Effects of Different Thinning Intensities on Carbon Storage in Pinus koraiensis Middle-Aged Plantations in Northeast China
Abstract
:1. Introduction
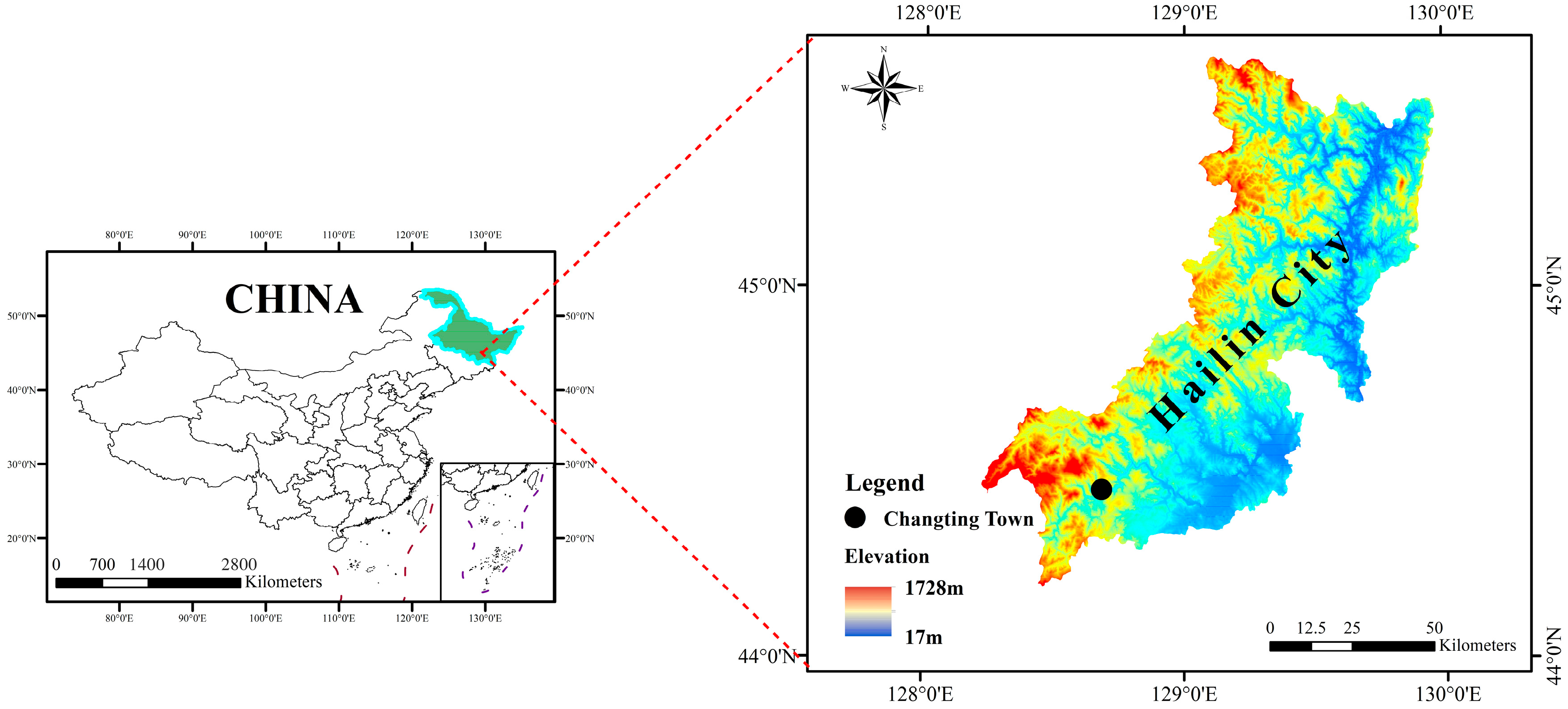
2. Materials and Methods
2.1. Experimental Procedures
2.2. Soil Collection
2.3. Soil Bulk Density Calculation
2.4. Vegetation Sample Collection
2.5. Determination of Conventional Soil and Vegetation Total Carbon
2.6. Statistical Analyses
3. Results
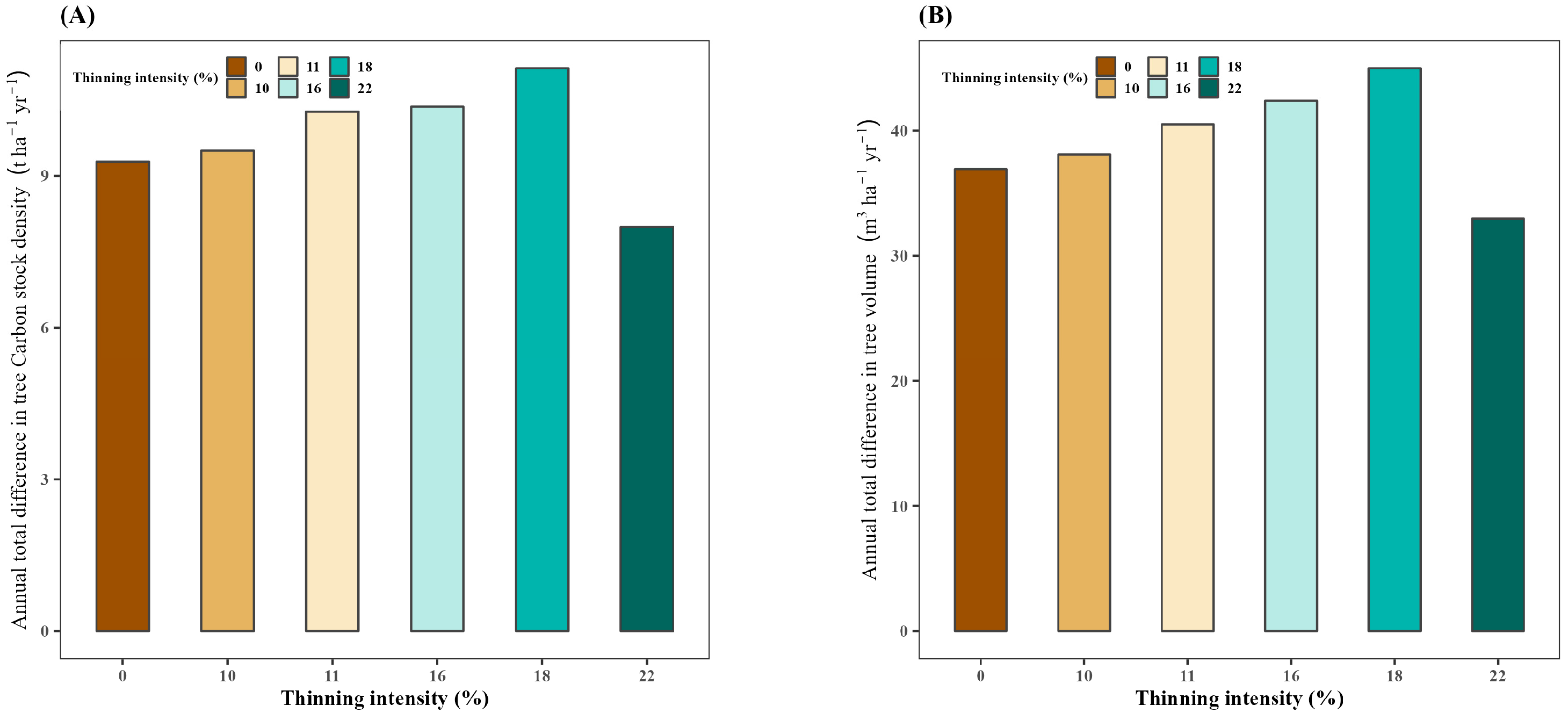
3.1. Effect of Thinning on Annual Total Difference in Tree Carbon Stock Density
3.2. Effect of Thinning on Annual Total Difference in Tree Volume
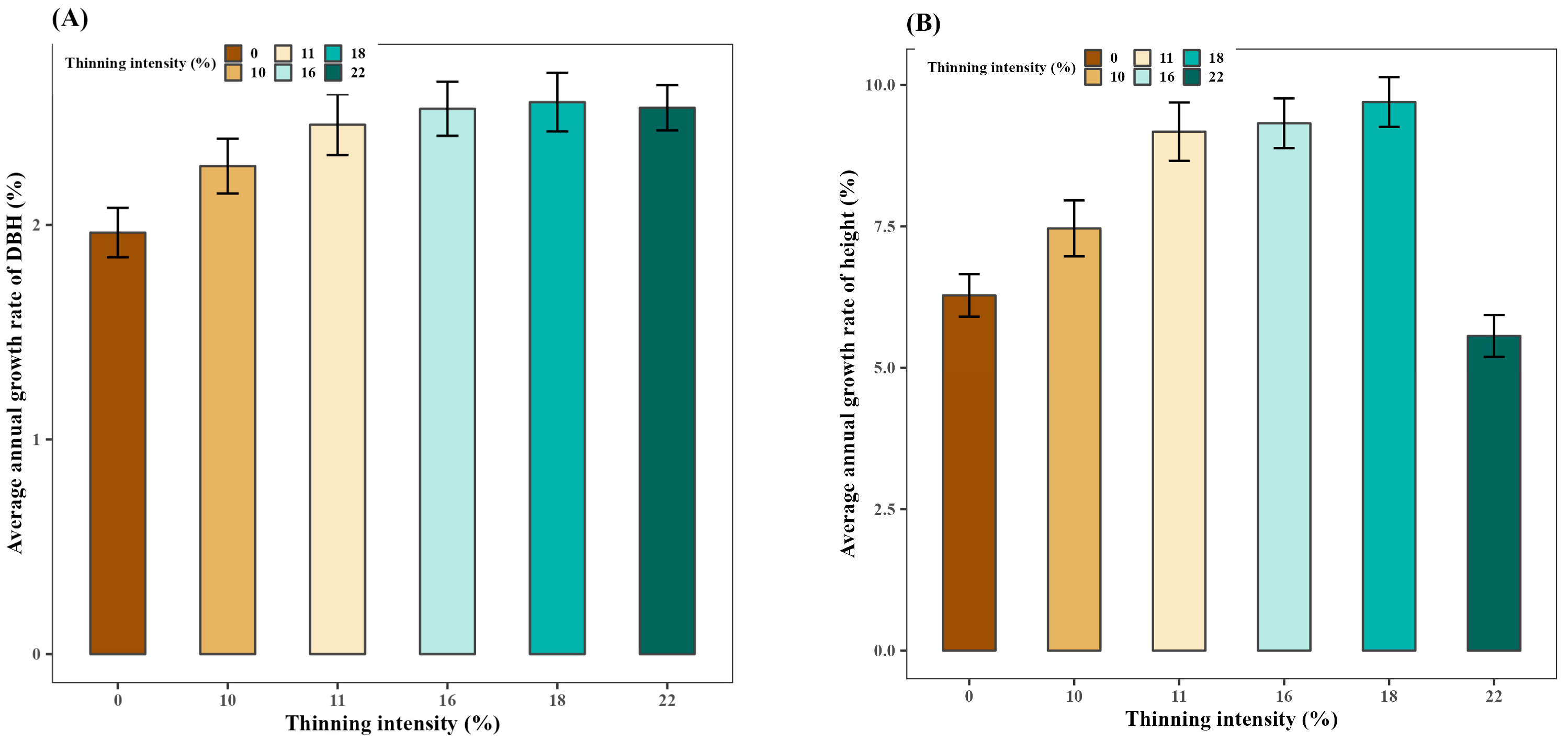
3.3. Effect of Thinning on the Average Annual Growth Rate of DBH and Height
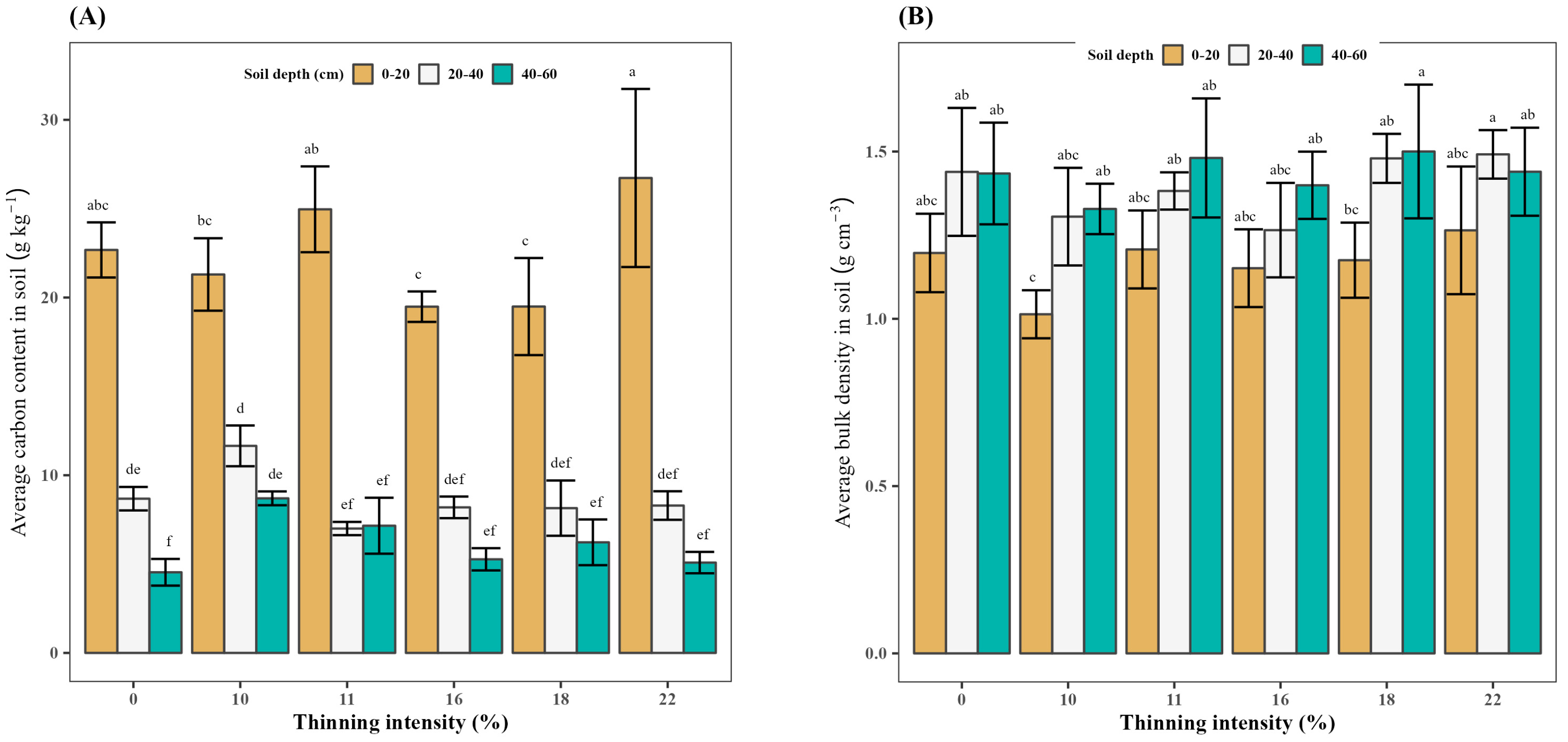
3.4. Effect of Thinning on the Average Carbon Content in Soil
3.5. Effect of Thinning on the Average Bulk Density in Soil
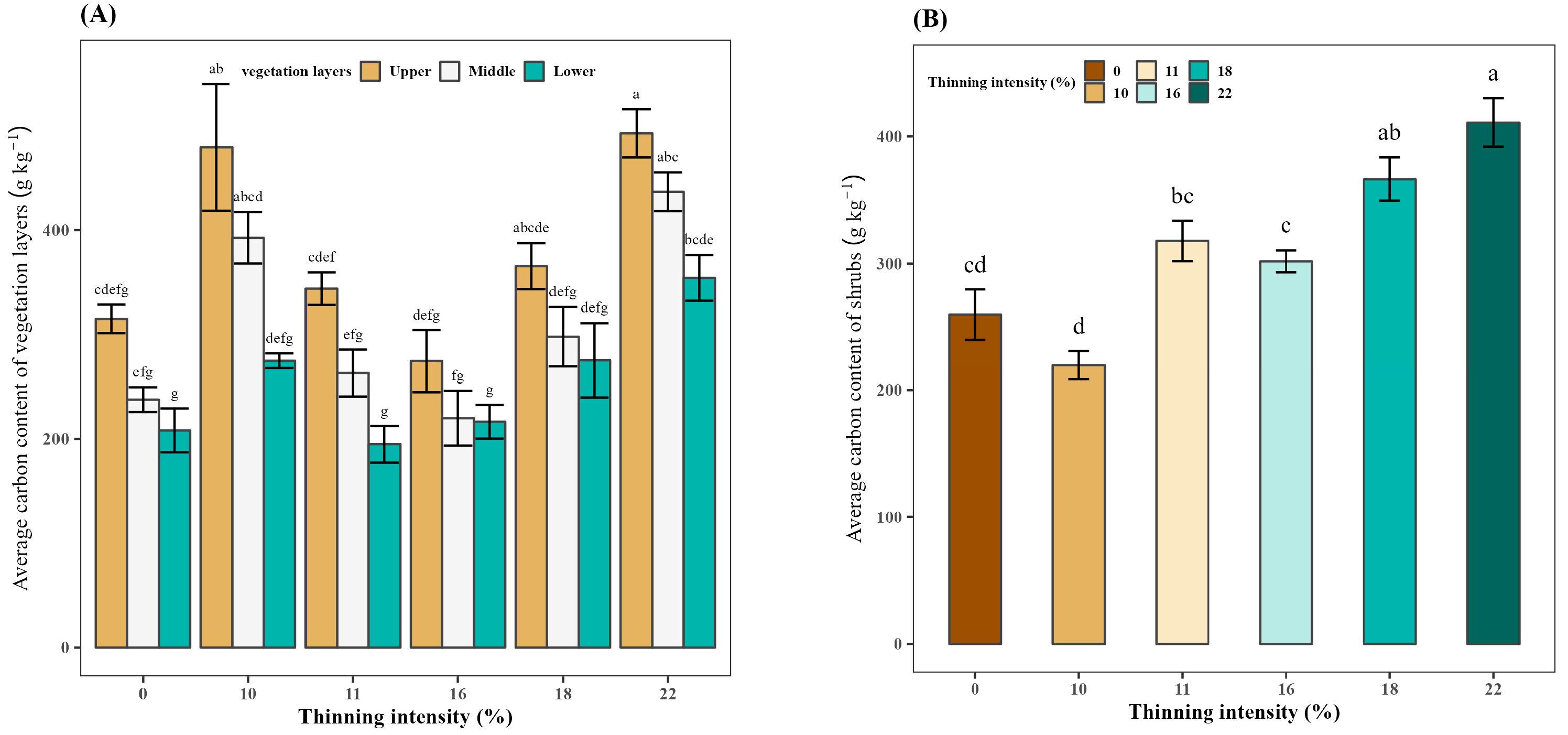
3.6. Effect of Thinning on the Average Carbon Content of Vegetation Layers
3.7. Effect of Thinning on the Average Carbon Content of Shrubs
3.8. Effect of Thinning on Carbon Storage of Tree Organs
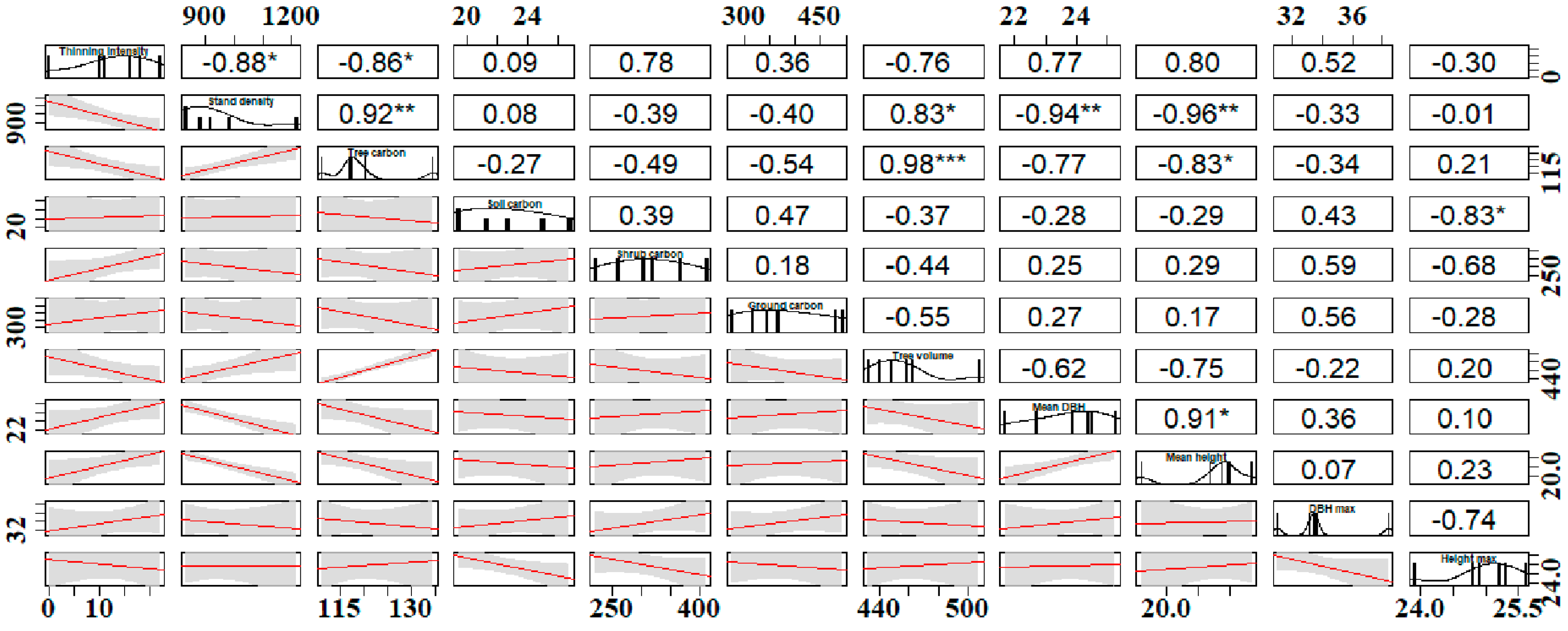
3.9. The Variation in Carbon Storage and Stand Factors
4. Discussion
4.1. Effect of Thinning on Tree Carbon Storage
4.2. Effect of Thinning on Tree Stem Volume
4.3. Effect of Thinning on Tree Growth
4.4. Effect of Thinning on Soil Carbon
4.5. Effect of Thinning on Vegetation and Shrub Layer
4.6. The Effect of Stand Factors on Carbon Storage
4.7. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peres, C.A.; Gardner, T.A.; Barlow, J.; Zuanon, J.; Michalski, F.; Lees, A.C.; Vieira, I.C.G.; Moreira, F.M.S.; Feeley, K.J. Biodiversity conservation in human-modified Amazonian forest landscapes. Biol. Conserv. 2010, 143, 2314–2327. