Mixed Chinese Fir Plantations Alter the C, N, and P Resource Limitations Influencing Microbial Metabolism in Soil Aggregates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Site and Sampling
2.2. Soil Aggregate Fractionation
2.3. Soil Chemical Analyses
2.4. Soil Microbial Indicators
2.5. Quantification of Microbial Metabolic Limitations
2.6. Statistics and Analysis
3. Results
3.1. Variations in Soil Aggregates and Nutrients
3.2. Soil Microbial Activity and Extracellular Enzymes
3.3. Enzyme Stoichiometric Characteristics
3.4. Resource Limitations and Influencing Factors of Microbial Metabolism
4. Discussion
4.1. Mixed Plantations Improve Soil Aggregate Structure and Stability as Well as Soil Nutrient Content
4.2. Soil Aggregates Drive Changes in Microbial Resource Limitations in Mixed Stands
4.3. Differences and Similarities in Regulatory Pathways of Microbial Resource Limitation in Different Plantations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Rabot, E.; Wiesmeier, M.; Schluter, S.; Vogel, H.J. Soil structure as an indicator of soil functions: A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- Zhou, B.; Peng, D.; Zhao, Q.X.; Yangnan, S.Y.; Yang, S.Q.; Yang, F.; Qu, G.Y.; Tang, W.W.; Ou, J.P.; Xiang, W.H.; et al. Improvements in timber production of Chinese fir (Cunninghamia lanceolata) per unit forest area in China via tree breeding: Status and challenges. Dendrobiology 2020, 83, 43–51. [Google Scholar] [CrossRef]
- Farooq, T.H.; Yan, W.; Rashid, M.H.U.; Tigabu, M.; Gilani, M.M.; Zou, X.H.; Wu, P.F. Chinese fir (Cunninghamia Lanceolata) a green gold of China with continues decline in its productivity over the successive rotations: A review. Appl. Ecol. Environ. Res. 2019, 17, 11055–11067. [Google Scholar] [CrossRef]
- Guan, F.Y.; Tang, X.L.; Fan, S.H.; Zhao, J.C.; Peng, C. Changes in soil carbon and nitrogen stocks followed the conversion from secondary forest to Chinese fir and Moso bamboo plantations. Catena 2015, 133, 455–460. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, Y.J.; Saeed, S.; Zhang, B.; Luo, M. The difference of soil properties between pure and mixed Chinese fir (Cunninghamia lanceolata) plantations depends on tree species. Glob. Ecol. Conserv. 2020, 22, e01009. [Google Scholar] [CrossRef]
