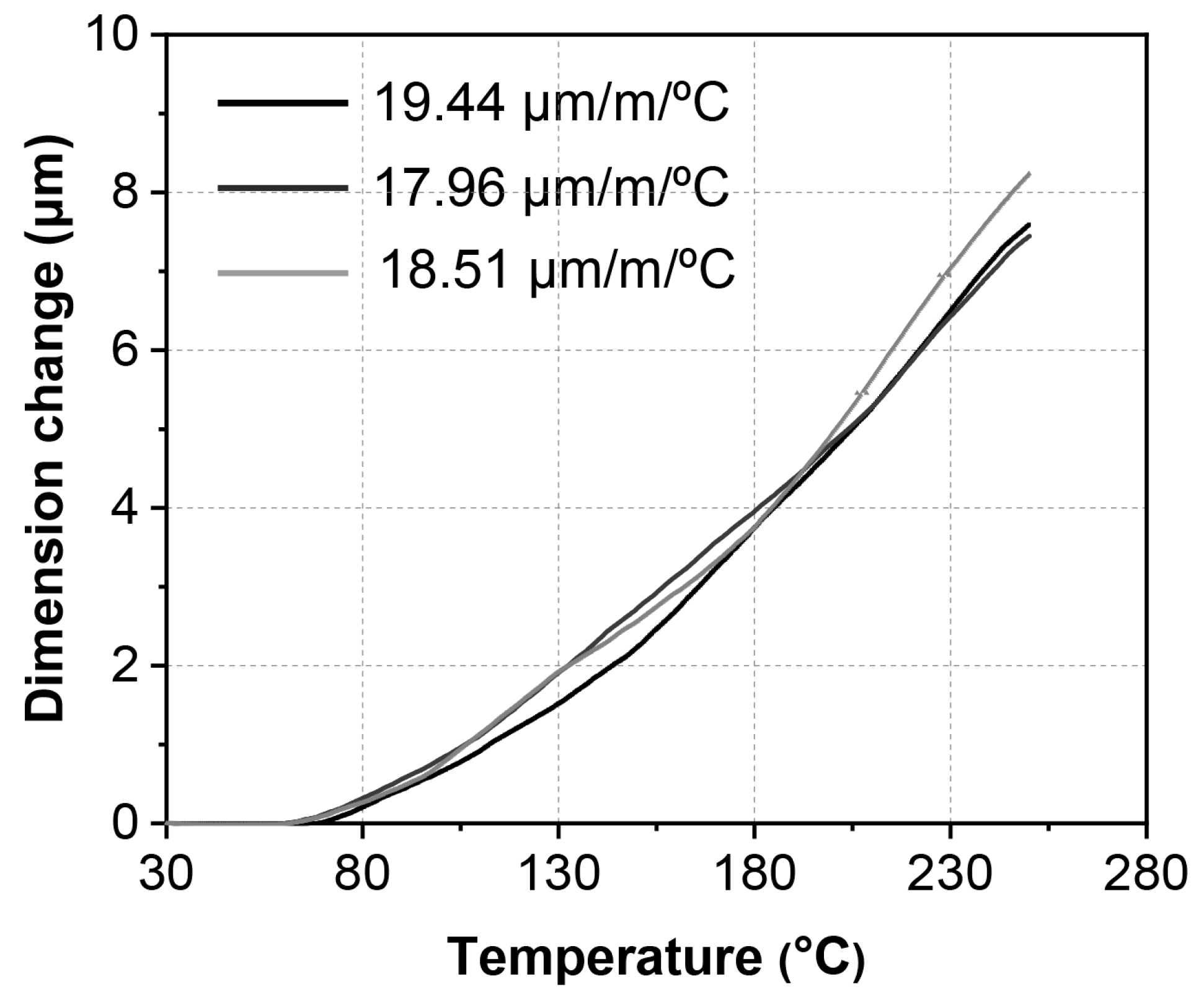
3.4. CTE of ζ (Ag3In) Phase from 30 °C to 250 °C
4. Discussion
5. Conclusions
- (1)
- Pure and densified ζ (Ag3In) bulk samples were fabricated by being cast in a water-cooled steel mold, followed by undergoing heat treatment at 520 °C for 30 h. Both the as-cast and heat-treated samples comprised the ζ (Ag3In) phase, and no phase transition was found. The heat treatment process was used for homogenization.