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Nitanan, K.M.; Shuib, A.; Sridar, R.; Kunjuraman, V.; Zaiton, S.; Herman, M.A.S. The total economic value of forest ecosystem services in the tropical forests of Malaysia. Int. For. Rev. 2020, 22, 485–503. [Google Scholar] [CrossRef]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Barbaro, L.; Castagneyrol, B.; Forrester, D.I.; Gardiner, B.; Ramon Gonzalez-Olabarria, J.; Lyver, P.O.B.; Meurisse, N.; Oxbrough, A.; Taki, H.; et al. Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers. Conserv. 2017, 26, 3005–3035. [Google Scholar] [CrossRef]
- Canadell, J.G.; Raupach, M.R. Managing forests for climate change mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.K.; Raghubanshi, A.S. Assessment of carbon density and accumulation in mono- and multi-specific stands in Teak and Sal forests of a tropical dry region in India. For. Ecol. Manag. 2015, 339, 11–21. [Google Scholar] [CrossRef]
- Sharma, C.M.; Mishra, A.K.; Krishan, R.; Tiwari, O.P.; Rana, Y.S. Variation in vegetation composition, biomass production, and carbon storage in ridge top forests of high mountains of Garhwal Himalaya. J. Sustain. For. 2016, 35, 119–132. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Valentini, R.; Matteucci, G.; Dolman, A.J.; Schulze, E.D.; Rebmann, C.; Moors, E.J.; Granier, A.; Gross, P.; Jensen, N.O.; Pilegaard, K.; et al. Respiration as the main determinant of carbon balance in European forests. Nature 2000, 404, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-S.; Yang, Q.-P.; Zhang, W.-D.; Zheng, W.-H.; Chi, Y.-G.; Xu, M.; Fang, Y.-T.; Gessler, A.; Li, M.-H.; Wang, S.-L. Thinning effect on photosynthesis depends on needle ages in a Chinese fir (Cunninghamia lanceolata) plantation. Sci. Total Environ. 2017, 580, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Schaedel, M.S.; Larson, A.J.; Affleck, D.L.R.; Belote, T.; Goodburn, J.M.; Page-Dumroese, D.S. Early forest thinning changes aboveground carbon distribution among pools, but not total amount. For. Ecol. Manag. 2017, 389, 187–198. [Google Scholar] [CrossRef]