- Feng, Y.; Schmid, B.; Loreau, M.; Forrester, D.I.; Fei, S.; Zhu, J.; Tang, Z.; Zhu, J.; Hong, P.; Ji, C.; et al. Multispecies forest plantations outyield monocultures across a broad range of conditions. Science 2022, 376, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Huang, Y.Z.; Ye, S.M. Distribution of organic carbon and nutrients in soil aggregates under different stand types of Cunninghamia lanceolata in southern Guangxi of China. Soil Sci. Plant Nutr. 2021, 67, 427–438. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhang, Q.C.; Wang, S.Q.; Jiang, C.Y.; Lan, Y.H.; Zhang, H.; Ye, S.M. Mixed plantations induce more soil macroaggregate formation and facilitate soil nitrogen accumulation. Forests 2023, 14, 735. [Google Scholar] [CrossRef]
- Holden, S.R.; Treseder, K.K. A meta-analysis of soil microbial biomass responses to forest disturbances. Front. Microbiol. 2013, 4, 163. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Calabrese, S.; Mohanty, B.P.; Malik, A.A. Soil microorganisms regulate extracellular enzyme production to maximize their growth rate. Biogeochemistry 2022, 158, 303–312. [Google Scholar] [CrossRef]
- Zheng, H.F.; Vesterdal, L.; Schmidt, I.K.; Rousk, J. Ecoenzymatic stoichiometry can reflect microbial resource limitation, substrate quality, or both in forest soils. Soil Biol. Biochem. 2022, 167, 108613. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Belnap, J.; Findlay, S.G.; Shah, J.J.F.; Hill, B.H.; Kuehn, K.A.; Kuske, C.R.; Litvak, M.E.; Martinez, N.G.; Moorhead, D.L.; et al. Extracellular enzyme kinetics scale with resource availability. Biogeochemistry 2014, 121, 287–304. [Google Scholar] [CrossRef]
- Wang, S.Q.; Li, T.X.; Zheng, Z.C. Effects of tea plantation age on soil aggregate-associated C- and N-cycling enzyme activities in the hilly areas of Western Sichuan, China. Catena 2018, 171, 145–153. [Google Scholar] [CrossRef]
- Xue, L.; Sun, B.; Yang, Y.; Jin, B.; Zhuang, G.; Bai, Z.; Zhuang, X. Efficiency and mechanism of reducing ammonia volatilization in alkaline farmland soil using Bacillus amyloliquefaciens biofertilizer. Environ. Res. 2021, 202, 111672. [Google Scholar] [CrossRef] [PubMed]
- Darch, T.; Blackwell, M.S.; Chadwick, D.; Haygarth, P.M.; Hawkins, J.M.; Turner, B.L. Assessment of bioavailable organic phosphorus in tropical forest soils by organic acid extraction and phosphatase hydrolysis. Geoderma 2016, 284, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.W.; Yu, G.R.; Zhang, X.Y.; He, N.P.; Wang, Q.F.; Wang, S.Z.; Wang, R.L.; Zhao, N.; Jia, Y.L.; Wang, C.Y. Soil enzyme activity and stoichiometry in forest ecosystems along the North-South Transect in eastern China (NSTEC). Soil Biol. Biochem. 2017, 104, 152–163. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2013, 117, 101–113. [Google Scholar] [CrossRef]