- (2)
- As the temperature increased from 30 °C to 200 °C, the hardness of the ζ (Ag3In) phase decreased linearly from 1.78 GPa to 1.46 GPa. Correspondingly, the Young’s modulus decreased linearly from 82.3 GPa to 66.5 GPa. Both values are lower than those of the other commonly used IMCs. Furthermore, the ζ (Ag3In) phase exhibited relatively good thermostability in terms of these properties.
- (3)
- The average CTE of the ζ (Ag3In) phase over the temperature range of 70–250 °C was approximately 18.63 ± 0.61 μm/m/°C, which is similar to that of Cu3Sn, Cu6Sn5, and Ag3Sn, placing it at a moderate level among the other IMCs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millan, J. A Review of WBG Power Semiconductor Devices. In Proceedings of the CAS 2012 (International Semiconductor Conference), Sinaia, Romania, 15–17 October 2012; pp. 57–66. [Google Scholar]
- Baliga, B.J. Fundamentals of Power Semiconductor Devices; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2010; ISBN 978-0-387-47314-7. [Google Scholar]
- Chu, S.; Cui, Y.; Liu, N. The Path towards Sustainable Energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Jain, H.; Rajawat, S.; Agrawal, P. Comparision of Wide Band Gap Semiconductors for Power Electronics Applications. In Proceedings of the International Conference on Recent Advances in Microwave Theory and Applications, Jaipur, Rajasthan, India, 21–24 November 2008; pp. 878–881. [Google Scholar]
- Buttay, C.; Planson, D.; Allard, B.; Bergogne, D.; Bevilacqua, P.; Joubert, C.; Lazar, M.; Martin, C.; Morel, H.; Tournier, D.; et al. State of the Art of High Temperature Power Electronics. Mater. Sci. Eng. B 2011, 176, 283–288. [Google Scholar] [CrossRef]
- Manikam, V.R.; Cheong, K.Y. Die Attach Materials for High Temperature Applications: A Review. IEEE Trans. Compon. Packag. Manufact. Technol. 2011, 1, 457–478. [Google Scholar] [CrossRef]
- Chidambaram, V.; Hattel, J.; Hald, J. High-Temperature Lead-Free Solder Alternatives. Microelectron. Eng. 2011, 88, 981–989. [Google Scholar] [CrossRef]
- Cook, G.O.; Sorensen, C.D. Overview of Transient Liquid Phase and Partial Transient Liquid Phase Bonding. J. Mater. Sci. 2011, 46, 5305–5323. [Google Scholar] [CrossRef]
- Bosco, N.S.; Zok, F.W. Strength of Joints Produced by Transient Liquid Phase Bonding in the Cu–Sn System. Acta Mater. 2005, 53, 2019–2027. [Google Scholar] [CrossRef]
- Lis, A. High Power Electronics Packaging by Transient Liquid Phase Bonding; ETH Zurich: Zurich, Switzerland, 2015. [Google Scholar]
- Chu, K.; Sohn, Y.; Moon, C. A Comparative Study of Cn/Sn/Cu and Ni/Sn/Ni Solder Joints for Low Temperature Stable Transient Liquid Phase Bonding. Scr. Mater. 2015, 109, 113–117. [Google Scholar] [CrossRef]
- Giudice, S.; Bosshard, C. Au-Sn Transient Liquid Phase Bonding for Hermetic Sealing and Getter Activation. In Proceedings of the Eurpoean Microelectronics Packaging Conference (EMPC), Grenoble, France, 9–12 September 2013; pp. 1–5. [Google Scholar]
- Chen, Y.; Liang, S.; Liu, C.; Liu, C.; Zhou, Z. Interfacial Characteristics and Mechanical Properties of Cu/Ga/Cu Interconnects by Transient Liquid Phase Bonding. In Proceedings of the IEEE 23rd Electronics Packaging Technology Conference (EPTC), Virtual Conference, 7–9 December 2021; pp. 451–459. [Google Scholar]
- Lin, W.P.; Sha, C.-H.; Lee, C.C. 40 μm Flip-Chip Process Using Ag–in Transient Liquid Phase Reaction. IEEE Trans. Compon. Packag. Manuf. Technol. 2012, 2, 903–908. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, L.; Xu, Y.H.; Zhang, Y.C.; Lu, K.J.; Xu, F.; Gao, H.M. Study on Microstructure and Shear Property of Cu/In-xCu/Cu Transient Liquid Phase Bonding Joints. J. Electron. Mater. 2021, 50, 217–223. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Huo, F.; Kariya, K.; Masago, N.; Nishikawa, H. Novel Transient Liquid Phase Bonding Method Using In-Coated Cu Sheet for High-Temperature Die Attach. Mater. Res. Bull. 2022, 149, 111713. [Google Scholar] [CrossRef]
- Liu, X.; Huo, F.; Wang, J.; Tatsumi, H.; Jin, Z.; Cheng, Z.; Nishikawa, H. Interfacial Reactions between In and Ag during Solid Liquid Interdiffusion Process. Surf. Interfaces, under Review.