- Laporte, M.F.; Duchesne, L.C.; Morrison, I.K. Effect of clearcutting, selection cutting, shelterwood cutting and microsites on soil surface CO2 efflux in a tolerant hardwood ecosystem of northern Ontario. For. Ecol. Manag. 2003, 174, 565–575. [Google Scholar] [CrossRef]
- Concilio, A.; Ma, S.Y.; Li, Q.L.; LeMoine, J.; Chen, J.Q.; North, M.; Moorhead, D.; Jensen, R. Soil respiration response to prescribed burning and thinning in mixed-conifer and hardwood forests. Can. J. For. Res. 2005, 35, 1581–1591. [Google Scholar] [CrossRef]
- Ohashi, M.; Gyokusen, K.; Saito, A. Measurement of carbon dioxide evolution from a Japanese cedar (Cryptomeria japonica D. Don) forest floor using an open-flow chamber method. For. Ecol. Manag. 1999, 123, 105–114. [Google Scholar] [CrossRef]
- Lee, J.; Morrison, I.K.; Leblanc, J.-D.; Dumas, M.T.; Cameron, D.A. Carbon sequestration in trees and regrowth vegetation as affected by clearcut and partial cut harvesting in a second-growth boreal mixedwood. For. Ecol. Manag. 2002, 169, 83–101. [Google Scholar] [CrossRef]
- Zou, X.; Zheng, Z.; Yang, C.; Yang, M.; Guo, Z.; Wang, Y.; Huang, Z.; Zhu, L.; Xu, L.; Lin, K. Initial effects of crop tree release and traditional thinning on productivity and carbon storage of Cunninghamia lanceolata plantation. Front. Glob. Chang. 2023, 6, 1288613. [Google Scholar] [CrossRef]
- Gao, T.; Song, X.; Ren, Y.; Liu, H.; Qu, H.; Dong, X. Thinning intensity affects carbon sequestration and release in seasonal freeze-thaw areas. J. For. Res. 2023, 34, 993–1006. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Xu, C.; Hao, M.; Choe, C.; He, H. Thinning can increase shrub diversity and decrease herb diversity by regulating light and soil environments. Front. Plant Sci. 2022, 13, 948648. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, K.; Song, C.; Cui, C.; Peng, L.; Zheng, H.; Zheng, M.; Ren, Z.; Qiu, M. Short-term effects of thinning on carbon storage in Chinese fir plantation ecosystems. J. Nanjing For. Univ. Nat. Sci. Ed. 2022, 46, 65–73. [Google Scholar] [CrossRef]