- Chen, X.; Feng, J.; Ding, Z.; Tang, M.; Zhu, B. Changes in soil total, microbial and enzymatic C-N-P contents and stoichiometry with depth and latitude in forest ecosystems. Sci. Total Environ. 2022, 816, 151583. [Google Scholar] [CrossRef] [PubMed]
- Soong, J.L.; Fuchslueger, L.; Maranon-Jimenez, S.; Torn, M.S.; Janssens, I.A.; Penuelas, J.; Richter, A. Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling. Glob. Chang. Biol. 2019, 26, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Townsend, A.R.; Schmidt, S.K. Phosphorus limitation of microbial processes in moist tropical forests: Evidence from short-term laboratory incubations and field studies. Ecosystems 2002, 5, 680–691. [Google Scholar] [CrossRef]
- Amézketa, E. Soil aggregate stability: A review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2017, 181, 104–136. [Google Scholar] [CrossRef]
- Xu, H.D.; Yuan, H.J.; Yu, M.K.; Cheng, X.R. Large macroaggregate properties are sensitive to the conversion of pure plantation to uneven-aged mixed plantations. Catena 2020, 194, 104724. [Google Scholar] [CrossRef]
- Han, S.; Delgado-Baquerizo, M.; Luo, X.S.; Liu, Y.R.; Van Nostrand, J.D.; Chen, W.L.; Zhou, J.Z.; Huang, Q.Y. Soil aggregate size-dependent relationships between microbial functional diversity and multifunctionality. Soil Biol. Biochem. 2021, 154, 108143. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Wang, S.Q.; Jiang, C.Y.; Cui, Y.H.; Fan, R.Y.; Lan, Y.H.; Zhang, Q.C.; Ye, S.M. Tree-litter-soil system C:N:P stoichiometry and tree organ homeostasis in mixed and pure Chinese fir stands in south subtropical China. Front. For. Glob. Chang. 2024, 7, 1293439. [Google Scholar] [CrossRef]
- Bach, E.M.; Hofmockel, K.S. Soil aggregate isolation method affects measures of intra-aggregate extracellular enzyme activity. Soil Biol. Biochem. 2014, 69, 54–62. [Google Scholar] [CrossRef]
- John, B.; Yamashita, T.; Ludwig, B.; Flessa, H. Storage of organic carbon in aggregate and density fractions of silty soils under different types of land use. Geoderma 2005, 128, 63–79. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 961–1010. [Google Scholar]
- Bremner, J.M. Nitrogen-total. Methods Soil Anal. Part. 3 Chem. Methods 1996, 5, 1085–1121. [Google Scholar]
- Bo, F.J.; Zhang, Y.X.; Chen, H.Y.H.; Wang, P.G.; Ren, X.M.; Guo, J.P. The C:N:P stoichiometry of planted and natural larix principis-rupprechtii stands along altitudinal gradients on the Loess Plateau, China. Forests 2020, 11, 363. [Google Scholar] [CrossRef]
- Tirol-Padre, A.; Ladha, J.K. Assessing the reliability of permanganate-oxidizable carbon as an index of soil labile carbon. Soil Sci. Soc. Am. J. 2004, 68, 969–978. [Google Scholar] [CrossRef]