- Liu, X.; Jin, Z.; Tatsumi, H.; Nishikawa, H. Mechanical Properties of Transient Liquid Phase Bonded Joints by Using Ag-In Sandwich Structure. In Proceedings of the IEEE 24th Electronics Packaging Technology Conference (EPTC), Singapore, 7 December 2022; pp. 71–75. [Google Scholar]
- Tsai, C.H.; Huang, W.C.; Chew, L.M.; Schmitt, W.; Li, J.; Nishikawa, H.; Kao, C.R. Low-Pressure Micro-Silver Sintering with the Addition of Indium for High-Temperature Power Chips Attachment. J. Mater. Res. Technol. 2021, 15, 4541–4553. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Nwoke, D.; Barlow, F.D.; Lee, C.C. Thermal Cycling Reliability Study of Ag–In Joints between Si Chips and Cu Substrates Made by Fluxless Processes. IEEE Trans. Compon. Packag. Manuf. Technol. 2014, 4, 1420–1426. [Google Scholar] [CrossRef]
- Marchenko, A.V. Thermo-Mechanical Properties of Materials. Cold. Reg. Sci. Technol. 2008. [Google Scholar]
- Chen, C.; Nagao, S.; Zhang, H.; Jiu, J.; Sugahara, T.; Suganuma, K.; Iwashige, T.; Sugiura, K.; Tsuruta, K. Mechanical Deformation of Sintered Porous Ag Die Attach at High Temperature and Its Size Effect for Wide-Bandgap Power Device Design. J. Electron. Mater. 2017, 46, 1576–1586. [Google Scholar] [CrossRef]
- Chen, C.; Choe, C.; Zhang, Z.; Kim, D.; Suganuma, K. Low-Stress Design of Bonding Structure and Its Thermal Shock Performance (−50 to 250 °C) in SiC/DBC Power Die-Attached Modules. J. Mater. Sci. Mater. Electron. 2018, 29, 14335–14346. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, F.; Liu, S.; Hu, X.; Liu, C.; Zhou, Z.; Wang, Z.; Robertson, S.; Liu, L. Creep Behavior of Intermetallic Compounds at Elevated Temperatures and Its Effect on Fatigue Life Evaluation of Cu Pillar Bumps. Intermetallics 2022, 144, 107526. [Google Scholar] [CrossRef]
- Liu, X.; Tatsumi, H.; Wang, J.; Jin, Z.; Cheng, Z.; Nishikawa, H. Analysis of microstructures and fractures in Ag-In transient liquid phase bonded joints. Mater. Sci. Eng. A, under Review.