- Malmsheimer, R.W.; Bowyer, J.L.; Fried, J.S.; Gee, E.; Izlar, R.L.; Miner, R.A.; Munn, I.A.; Oneil, E.; Stewart, W.C. Managing Forests because Carbon Matters: Integrating Energy, Products, and Land Management Policy. J. For. 2011, 109, S7–S48. [Google Scholar] [CrossRef]
- Taki, H.; Inoue, T.; Tanaka, H.; Makihara, H.; Sueyoshi, M.; Isono, M.; Okabe, K. Responses of community structure, diversity, and abundance of understory plants and insect assemblages to thinning in plantations. For. Ecol. Manag. 2010, 259, 607–613. [Google Scholar] [CrossRef]
- Aldea, J.; Bravo, F.; Bravo-Oviedo, A.; Ruiz-Peinado, R.; Rodriguez, F.; del Rio, M. Thinning enhances the species-specific radial increment response to drought in Mediterranean pine-oak stands. Agr. For. Meteorol. 2017, 237, 371–383. [Google Scholar] [CrossRef]
- Guan, H.; Dong, X.; Zhang, T.; Wang, Z.; Ruan, J. Comparing the Stand Structure of a Secondary Mixed Forest with Three Broad-Leaved Forests in China’s Greater Khingan Mountains. Pol. J. Environ. Stud. 2020, 29, 3127–3141. [Google Scholar] [CrossRef]
- Fantini, A.C.; Schuch, C.; Siminski, A.; Siddique, I. Small-scale Management of Secondary Forests in the Brazilian Atlantic Forest. Floresta Ambiente 2019, 26, e20170690. [Google Scholar] [CrossRef]
- Nicoll, B.C.; Connolly, T.; Gardiner, B.A. Changes in Spruce Growth and Biomass Allocation Following Thinning and Guying Treatments. Forests 2019, 10, 253. [Google Scholar] [CrossRef]
- Xu, J. The 8th forest resources inventory results and analysis in China. For. Econ. 2014, 3, 6–8. [Google Scholar]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vazquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Wang, C.K. Biomass allometric equations for 10 co-occurring tree species in Chinese temperate forests. For. Ecol. Manag. 2006, 222, 9–16. [Google Scholar] [CrossRef]
- Hu, H.; Luo, B.; Wei, S.; Wei, S.; Sun, L.; Luo, S.; Ma, H. Biomass carbon density and carbon sequestration capacity in seven typical forest types of the Xiaoxing’an Mountains, China. Chin. J. Plant Ecol. 2015, 39, 140–158. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Tao, Y.; Gu, Y. Study on the tree volume table compilation of Korean pine in Liaoning province. Liaoning For. Sci. Technol. 2007, 2, 61–62. [Google Scholar]
