Morpho-Anatomical Characteristics and Volatile Profiles of Pinus nigra J.F.Arnold from the Balkan Peninsula and Southern Carpathians
Abstract
:1. Introduction
2. Materials and Methods
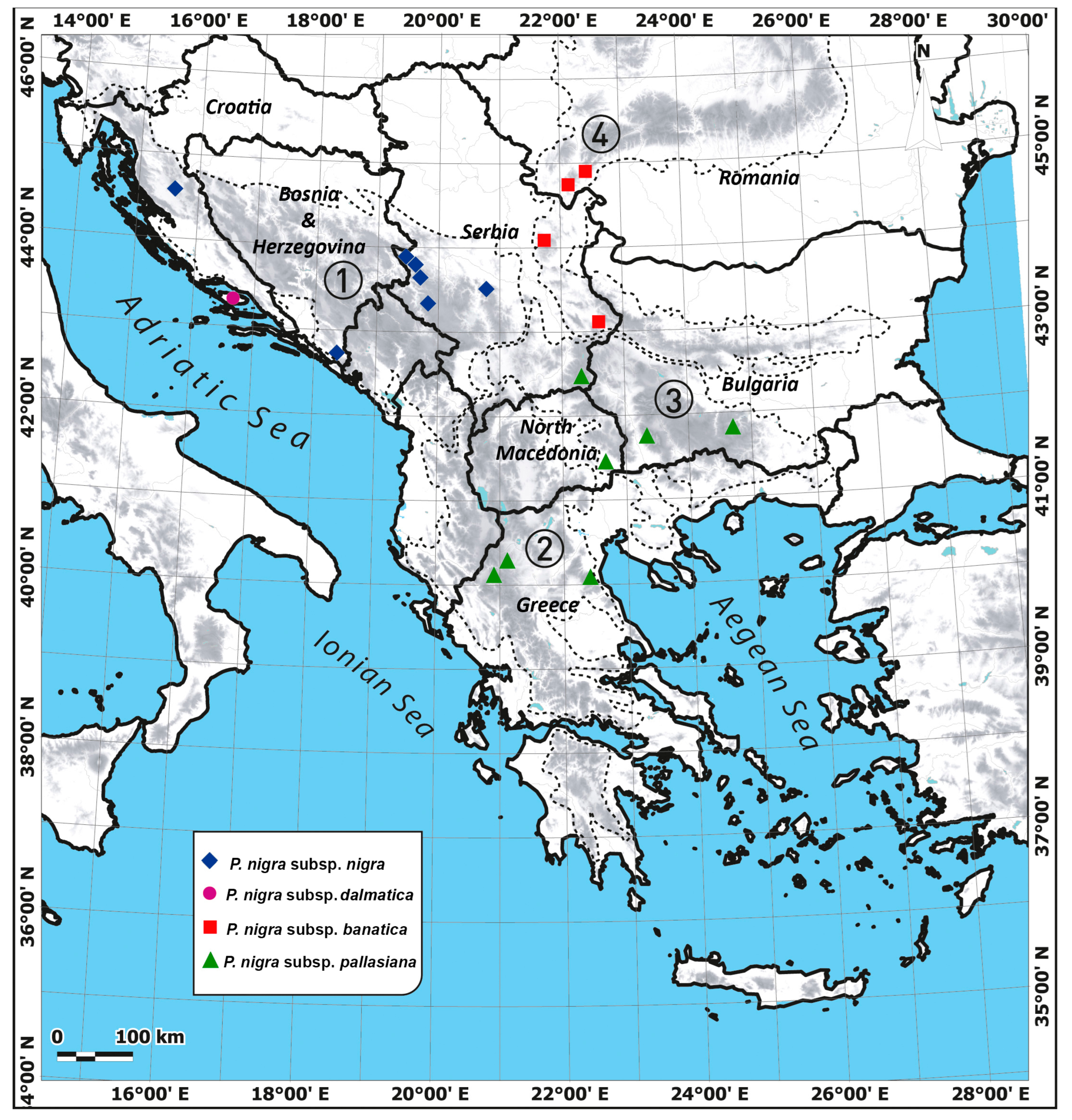
2.1. Plant Material
2.2. Morpho-Anatomical Characteristics of Needles
2.3. Static Headspace (HS)
2.4. Gas Chromatography-Mass Spectrometry/Flame Ionization Detector (GC-MS/FID) Analyses
2.5. Statistical Analyses
3. Results
3.1. Morpho-Anatomical Characteristics of Needles
3.2. HS Needle Volatiles
4. Discussion
4.1. Variability of Morpho-Anatomical Characteristics of Needles
4.2. Variability of HS Needle Volatiles
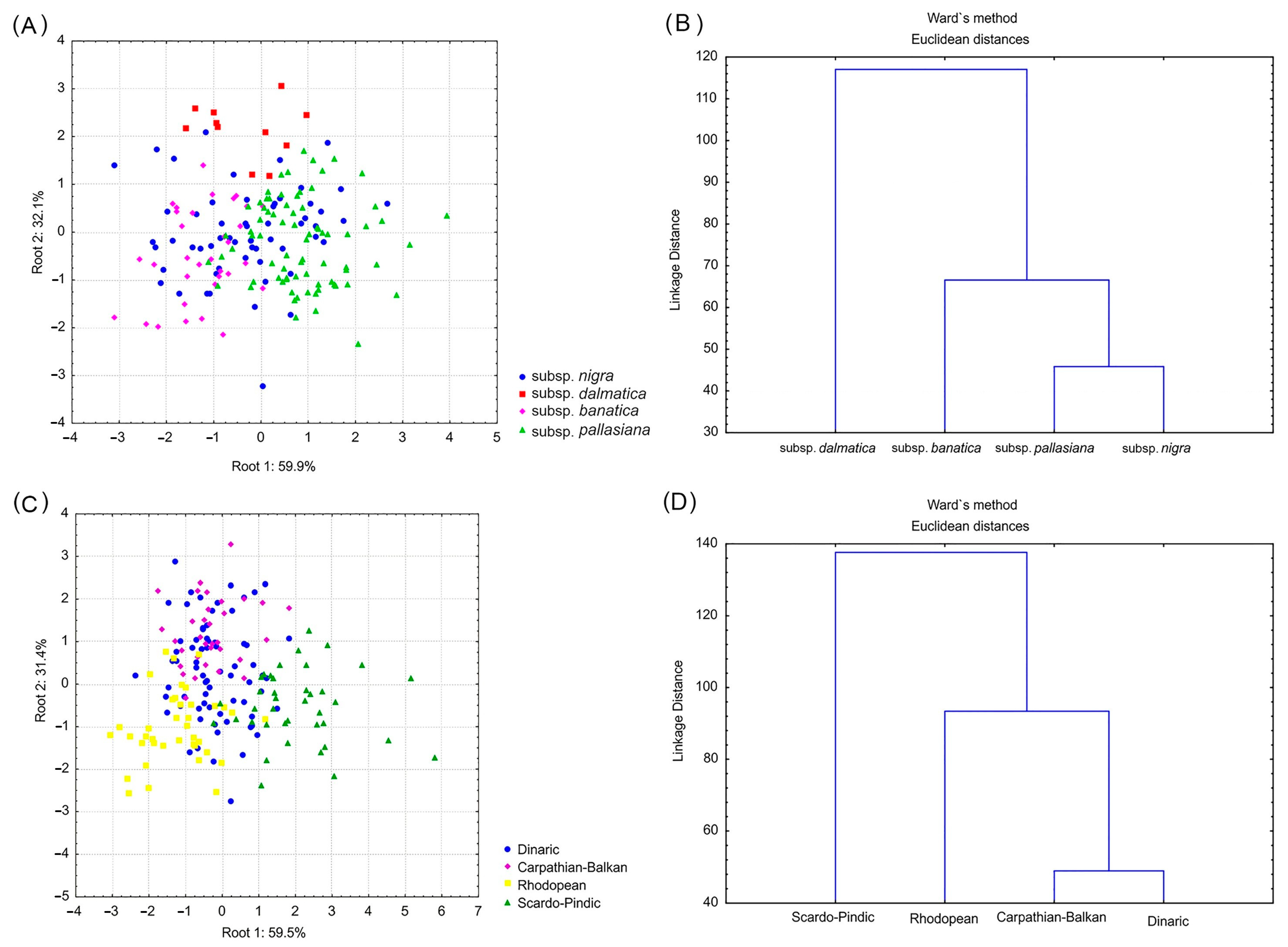
4.3. Taxonomic Consideration
- (1)
- The first group included the population from an island of Dalmatia, which corresponds to P. nigra subsp. dalmatica (Dalmatian black pine). This taxon is endemic to the Dalmatian coast and islands in Croatia, but with doubtful data on its precise distribution [21,22]. The distinctiveness of its populations was already suggested based on the morpho-anatomical characteristics of the needles [21], but they showed a lack of significant differentiation from populations of P. nigra subsp. nigra at the level of cuticular wax compounds [20]. If we consider three comprehensive molecular studies of P. nigra, we can notice that the situation is similar to that of phenotypic markers. Namely, some markers indicated that P. nigra subsp. dalmatica represents a distinct genetic lineage of black pine [16], while others failed to support such a hypothesis [15,17].