- Liu, X.; Tatsumi, H.; Jin, Z.; Chen, Z.; Nishikawa, H. Fabrication and Thermo-Mechanical Properties of Ag9In4 Intermetallic Compound. Intermetallics 2023, 162, 108028. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Jendrzejczyk, D.; Fitzner, K. Thermodynamic Properties of Liquid Silver–Indium Alloys Determined from e.m.f. Measurements. Thermochimi. Acta 2005, 433, 66–71. [Google Scholar] [CrossRef]
- Chavoshi, S.Z.; Xu, S. Temperature-Dependent Nanoindentation Response of Materials. MRS Commun. 2018, 8, 15–28. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.K.; Paufler, P. Micro- and Nanoindentation Techniques for Mechanical Characterisation of Materials. Int. Mater. Rev. 2006, 51, 209–245. [Google Scholar] [CrossRef]
- Mu, D.; Huang, H.; McDonald, S.D.; Nogita, K. Creep and Mechanical Properties of Cu6Sn5 and (Cu,Ni)6Sn5 at Elevated Temperatures. J. Electron. Mater. 2013, 42, 304–311. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, F.; Guo, M. Investigation of Elevated Temperature Mechanical Properties of Intermetallic Compounds in the Cu–Sn System Using Nanoindentation. J. Electron. Packag. 2020, 142, 021004. [Google Scholar] [CrossRef]
- Yang, P.F.; Lai, Y.S.; Jian, S.R.; Chen, J.; Chen, R.S. Nanoindentation Identifications of Mechanical Properties of Cu6Sn5, Cu3Sn, and Ni3Sn4 Intermetallic Compounds Derived by Diffusion Couples. Mater. Sci. Eng. A 2008, 485, 305–310. [Google Scholar] [CrossRef]
- Fahim, A.; Ahmed, S.; Suhling, J.C.; Lall, P. Mechanical Characterization of Intermetallic Compounds in SAC Solder Joints at Elevated Temperatures. In Proceedings of the 17th IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), San Diego, CA, USA, 29 May–1 June 2018; pp. 1081–1090. [Google Scholar]