- Roberts, T.L.; Ross, W.J.; Norman, R.J.; Slaton, N.A.; Wilson, C.E. Predicting nitrogen fertilizer needs for rice in Arkansas using alkaline hydrolyzable- nitrogen. Soil Sci. Soc. Am. J. 2011, 75, 1161–1171. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Vance, E.D.; Brggek, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Dahlin, S.; Witter, E.; Martensson, A.; Turner, A.; Baath, E. Where’s the limit? Changes in the microbiological properties of agricultural soils at low levels of metal contamination. Soil Biol. Biochem. 1997, 29, 1405–1415. [Google Scholar] [CrossRef]
- Tabatabai, M.; Weaver, R. Methods of Soil: Analysis Microbiological and Biochemical Properties; Part 2; Soil Science Society of America: Madison, WI, USA, 1994. [Google Scholar]
- Gianfreda, L.; Sannino, F.; Ortega, N.; Nannipieri, P. Activity of free and immobilized urease in soil: Effects of pesticides. Soil Biol. Biochem. 1994, 26, 777–784. [Google Scholar] [CrossRef]
- Georgea, T.S.; Gregorya, P.J.; Wooda, M.; Reada, D.; Bureshb, R.J. Phosphatase activity and organic acids in the rhizosphere of potential agroforestry species and maize. Soil Biol. Biochem. 2002, 34, 1487–1494. [Google Scholar] [CrossRef]
- Zhou, L.H.; Liu, S.S.; Shen, H.H.; Zhao, M.Y.; Xu, L.C.; Xing, A.J.; Fang, J.Y. Soil extracellular enzyme activity and stoichiometry in China’s forests. Funct. Ecol. 2020, 34, 1461–1471. [Google Scholar] [CrossRef]
- Liu, M.; Gan, B.; Li, Q.; Xiao, W.; Song, X. Effects of nitrogen and phosphorus addition on soil extracellular enzyme activity and stoichiometry in Chinese fir (Cunninghamia lanceolata) forests. Front. Plant Sci. 2022, 13, 834184. [Google Scholar] [CrossRef]
- Urbach, N.; Ahlemann, F. Structural equation modeling in information systems research using partial least squares. J. Inf. Technol. Theory Appl. 2010, 11, 5–40. [Google Scholar]
- Diamantopoulos, A.; Siguaw, J.A. Formative versus reflective indicators in organizational measure development: A comparison and empirical illustration. Br. J. Manag. 2006, 17, 263–282. [Google Scholar]
- Chin, W.W. The Partial Least Squares Approach to Structural Equation Modeling; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1998; pp. 1295–1336. [Google Scholar]
- Hair, J.F.; Risher, J.J.; Sarstedt, M.; Ringle, C.M. When to use and how to report the results of PLS-SEM. Eur. Bus. Rev. 2019, 31, 2–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, C.F.; Xie, D.T.; Gao, M.; Zeng, X.B. Effects of land use patterns on soil aggregate stability in Sichuan Basin, China. Particuology 2008, 6, 157–166. [Google Scholar] [CrossRef]