- (2)
- The second group consisted of populations from Greece. These populations (together with neighboring populations from southernmost North Macedonia) have also shown a very sharp differentiation from other Balkan populations based on cuticular wax compounds [20]. Moreover, according to Naydenov et al. [17], populations from western Greece present one of five genetic groups of P. nigra (1. Morocco-Spain; 2. France-Corsica; 3. Western Greece; 4. Northern Turkey; and 5. the remaining part of the black pine natural range) based on mtDNA. In older literature sources [4,10], Greek populations were usually attributed to P. nigra subsp. pallasiana (Crimean black pine). This taxon is mainly distributed in Turkey and the Caucasus, but regarding the European distribution, Jalas and Suominen [4] showed that it appears across the eastern and central Balkans, Southern Carpathians, Crimea, and the European part of Turkey. On the other side, according to POWO [13], Greek populations (together with other Balkan and the Southern Carpathian populations) should be ascribed to P. nigra subsp. nigra, since P. nigra subsp. pallasiana has extremely small distribution range on the European continent (Crimea and the European part of Turkey). However, in the light of the most recent results of Naydenov et al. [17], who presented the populations from western Greece, northern Turkey, and the main area of P. nigra distribution as three distinct genetic groups, one can speculate that there is an additional subspecies of P. nigra within this region that corresponds to populations from Greece. This assumption is somewhat supported by the study of Scotti-Saintagne et al. [16], who, although they did not include any population from Greece, also identified three genetic lineages within the distribution region of subspecies nigra and pallasiana (Central Europe, Crimea with Turkey, and Cyprus) using a set of different molecular markers.
- (3)
- The third group included populations from the remaining part of the Balkans and the Southern Carpathians, corresponding to P. nigra subsp. nigra. Therefore, the combined results of all performed analyses have clearly shown that populations of P. nigra subsp. banatica (Banat black pine) belong to the same group as populations of P. nigra subsp. nigra, so the hypothesis about their separation at the rank of distinct subspecies was not upheld. Banat black pine is endemic to the southwestern Romania and northeastern Serbia (the Carpathian-Balkan mountain system), but with very controversial data on its taxonomic position [20]. For instance, populations of P. nigra from the Southern Carpathians are regarded as a separate subspecies (P. nigra subsp. banatica) in the Flora Romania [18], while in the Flora Europaea [10], they are ascribed to P. nigra subsp. pallasiana (without stating any distinct taxonomic position). Considering the point of view that P. nigra subsp. pallasiana appears in Europe only in Crimea and the European part of Turkey, current floristic databases [11,12,13] treat Banat black pine taxa as synonyms of P. nigra subsp. nigra. Anyway, based on the recent phytochemical [20] and molecular data [16,17,46], all populations of P. nigra from Serbia, Romania, Bulgaria, Bosnia, and Herzegovina and continental Croatia belong to a single genetic lineage that corresponds to P. nigra subsp. nigra, matching the results of the present study.