- Hertzberg, R.W.; Hauser, F.E. Deformation and Fracture Mechanics of Engineering Materials. J. Eng. Mater. Technol. 1977, 99, 96. [Google Scholar] [CrossRef]
- Tsai, I.; Wu, E.; Yen, S.F.; Chuang, T.H. Mechanical Properties of Intermetallic Compounds on Lead-Free Solder by Moiré Techniques. J. Electron. Mater. 2006, 35, 1059–1066. [Google Scholar] [CrossRef]
- Fields, R.; Low, S., III. Physical and Mechanical Properties of Intermetallic Compounds Commonly Found in Solder Joints. Available online: https://www.metallurgy.nist.gov/mechanical_properties/solder_paper.html (accessed on 31 August 2023).
- Lis, A.; Kicin, S.; Brem, F.; Leinenbach, C. Thermal Stress Assessment for Transient Liquid-Phase Bonded Si Chips in High-Power Modules Using Experimental and Numerical Methods. J. Electron. Mater. 2017, 46, 729–741. [Google Scholar] [CrossRef]
- Hung, T.Y.; Huang, C.J.; Lee, C.C.; Wang, C.C.; Lu, K.C.; Chiang, K.N. Investigation of Solder Crack Behavior and Fatigue Life of the Power Module on Different Thermal Cycling Period. Microelectron. Eng. 2013, 107, 125–129. [Google Scholar] [CrossRef]

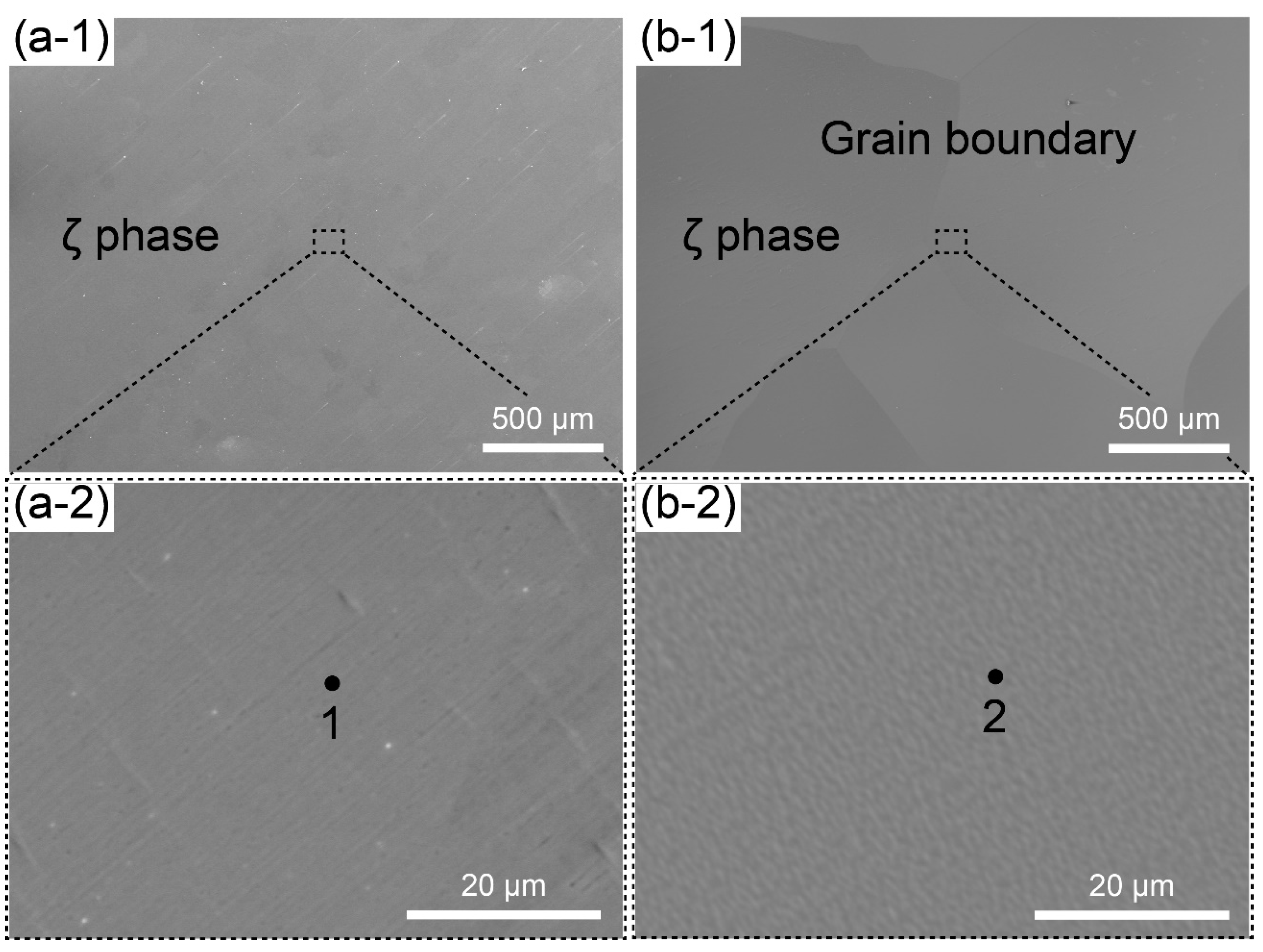



| 1 | 2 | |
|---|---|---|
| Ag (at. %) | 75.86 | 76.07 |
| In (at. %) | 24.14 | 23.93 |
| Estimated phase | ζ (Ag3In) | ζ (Ag3In) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Tatsumi, H.; Jin, Z.; Chen, Z.; Nishikawa, H. Thermomechanical Properties of Zeta (Ag3In) Phase. Materials 2023, 16, 7115. https://doi.org/10.3390/ma16227115
Liu X, Tatsumi H, Jin Z, Chen Z, Nishikawa H. Thermomechanical Properties of Zeta (Ag3In) Phase. Materials. 2023; 16(22):7115. https://doi.org/10.3390/ma16227115
Chicago/Turabian StyleLiu, Xunda, Hiroaki Tatsumi, Zhi Jin, Zhong Chen, and Hiroshi Nishikawa. 2023. "Thermomechanical Properties of Zeta (Ag3In) Phase" Materials 16, no. 22: 7115. https://doi.org/10.3390/ma16227115