- Chen, L. Soil Experiment and Practice Course; Northeast Forestry University Press: Harbin, China, 2005. [Google Scholar]
- Mu, C.; Lu, H.; Wang, B.; Bao, X.; Cui, W. Short-term effects of harvesting on carbon storage of boreal Larix gmelinii Carex schmidtii forested wetlands in Daxing’anling, northeast China. For. Ecol. Manag. 2013, 293, 140–148. [Google Scholar] [CrossRef]
- Ming, A.; Zhang, Z.; Chen, H.; Zhang, X.; Tao, Y.; Su, Y. Effects of Thinning on the Biomass and Carbon Storage in Pinus massoniana Plantation. Sci. Silvae Sin. 2013, 49, 1–6. [Google Scholar] [CrossRef]
- Han, F.; Tian, X.; Dang, K.; Cao, T. Impact of Thinning on Carbon Storage for Pinus tabulaeformis Stands in Shaanxi Qiaoshan. J. Northwest For. Univ. 2015, 30, 184–191. [Google Scholar]
- Mäkinen, H.; Isomäki, A. Thinning intensity and growth of Scots pine stands in Finland. For. Ecol. Manag. 2004, 201, 311–325. [Google Scholar] [CrossRef]
- Navarro, F.B.; Jimenez, M.N.; Canadas, E.M.; Gallego, E.; Terron, L.; Ripoll, M.A. Effects of different intensities of overstory thinning on tree growth and understory plant-species productivity in a semi-arid Pinus halepensis Mill. afforestation. For. Syst. 2010, 19, 410–417. [Google Scholar] [CrossRef]
- Woodruff, D.R.; Bond, B.J.; Ritchie, G.A.; Scott, W. Effects of stand density on the growth of young Douglas-fir trees. Can. J. For. Res. 2002, 32, 420–427. [Google Scholar] [CrossRef]
- Bae, S.-W.; Hwang, J.-H.; Lee, S.-T.; Kim, H.-S.; Jeong, J.-M. Changes in soil temperature, moisture content, light availability and diameter growth after thinning in Korean pine (Pinus koraiensis) plantation. J. Korean Soc. For. Sci. 2010, 99, 397–403. [Google Scholar]
- Ruano, I.; Rodriguez-Garcia, E.; Bravo, F. Effects of pre-commercial thinning on growth and reproduction in post-fire regeneration of Pinus halepensis Mill. Ann. For. Sci. 2013, 70, 357–366. [Google Scholar] [CrossRef]