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckert, A.J.; Hall, B.D. Phylogeny, historical biogeography, and patterns of diversification for Pinus (Pinaceae): Phylogenetic tests of fossil-based hypotheses. Mol. Phylogenet. Evol. 2006, 40, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Mirov, N.T. The Genus Pinus; Ronald Press: New York, NY, USA, 1967. [Google Scholar]
- Vacek, Z.; Cukor, J.; Vacek, S.; Gallo, J.; Bažant, V.; Zeidler, A. Role of black pine (Pinus nigra J. F. Arnold) in European forests modified by climate change. Eur. J. For. Res. 2023, 142, 1239–1258. [Google Scholar] [CrossRef]
- Jalas, J.; Suominen, J. Atlas Florae Europaeae. 2 Gymnospermae (Pinaceae to Ephedraceae); The Committee for Mapping the Flora of Europe and Societas Biologica Fennica Vanamo: Helsinki, Finland, 1973. [Google Scholar]
- Vidaković, M. Conifers. Morphology and Variation; Grafički Zavod Hrvatske: Zagreb, Croatia, 1991. [Google Scholar]
- Rubio-Moraga, A.; Candel-Perez, D.; Lucas-Borja, M.E.; Tíscar, P.A.; Viñegla, B.; Linares, J.C.; Gómez-Gómez, L.; Ahrazem, O. Genetic diversity of Pinus nigra Arn. populations in Southern Spain and Northern Morocco revealed by inter-simple sequence repeat profiles. Int. J. Mol. Sci. 2012, 13, 5645–5658. [Google Scholar] [CrossRef] [PubMed]
- Fukarek, P. Prilog poznavanju crnoga bora (Pinus nigra Arn.)/A contribution to the knowledge of black pine (Pinus nigra Arn.). Rad. Poljopr.—Šumarsk. Fak. Univ. U Sarajev. 1958, 6, 93–146. [Google Scholar] [CrossRef]
- Dobrinov, I.; Doykov, G.; Gagov, V. State of Forest Genetic Pool in Bulgaria; Zemizdat: Sofia, Bulgaria, 1982. [Google Scholar]
- Naydenov, K.D.; Naydenov, M.K.; Alexandrov, A.; Vasilevski, K.; Hinkov, G.; Metevski, V.; Nikolić, B.; Goudiaby, V.; Riegert, D.; Paitaridou, D.; et al. Ancient genetic bottleneck and Plio-Pleistocene climatic changes imprinted the phylobiogeography of European Black Pine populations. Eur. J. For. Res. 2017, 136, 767–786. [Google Scholar] [CrossRef]
- Gaussen, H.; Heywood, V.H.; Charter, A.O. Pinus L. In Flora Europea; Tutin, T.G., Burges, N.A., Chater, A.O., Edmondson, J.R., Heywood, V.H., Moore, D.M., Valentine, D.H., et al., Eds.; Cambridge University Press: Cambridge, UK, 1993; Volume 1, pp. 40–44. [Google Scholar]
- Euro+Med 2006+ [Continuously Updated]: Euro+Med PlantBase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://www.europlusmed.org (accessed on 12 February 2024).
- WFO: World Flora Online. Published on the Internet. 2023. Available online: http://www.worldfloraonline.org (accessed on 12 February 2024).
- POWO: Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. 2024. Available online: http://www.plantsoftheworldonline.org/ (accessed on 12 February 2024).
- Jaramillo-Correa, J.P.; Grivet, D.; Terrab, A.; Kurt, Y.; De-Lucas, A.I.; Wahid, N.; Vendramin, G.G.; González-Martínez, S.C. The Strait of Gibraltar as a major biogeographic barrier in Mediterranean conifers: A comparative phylogeographic survey. Mol. Ecol. 2010, 19, 5452–5468. [Google Scholar] [CrossRef] [PubMed]