- Regelink, I.C.; Stoof, C.R.; Rousseva, S.; Weng, L.; Lair, G.J.; Kram, P.; Nikolaidis, N.P.; Kercheva, M.; Banwart, S.; Comans, R.N.J. Linkages between aggregate formation, porosity and soil chemical properties. Geoderma 2015, 247–248, 24–37. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Li, D.; Liu, Z.; Wen, L.; Huang, Z.; Jiang, D.; Lu, Y. Soil aggregate stability and its response to overland flow in successive Eucalyptus plantations in subtropical China. Sci. Total Environ. 2022, 807, 151000. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Zhou, H.C.; Sheng, R.; Qin, H.L.; Hou, H.J.; Liu, Y.; Chen, A.L.; Chen, C.L.; Wei, W.X. Differences in the nitrous oxide emission and the nitrifier and denitrifier communities among varying aggregate sizes of an arable soil in China. Geoderma 2021, 389, 114970. [Google Scholar] [CrossRef]
- Xue, B.; Huang, L.; Huang, Y.A.; Yin, Z.Y.; Li, X.K.; Lu, J.W. Effects of organic carbon and iron oxides on soil aggregate stability under different tillage systems in a rice-rape cropping system. Catena 2019, 177, 1–12. [Google Scholar] [CrossRef]
- Ouyang, W.; Wu, Y.Y.; Hao, Z.C.; Zhang, Q.; Bu, Q.W.; Gao, X. Combined impacts of land use and soil property changes on soil erosion in a mollisol area under long-term agricultural development. Sci. Total Environ. 2018, 613, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Zhu, H.S.; Wei, X.R.; Liu, B.Y.; Shao, M.G. Soil erosion leads to degradation of hydraulic properties in the agricultural region of Northeast China. Agr. Ecosyst. Environ. 2021, 314, 107388. [Google Scholar] [CrossRef]
- Guo, J.H.; Feng, H.L.; McNie, P.; Liu, Q.Y.; Xu, X.; Pan, C.; Yan, K.; Feng, L.; Goitom, E.A.; Yu, Y.C. Species mixing improves soil properties and enzymatic activities in Chinese fir plantations: A meta-analysis. Catena 2023, 220, 106723. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Huang, Y. Comparisons of litterfall, litter decomposition and nutrient return in a monoculture Cunninghamia lanceolata and a mixed stand in southern China. For. Ecol. Manag. 2008, 255, 1210–1218. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.J.; Zhang, W.W.; Zhang, Q.; Lu, D.L.; Zhang, Y.K.; Zheng, X.; Xu, S.; Wang, G.G. Litter decomposition and nutrient release from monospecific and mixed litters: Comparisons of litter quality, fauna and decomposition site effects. J. Ecol. 2022, 110, 1673–1686. [Google Scholar] [CrossRef]
- Pries, C.E.H.; Sulman, B.N.; West, C.; O’Neill, C.; Poppleton, E.; Porras, R.C.; Castanha, C.; Zhu, B.; Wiedemeier, D.B.; Torn, M.S. Root litter decomposition slows with soil depth. Soil Biol. Biochem. 2018, 125, 103–114. [Google Scholar] [CrossRef]