- Valinger, E.; Elfving, B.; Mörling, T. Twelve-year growth response of Scots pine to thinning and nitrogen fertilisation. For. Ecol. Manag. 2000, 134, 45–53. [Google Scholar] [CrossRef]
- Harrington, C.A.; Reukema, D.L. Initial shock and long-term stand development following thinning in a Douglas-fir plantation. For. Sci. 1983, 29, 33–46. [Google Scholar] [CrossRef]
- Roberts, S.D.; Harrington, C.A. Individual tree growth response to variable-density thinning in coastal Pacific Northwest forests. For. Ecol. Manag. 2008, 255, 2771–2781. [Google Scholar] [CrossRef]
- Vernon, M.J.; Sherriff, R.L.; van Mantgem, P.; Kane, J.M. Thinning, tree-growth, and resistance to multi-year drought in a mixed-conifer forest of northern California. For. Ecol. Manag. 2018, 422, 190–198. [Google Scholar] [CrossRef]
- Li, Y.; Turnblom, E.C.; Briggs, D.G. Effects of density control and fertilization on growth and yield of young Douglas-fir plantations in the Pacific Northwest. Can. J. For. Res. 2007, 37, 449–461. [Google Scholar] [CrossRef]
- Tong, Q.J.; Zhang, S.Y.; Thompson, M. Evaluation of growth response, stand value and financial return for pre-commercially thinned jack pine stands in Northwestern Ontario. For. Ecol. Manag. 2005, 209, 225–235. [Google Scholar] [CrossRef]
- Brix, H. Fertilization and Thinning Effect on a Douglas-Fir Ecosystem at Shawnigan Lake: A Synthesis of Project Results; FRDA Research Program, Research Branch, BC Ministry of Forests and Lands: Victoria, Canada, 1992. [Google Scholar]
- Lei, L.; Xiao, W. Uncertainty Effect of Forest Harvest on Soil Carbon Pool: A Review. For. Res. 2015, 28, 892–899. [Google Scholar] [CrossRef]
- Gong, C.; Tan, Q.; Liu, G.; Xu, M. Forest thinning increases soil carbon stocks in China. For. Ecol. Manag. 2021, 482, 118812. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, M.-X.; Wang, Z.; Ma, X.-X.; Qiu, Y.-J. Effects of revegetation on organic carbon storage in deep soils in hilly Loess Plateau region of Northwest China. Ying Yong Sheng Tai Xue Bao=J. Appl. Ecol. 2012, 23, 2721–2727. [Google Scholar] [CrossRef]