- Naydenov, K.D.; Naydenov, M.K.; Alexandrov, A.; Vasilevski, K.; Gyuleva, V.; Matevski, V.; Nikolić, B.; Goudiaby, V.; Bogunić, F.; Paitaridou, D.; et al. Ancient split of major genetic lineages of European black pine: Evidence from chloroplast DNA. Tree Genet. Genomes 2016, 12, 68. [Google Scholar] [CrossRef]
- Scotti-Saintagne, C.; Giovannelli, G.; Scotti, I.; Roig, A.; Spanu, I.; Vendramin, G.G.; Guibal, F.; Fady, B. Recent, Late Pleistocene fragmentation shaped the phylogeographic structure of the European black pine (Pinus nigra Arnold). Tree Genet. Genomes 2019, 15, 76. [Google Scholar] [CrossRef]
- Naydenov, K.D.; Naydenov, M.K.; Alexandrov, A.; Gurov, T.; Gyuleva, V.; Hinkov, G.; Ivanovska, S.; Tsarev, A.; Nikolić, B.; Goudiaby, V.; et al. Speciation and historical migration pattern interaction: Examples from P. nigra and P. sylvestris phylogeography. Eur. J. For. Res. 2023, 142, 1–26. [Google Scholar] [CrossRef]
- Ciocârlan, V. Flora Ilustrată a României; Pteridophyta et Spermatophyta; Editura Ceres: Bucureşti, Romania, 2000. [Google Scholar]
- Vidaković, M. Značenje anatomske građe iglica kod svojta crnog bora u Jugoslaviji. Šumar. List 1955, 79, 244–253. [Google Scholar]
- Mitić, Z.S.; Zlatković, B.K.; Jovanović, S.Č.; Nikolić, J.S.; Nikolić, B.M.; Stojanović, G.S.; Marin, P.D. Diversity of needle n-alkanes, primary alcohols and diterpenes in Balkan and Carpathian native populations of Pinus nigra J.F. Arnold. Biochem. Syst. Ecol. 2018, 80, 46–54. [Google Scholar] [CrossRef]
- Liber, Z.; Nikolić, T.; Mitić, B. Intra- and interpopulation relationships and taxonomic status of Pinus nigra Arnold in Croatia according to morphology and anatomy of needles. Acta Soc. Bot. Pol. 2002, 71, 141–147. [Google Scholar] [CrossRef]
- Liber, Z.; Nikolić, T.; Mitić, B.; Šatović, Z. RAPD markers and black pine (Pinis nigra Arnold) intraspecies taxonomy—Evidence from the study of nine populations. Acta Soc. Bot. Pol. 2003, 72, 249–257. [Google Scholar] [CrossRef]
- Boratyńska, K.; Dzialuk, A.; Lewandowski, A.; Marcysiak, K.; Jasiñska, K.A.; Sobierajska, K.; Tomaszewski, D.; Burczyk, J.; Boratyński, A. Geographic distribution of quantitative traits variation and genetic variability in natural populations of Pinus mugo in Central Europe. Dendrobiology 2014, 72, 65–84. [Google Scholar] [CrossRef]
- Boratyńska, K.; Jasińska, A.K.; Boratyński, A. Taxonomic and geographic differentiation of Pinus mugo complex on the needle characteristics. Syst. Biodivers. 2015, 13, 581–595. [Google Scholar] [CrossRef]
- Köbölkuti, Z.A.; Tóth, E.G.; Ladányi, M.; Höhn, M. Morphological and anatomical differentiation in peripheral Pinus sylvestris L. populations from the Carpathian region. Dendrobiology 2017, 77, 105–117. [Google Scholar] [CrossRef]
- Bozkurt, A.E.; Coşkunçelebi, K.; Terzioğlu, S. Variation in needle anatomy of Scots pine (Pinus sylvestris L.) populations according to habitat and altitudinal zones in Turkiye. Šumarski List 2023, 147, 215–225. [Google Scholar]
- Sutinen, S.; Koivisto, L. Microscopic structure of conifer needles as a diagnostic tool in the field. In Bioindicators of Environmental Health; Munawar, M., Haenninen, O., Roy, S., Munawar, N., Eds.; SPB Academic Publishing bv: Amsterdam, The Netherlands, 1995; pp. 73–81. [Google Scholar]