- Frouz, J.; Livecková, M.; Albrechtová, J.; Chronáková, A.; Cajthaml, T.; Pizl, V.; Hánel, L.; Stary, J.; Baldrian, P.; Lhotáková, Z.; et al. Is the effect of trees on soil properties mediated by soil fauna? A case study from post-mining sites. For. Ecol. Manag. 2013, 309, 87–95. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.R.; Huang, Y.T.; Fu, S.L.; Wang, J.X.; Ming, A.G.; Li, X.Z.; Yao, M.J.; Li, H. Tree species mixture inhibits soil organic carbon mineralization accompanied by decreased r-selected bacteria. Plant Soil 2018, 431, 203–216. [Google Scholar] [CrossRef]
- Zhang, W.; You, Y.M.; Su, X.Y.; Yan, J.L.; Gao, G.; Ming, A.A.; Shen, W.J.; Huang, X.M. Introducing N2-fixing tree species into Eucalyptus plantations promotes soil organic carbon sequestration in aggregates by increasing microbial carbon use efficiency. Catena 2023, 231, 107321. [Google Scholar] [CrossRef]
- Pereira, E.L.; Santos, S.A.P.; Arrobas, M.; Patricio, M.S. Microbial biomass and N mineralization in mixed plantations of broadleaves and nitrogen-fixing species. For. Syst. 2011, 20, 516–524. [Google Scholar] [CrossRef]
- Bai, S.H.; Gallart, M.; Singh, K.; Hannet, G.; Komolong, B.; Yinil, D.; Field, D.J.; Muqaddas, B.; Wallace, H.M. Leaf litter species affects decomposition rate and nutrient release in a cocoa plantation. Agric. Ecosyst. Environ. 2022, 324, 107705. [Google Scholar] [CrossRef]
- Qi, D.; Feng, F.; Lu, C.; Fu, Y. C:N:P stoichiometry of different soil components after the transition of temperate primary coniferous and broad-leaved mixed forests to secondary forests. Soil Till Res. 2022, 216, 105260. [Google Scholar] [CrossRef]
- Schmidt, M.; Veldkamp, E.; Corre, M.D. Tree species diversity effects on productivity, soil nutrient availability and nutrient response efficiency in a temperate deciduous forest. For. Ecol. Manag. 2015, 338, 114–123. [Google Scholar] [CrossRef]
- Jing, X.; Chen, X.; Tang, M.; Ding, Z.; Jiang, L.; Li, P.; Ma, S.; Tian, D.; Xu, L.; Zhu, J.; et al. Nitrogen deposition has minor effect on soil extracellular enzyme activities in six Chinese forests. Sci. Total Environ. 2017, 607–608, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Du, E.; Jiang, L.; Ma, S.; Zeng, W.; Zou, A.; Feng, C.; Xu, L.; Xing, A.; Wang, W.; et al. Responses of forest ecosystems to increasing N deposition in China: A critical review. Environ. Pollut. 