- Fang, X.; Tian, D.; Xiang, W. Effects of thinning on carbon stroage and its spatial distributions in Chinese Fir plantation ecosystem. J. Cent. S. Univ. For. Technol. 2010, 30, 47–53. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, W.; Zhou, L.; Yu, D.; Zhou, Y.; Qin, S.; Dai, L. Carbon storage and its distribution pattern in the natural Larix gmelin?? forests on Daxing’an mountains. Acta Ecol. Sin. 2015, 35, 189–195. [Google Scholar] [CrossRef]
- Wei, Y.; Li, M.; Chen, H.; Lewis, B.J.; Yu, D.; Zhou, L.; Zhou, W.; Fang, X.; Zhao, W.; Dai, L. Variation in Carbon Storage and Its Distribution by Stand Age and Forest Type in Boreal and Temperate Forests in Northeastern China. PLoS ONE 2013, 8, e72201. [Google Scholar] [CrossRef]
- Dong, L.; Liu, H.; Wang, C.; Zhao, J.; Gao, Y.; Huang, X.; Xiao, Y. Short-term and Long-term Effects of Thinning on Carbon Storage of Quercus mongolica Secondary Forests. J. Shenyang Agric. Univ. 2019, 50, 614–620. [Google Scholar]
- Zhao, P.; Song, C.; Su, R.; Hai, L.; Zhou, M.; Wei, J.; Wang, S. Review in the research of litter fall in forest ecosystems. J. Inn. Mong. Agric. Univ. 2009, 30, 292–299. [Google Scholar]
- Haibo, F.; Dalun, T.; Wenxing, K. Biomass dynamics of a thinned Chinese fir plantation ecosystem. J. Cent. S. For. Coll. 1999, 19, 16–19. [Google Scholar] [CrossRef]
- Lin, J.-C.; Chiu, C.-M.; Lin, Y.-J.; Liu, W.-Y. Thinning Effects on Biomass and Carbon Stock for Young Taiwania Plantations. Sci. Rep. 2018, 8, 3070. [Google Scholar] [CrossRef]
- Anderson, S.; Knapp, B.O.; Kabrick, J.M. Stand-Density Effects on Aboveground Carbon Dynamics in Secondary Pinus and Quercus Forests of Central USA. For. Sci. 2023, 69, 213–227. [Google Scholar] [CrossRef]
- Bonnor, G. The influence of stand density on the correlation of stem diameter with crown width and height for lodgepole pine. For. Chron. 1964, 40, 347–349. [Google Scholar] [CrossRef]