- Lin, J.; Jach, M.E.; Ceulemans, R. Stomatal density and needle anatomy of Scots pine (Pinus sylvestris) are affected by elevated CO2. New Phytol. 2001, 150, 665–674. [Google Scholar] [CrossRef]
- Harborne, J.B. Comparative Biochemistry of the Flavonoids; Academic Press: London, UK; New York, NY, USA, 1967. [Google Scholar]
- Hanover, J.W. Applications of terpene analysis in forest genetics. New For. 1992, 6, 159–178. [Google Scholar] [CrossRef]
- Gerber, S.; Baradat, P.; Marpeau, A.; Arbez, M. Geographic variation in terpene composition of Pinus nigra Arn. For. Genet. 1995, 2, 1–10. [Google Scholar]
- Rafii, Z.A.; Dodd, R.S.; Zavarin, E. Genetic diversity in foliar terpenoids among natural populations of European black pine. Biochem. Syst. Ecol. 1996, 24, 325–339. [Google Scholar] [CrossRef]
- Bojović, S.; Jurc, M.; Drazić, D.; Pavlović, P.; Mitrović, M.; Djurdjević, L.; Dodd, R.S.; Afzal-Raffi, Z.A.; Barbero, M. Origin identification of Pinus nigra populations in southwestern Europe using terpene composition variations. Trees 2005, 19, 531–538. [Google Scholar] [CrossRef]
- Naydenov, K.D.; Tremblay, F.M.; Fenton, N.J.; Alexandrov, A. Structure of Pinus nigra Arn. populations in Bulgaria revealed by chloroplast microsatellites and terpene analysis: Provenance tests. Biochem. Syst. Ecol. 2006, 34, 562–574. [Google Scholar] [CrossRef]
- Šarac, Z.; Bojović, S.; Nikolić, B.; Tešević, V.; Đorđević, I.; Marin, P.D. Chemotaxonomic significance of the terpene composition in natural populations of Pinus nigra J.F. Arnold from Serbia. Chem. Biodivers. 2013, 10, 1507–1520. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, B.; Ljujić, J.; Bojović, S.; Mitić, Z.; Rajčević, N.; Tešević, V.; Marin, P.D. Headspace volatiles isolated from twigs of Picea omorika from Serbia. Arch. Biol. Sci. 2020, 72, 445–452. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Jovanović, S.Č.; Zlatković, B.K.; Milanovici, S.J.; Nikolić, B.M.; Petrović, G.M.; Stojanović, G.S.; Marin, P.D. Variation of needle volatiles in native populations of Pinus mugo—Evidence from multivariate statistical analysis. Plant Biosyst. 2021, 155, 700–710. [Google Scholar] [CrossRef]
- Nikolić, J.S.; Zlatković, B.K.; Jovanović, S.Č.; Stojanović, G.S.; Marin, P.D.; Mitić, Z.S. Needle volatiles as chemophenetic markers in differentiation of natural populations of Abies alba, A. x borisii-regis, and A. cephalonica. Phytochemistry 2021, 183, 112612. [Google Scholar] [CrossRef] [PubMed]
- Reale, S.; Pace, L.; D’Archivio, A.A.; De Angelis, F.; Marcozzi, G. Volatiles fingerprint of Artemisia umbelliformis subsp. eriantha by headspace-solid phase microextraction GC-MS. Nat. Prod. Res. 2014, 28, 61–66. [Google Scholar] [CrossRef]
- Nikolić, B.; Bojović, S.; Marin, P.D. Variability of morpho-anatomical characteristics of the needles of Picea omorika from natural populations in Serbia. Plant Biosyst. 2015, 149, 61–67. [Google Scholar] [CrossRef]
- Macchioni, F.; Cioni, P.L.; Flamini, G.; Morelli, I.; Maccioni, S.; Ansaldi, M. Chemical composition of essential oils from needles, branches and cones of Pinus pinea, P. halepensis, P. pinaster and P. nigra from central Italy. Flavour Fragr. J. 2003, 18, 139–143. [Google Scholar] [CrossRef]