2018, 243, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Bach, E.M.; Williams, R.J.; Hargreaves, S.K.; Yang, F.; Hofmockel, K.S. Greatest soil microbial diversity found in micro-habitats. Soil Biol. Biochem. 2018, 118, 217–226. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Or, D. Hydration and diffusion processes shape microbial community organization and function in model soil aggregates. Water Resour. Res. 2015, 51, 9804–9827. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Yan, J.; Han, X.Z.; Zou, W.X.; Chen, X.; Lu, X.C.; Feng, Y.T. Labile organic carbon fractions drive soil microbial communities after long-term fertilization. Glob. Ecol. Conserv. 2021, 32, e01867. [Google Scholar] [CrossRef]
- Wang, L.; Luo, X.; Xiong, X.; Chen, W.; Hao, X.; Huang, Q. Soil aggregate stratification of ureolytic microbiota affects urease activity in an inceptisol. J. Agric. Food Chem. 2019, 67, 11584–11590. [Google Scholar] [CrossRef]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Anderson, I.C.; Singh, B.K. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Wan, W.J.; Li, X.; Han, S.; Wang, L.; Luo, X.S.; Chen, W.L.; Huang, Q.Y. Soil aggregate fractionation and phosphorus fraction driven by long-term fertilization regimes affect the abundance and composition of P-cycling-related bacteria. Soil Till Res. 2020, 196, 104475. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.K.; Wang, H.; Chen, X.W.; Fu, Y. Effect of rainfall on soil aggregate breakdown and transportation on cultivated land in the black soil region of Northeast China. Sustainability 2022, 14, 11028. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, H.; Zheng, C.; Wu, X.; Zhao, Y.; Li, X.; Liu, H.; Dong, L.; Lu, Z.; Zhou, J.; et al. Bacteria life-history strategies and the linkage of soil C-N-P stoichiometry to microbial resource limitation differed in karst and non-karst plantation forests in southwest China. Catena 2023, 231, 107341. [Google Scholar] [CrossRef]
- Qiu, X.C.; Peng, D.L.; Tian, H.X.; Wang, H.B.; Liu, X.; Cao, L.; Li, Z.; Cheng, S. Soil ecoenzymatic stoichiometry and microbial resource limitation driven by thinning practices and season types in Larix principis-rupprechtii plantations in North China. For. Ecol. Manag. 2021, 482, 118880. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Jiang, M.; Shen, G.; Yu, J.; Wang, X.; Zhu, H.; Wu, Y.; Zhang, X. Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 2019, 458, 7–20. [Google Scholar] [CrossRef]
- Zimmermann, S.; Frey, B. Soil respiration and microbial properties in an acid forest soil: Effects of wood ash. Soil Biol. Biochem. 2002, 34, 1727–1737. [Google Scholar] [CrossRef]