- Sezik, E.; Üstün, O.; Demirci, B.; Başer, K.H.C. Composition of the essential oils of Pinus nigra Arnold from Turkey. Turk. J. Chem. 2010, 34, 313–325. [Google Scholar] [CrossRef]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Jovanović, S.Č.; Zlatković, B.K.; Nikolić, B.M.; Stojanović, G.S.; Marin, P.D. Needle terpenes as chemotaxonomic markers in Pinus: Subsections Pinus and Pinaster. Chem. Biodivers. 2017, 14, e1600453. [Google Scholar] [CrossRef] [PubMed]
- Rezzi, S.; Bighelli, A.; Mouillot, D.; Casanova, J. Composition and chemical variability of the needle essential oil of Pinus nigra subsp. laricio from Corsica. Flavour Fragr. J. 2001, 16, 379–383. [Google Scholar] [CrossRef]
- Šarac, Z.; Dodoš, T.; Rajčević, N.; Bojović, S.; Marin, P.D.; Aleksić, J.M. Genetic patterns in Pinus nigra from the central Balkans inferred from plastid and mitochondrial data. Silva Fenn. 2015, 49, 1415. [Google Scholar] [CrossRef]

| No. | Morpho-Anatomical Characteristics | F | p | P. nigra subsp. nigra n = 70 | P. nigra subsp. dalmatica n = 11 | P. nigra subsp. banatica n = 40 | P. nigra subsp. pallasiana n = 80 |
|---|---|---|---|---|---|---|---|
| X ± SD | X ± SD | X ± SD | X ± SD | ||||
| 1. | Needle length (cm) | 27.2 | *** | 10.1 ± 2.0 b | 6.0 ± 1.0 a | 8.6 ± 1.2 b | 12.2 ± 1.4 c |
| 2. | Needle width (μm) | 15.8 | *** | 1658.2 ± 213.9 a | 1838.0 ± 140.5 b | 1638.4 ± 153.8 a | 1731.9 ± 182.6 b |
| 3. | Needle thickness (μm) | 22.0 | *** | 941.9 ± 105.2 b | 1086.6 ± 67.2 c | 850.4 ± 116.0 a | 989.4 ± 93.2 c |
| 4. | Endodermis tube perimeter (μm) | 24.5 | *** | 2071.0 ± 290.8 a | 2455.5 ± 196.7 b | 1839.7 ± 203.2 a | 2214.0 ± 262.0 b |
| 5. | Number of resin ducts | 12.3 | *** | 4.9 ± 2.1 a | 8.4 ± 2.2 b | 4.4 ± 2.1 a | 6.3 ± 2.7 b |
| 6. | Resin duct diameter (μm) | 6.6 | *** | 114.3 ± 14.3 b | 121.4 ± 18.6 b | 115.1 ± 10.5 b | 107.9 ± 9.9 a |
| 7. | Distance between resin duct and endodermis tube (μm) | 0.8 | ns | 101.3 ± 13.4 | 105.8 ± 19.2 | 99.7 ± 15.1 | 108.6 ± 22.1 |
| 8. | Epidermis + cuticle thickness (μm) | 10.3 | *** | 32.0 ± 3.1 a | 36.3 ± 1.4 b | 31.3 ± 3.0 a | 29.1 ± 6.2 a |
| 9. | Hypodermis thickness (μm) | 4.3 | ** | 74.5 ± 15.1 ab | 77.0 ± 7.1 b | 68.6 ± 11.0 a | 80.2 ± 18.8 b |
| A Priori Groups | ||||
|---|---|---|---|---|
| P. nigra Taxa | Mountain Systems | |||
| Variables | CA1 | CA2 | CA1 | CA2 |
| Needle length | 0.742 | −0.393 | −0.527 | −0.908 |
| Needle width | −0.374 | −0.014 | −0.518 | 0.251 |
| Needle thickness | 0.297 | 0.370 | −0.734 | −0.520 |
| Endodermis tube perimeter | 0.636 | 0.603 | 0.909 | −0.409 |
| Number of resin ducts | −0.158 | 0.066 | 0.922 | 0.408 |
| Resin duct diameter | −0.271 | 0.103 | 0.323 | 0.369 |
| Distance between resin duct and endodermis tube | 0.181 | −0.444 | 0.187 | −0.126 |
| Epidermis + cuticle thickness | −0.163 | 0.411 | −0.816 | −0.051 |
| Hypodermis thickness | −0.371 | 0.107 | 0.780 | 0.571 |
| Eigenvalue | 0.662 | 0.355 | 1.205 | 0.635 |
| Cum.Prop. | 0.599 | 0.920 | 0.595 | 0.909 |
| No. | Compounds | Class of Compounds | RI | LI | F | p | P. nigra subsp. nigra n = 70 | P. nigra subsp. dalmatica n = 11 | P. nigra subsp. banatica n = 40 | P. nigra subsp. pallasiana n = 80 |
|---|---|---|---|---|---|---|---|---|---|---|
| X ± SD | X ± SD | X ± SD | X ± SD | |||||||