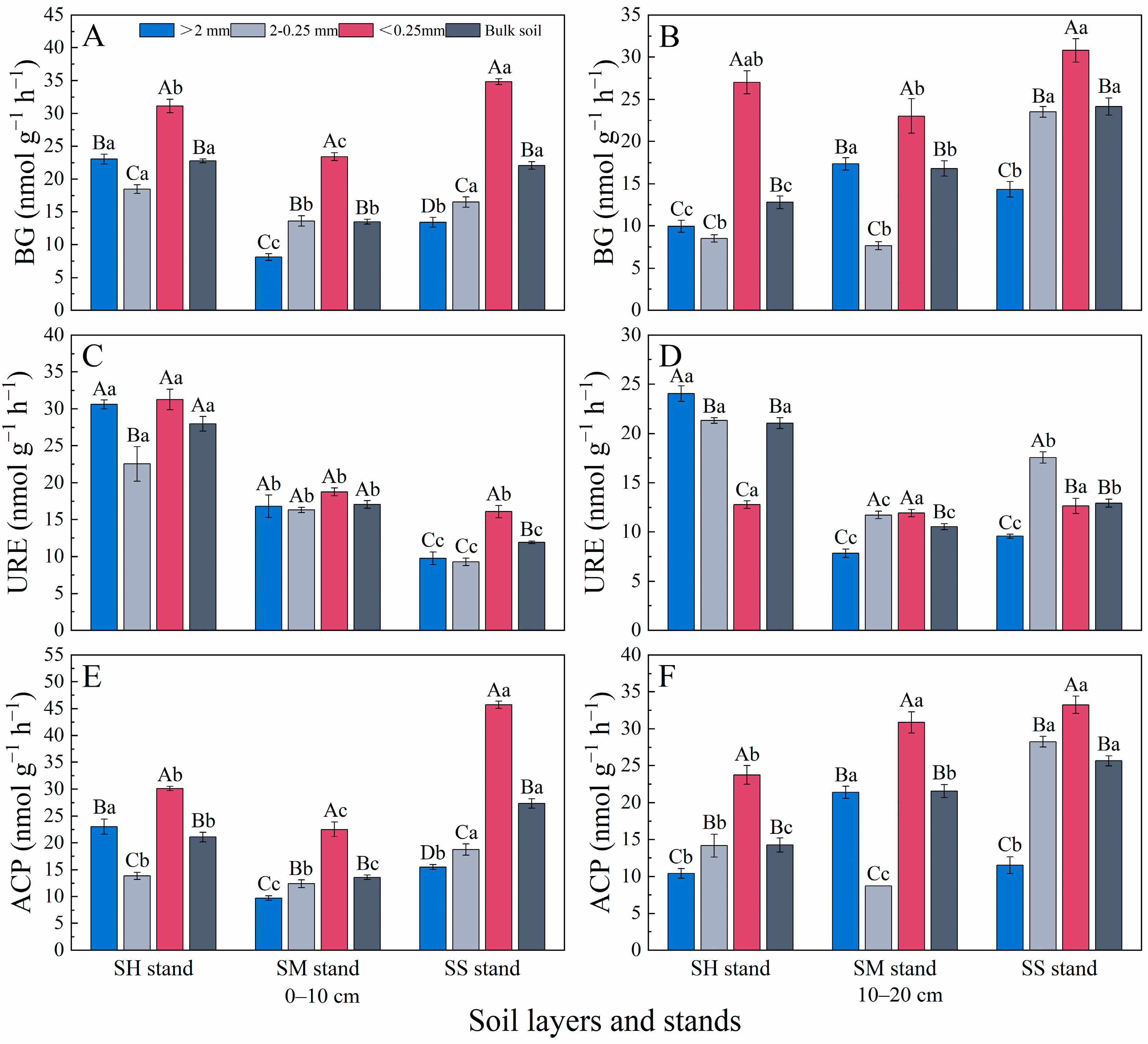


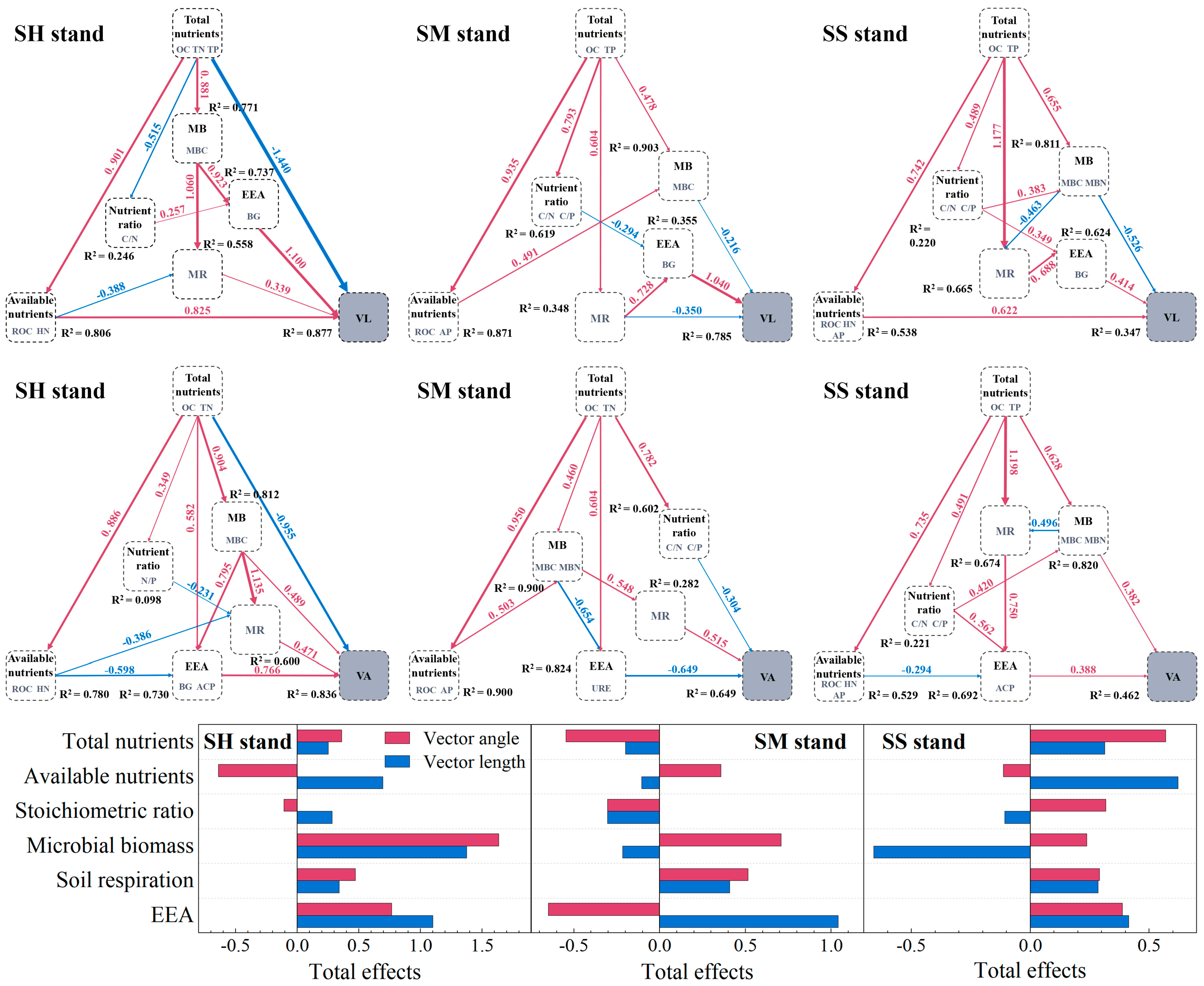
| Stand Type | Altitude | Slope Aspect | Slope | Crown Density | Density | DBH (cm) | Height (m) | Litter Mass | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (m) | (°) | (%) | Tree ha−1 | Chinese Fir | Mixed Species | Chinese Fir | Mixed Species | (kg ha−1) | ||
| SH | 730 | Southeast | 27 | 85 | 1215 ± 100.93 a | 17.22 ± 1.35 c | 24.06 ± 1.63 a | 13.84 ± 0.87 c | 13.58 ± 0.58 c | 4973.98 a |
| SM | 725 | Southeast | 23 | 85 | 1005 ± 222.49 a | 20.22 ± 2.10 b | 12.01 ± 2.56 d | 15.78 ± 1.35 ab | 14.49 ± 2.04 bc | 4527.88 a |
| SS | 728 | Southeast | 32 | 85 | 1095 ± 97.47 a | 21.00 ± 0.82 b | - | 16.28 ± 0.53 a | - | 2974.88 b |
| Soil Layer | Stand Type | Soil Aggregate Composition (%) | MWD (mm) | GMD | Bulk Density (g cm−3) | SWC (%) | ||
|---|---|---|---|---|---|---|---|---|
| >2 mm | 2–0.25 mm | <0.25 mm | (mm) | |||||
| 0–10 cm | SH | 51.94 ± 2.98 Aa | 33.29 ± 4.35 Ba | 14.77 ± 4.53 Cc | 2.21 ± 0.10 a | 1.48 ± 0.17 a | 1.24 ± 0.01 b | 24.57 ± 1.48 a |
| SM | 45.01 ± 3.95 Ab | 31.17 ± 3.39 Ba | 23.82 ± 4.22 Cb | 1.96 ± 0.13 b | 1.12 ± 0.14 b | 1.26 ± 0.02 b | 20.66 ± 1.96 b | |
| SS | 32.36 ± 3.04 Bc | 31.84 ± 2.00 Ca | 35.81 ± 5.00 Aa | 1.54 ± 0.12 c | 0.75 ± 0.11 c | 1.31 ± 0.02 a | 18.19 ± 1.53 c | |
| 10–20 cm | SH | 49.18 ± 1.41 Aa | 31.50 ± 3.35 Ba | 19.32 ± 4.33 Cc | 2.10 ± 0.07 a | 1.29 ± 0.14 a | 1.26 ± 0.01 b | 24.03 ± 0.93 a |
| SM | 33.09 ± 2.66 Bb | 27.88 ± 1.92 Cab | 39.03 ± 2.75 Ab | 1.52 ± 0.09 b | 0.70 ± 0.06 b | 1.27 ± 0.01 b | 24.13 ± 0.54 a | |
| SS | 29.73 ± 3.49 Bb | 24.81 ± 2.86 Bb | 45.46 ± 4.34 Aa | 1.38 ± 0.12 c | 0.58 ± 0.07 b | 1.33 ± 0.02 a | 21.98 ± 1.03 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Huang, Y.; Lan, Y.; He, Y.; Wang, S.; Jiang, C.; Cui, Y.; Fan, R.; Ye, S. Mixed Chinese Fir Plantations Alter the C, N, and P Resource Limitations Influencing Microbial Metabolism in Soil Aggregates. Forests 2024, 15, 724. https://doi.org/10.3390/f15040724
Zhang H, Huang Y, Lan Y, He Y, Wang S, Jiang C, Cui Y, Fan R, Ye S. Mixed Chinese Fir Plantations Alter the C, N, and P Resource Limitations Influencing Microbial Metabolism in Soil Aggregates. Forests. 2024; 15(4):724. https://doi.org/10.3390/f15040724
Chicago/Turabian StyleZhang, Han, Yongzhen Huang, Yahui Lan, Yaqin He, Shengqiang Wang, Chenyang Jiang, Yuhong Cui, Rongyuan Fan, and Shaoming Ye. 2024. "Mixed Chinese Fir Plantations Alter the C, N, and P Resource Limitations Influencing Microbial Metabolism in Soil Aggregates" Forests 15, no. 4: 724. https://doi.org/10.3390/f15040724