| 1. | Tricyclene | MH | 921 | 921 | 0.4 | ns | 0.0 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.0 ± 0.2 |
| 2. | α-Thujene | MH | 926 | 924 | 2.7 | ns | 1.2 ± 1.0 | 0.7 ± 0.6 | 0.8 ± 0.9 | 0.8 ± 0.7 |
| 3. | α-Pinene | MH | 934 | 932 | 7.7 | *** | 72.7 ± 10.2 a | 85.1 ± 8.7 b | 79.5 ± 6.5 a | 72.6 ± 11.7 a |
| 4. | Camphene | MH | 949 | 946 | 1.3 | ns | 1.1 ± 0.4 | 0.8 ± 0.4 | 1.2 ± 0.6 | 1.1 ± 0.6 |
| 5. | Sabinene | MH | 971 | 969 | 5.4 | ** | 0.1 ± 0.3 b | 0.0 ± 0.0 ab | 0.0 ± 0.1 ab | 0.0 ± 0.0 a |
| 6. | β-Pinene | MH | 980 | 974 | 2.3 | ns | 11.5 ± 10.7 | 5.9 ± 6.3 | 8.0 ± 6.6 | 13.2 ± 13.7 |
| 7. | Myrcene | MH | 990 | 988 | 9.9 | *** | 1.2 ± 0.4 c | 0.5 ± 0.4 a | 1.0 ± 0.5 bc | 0.9 ± 0.4 ab |
| 8. | Limonene + β-Phellandrene | MH | 1029 | 1024/1025 | 9.7 | *** | 2.9 ± 2.1 b | 1.4 ± 0.9 ab | 1.5 ± 0.8 a | 1.7 ± 0.8 a |
| 9. | (E)-β-ocimene | MH | 1044 | 1044 | 2.8 | * | 0.4 ± 0.4 b | 0.0 ± 0.0 a | 0.3 ± 0.5 b | 0.5 ± 0.6 b |
| 10. | Terpinolene | MH | 1087 | 1086 | 4.5 | ** | 0.7 ± 0.8 b | 0.1 ± 0.2 ab | 0.4 ± 0.6 ab | 0.3 ± 0.4 a |
| 11. | (E)-Caryophyllene | SH | 1420 | 1417 | 0.4 | ns | 2.2 ± 1.3 | 2.6 ± 1.5 | 2.1 ± 1.4 | 2.3 ± 1.5 |
| 12. | Germacrene D | SH | 1485 | 1484 | 4.0 | ** | 6.1 ± 3.3 b | 3.2 ± 2.1 a | 5.1 ± 2.7 b | 6.6 ± 3.5 b |
| Total | 99.9 ± 0.5 | 100.0 ± 0.9 | 99.9 ± 0.3 | 99.9 ± 0.3 | ||||||
| Monoterpene hydrocarbons (MH) Sesquiterpene hydrocarbons (SH) | 91.7 ± 3.8 | 94.1 ± 3.9 | 92.8 ± 3.4 | 91.0 ± 4.4 | ||||||
| 8.2 ± 3.9 | 5.8 ± 3.2 | 7.2 ± 3.4 | 8.9 ± 4.3 | |||||||
| A Priori Groups | ||||
|---|---|---|---|---|
| P. nigra Taxa | Mountain Systems | |||
| Variables | CA1 | CA2 | CA1 | CA2 |
| α-Thujene | 0.469 | 0.272 | 0.809 | 0.613 |
| α-Pinene | 5.956 | 6.360 | 8.735 | 0.546 |
| Camphene | 0.232 | 0.030 | 0.524 | 0.649 |
| β-Pinene | 5.935 | 6.518 | 8.614 | 0.622 |
| Myrcene | −0.340 | 0.342 | 0.426 | −0.030 |
| Limonene + β-Phellandrene | 0.463 | 1.640 | 1.913 | −0.254 |
| (E)-β-ocimene | 0.202 | −0.204 | 0.251 | 0.671 |
| Terpinolene | 0.096 | 0.948 | 0.584 | −0.847 |
| (E)-Caryophyllene | 1.272 | 0.865 | 1.337 | 0.329 |
| Germacrene D | 0.904 | 1.789 | 2.461 | 0.217 |
| Eigenvalue | 0.517 | 0.213 | 0.220 | 0.201 |
| Cum.Prop. | 0.630 | 0.889 | 0.405 | 0.775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitić, Z.S.; Nikolić, B.M.; Stojković, J.P.; Jevtović, S.Č.; Stojanović, G.S.; Zlatković, B.K.; Marin, P.D. Morpho-Anatomical Characteristics and Volatile Profiles of Pinus nigra J.F.Arnold from the Balkan Peninsula and Southern Carpathians. Forests 2024, 15, 739. https://doi.org/10.3390/f15050739
Mitić ZS, Nikolić BM, Stojković JP, Jevtović SČ, Stojanović GS, Zlatković BK, Marin PD. Morpho-Anatomical Characteristics and Volatile Profiles of Pinus nigra J.F.Arnold from the Balkan Peninsula and Southern Carpathians. Forests. 2024; 15(5):739. https://doi.org/10.3390/f15050739
Chicago/Turabian StyleMitić, Zorica S., Biljana M. Nikolić, Jelena P. Stojković, Snežana Č. Jevtović, Gordana S. Stojanović, Bojan K. Zlatković, and Petar D. Marin. 2024. "Morpho-Anatomical Characteristics and Volatile Profiles of Pinus nigra J.F.Arnold from the Balkan Peninsula and Southern Carpathians" Forests 15, no. 5: 739. https://doi.org/10.3390/f15050739