- Węgiel, A.; Bembenek, M.; Łacka, A.; Mederski, P.S. Relationship between stand density and value of timber assortments: A case study for Scots pine stands in north-western Poland. N. Z. J. For. Sci. 2018, 48, 12. [Google Scholar] [CrossRef]
- Allen, M.; Brunner, A.; Antón-Fernández, C.; Astrup, R. The relationship between volume increment and stand density in Norway spruce plantations. For. Int. J. For. Res. 2021, 94, 151–165. [Google Scholar] [CrossRef]

| Thinning Treatments | Tree Types | Tree Density (ha−1) | The Year after Thinning (2019) | Four Years after Thinning (2023) | ||
|---|---|---|---|---|---|---|
| DBH (cm) | H (m) | DBH (cm) | H (m) | |||
| 0% (CK) | All trees | 1216 | 20.1 ± 0.7 | 15.7 ± 0.2 | 21.7 ± 0.8 | 19.6 ± 0.3 |
| Korean pine | 1150 | 20.1 ± 0.7 | 15.8 ± 0.2 | 21.8 ± 0.8 | 19.6 ± 0.3 | |
| 10% | All trees | 883 | 22.3 ± 0.4 * | 16.1 ± 0.1 | 24.4 ± 0.4 * | 21.0 ± 0.4 * |
| Korean pine | 850 | 22.6 ± 0.4 * | 16.2 ± 0.1 | 24.6 ± 0.4 * | 21.0 ± 0.4 * | |
| 11% | All trees | 983 | 20.7 ± 0.5 | 15.2 ± 0.2 * | 22.7 ± 0.6 | 20.7 ± 0.3 * |
| Korean pine | 900 | 21.2 ± 0.5 | 15.4 ± 0.2 * | 23.3 ± 0.6 | 21.1 ± 0.3 * | |
| 16% | All trees | 833 | 23.0 ± 0.5 * | 15.6 ± 0.2 | 25.3 ± 0.5 * | 21.3 ± 0.2 * |
| Korean pine | 833 | 23.0 ± 0.5 * | 15.6 ± 0.2 | 25.3 ± 0.5 * | 21.3 ± 0.2 * | |
| 18% | All trees | 916 | 21.6 ± 0.6 | 15.1 ± 0.2 * | 23.9 ± 0.6 * | 21.0 ± 0.4 * |
| Korean pine | 883 | 21.8 ± 0.6 * | 15.2 ± 0.2 * | 24.1 ± 0.6 * | 21.2 ± 0.3 * | |
| 22% | All trees | 833 | 22.3 ± 0.6 * | 17.1 ± 0.2 * | 24.5 ± 0.6 * | 20.9 ± 0.3 * |
| Korean pine | 833 | 22.3 ± 0.6 * | 17.1 ± 0.2 * | 24.5 ± 0.6 * | 20.9 ± 0.3 * | |
| Tree Species | Component | Equation |
|---|---|---|
| Pinus koraiensis | Stem | B1 = 0.04665 × (D2H)0.90237 |
| Branch | B2 = 0.03123 × (D2H)0.61248 | |
| Leaf | B3 = 0.01235 × (D2H)0.64347 | |
| Root | B4 = 0.00925 × (D2H)0.73965 | |
| Betula dahurica | Stem | B1 = 0.01175 × (D2H)1.10252 |
| Branch | B2 = 0.01024 × (D2H)0.80547 | |
| Leaf | B3= 0.01347 × (D2H)0.64947 | |
| Root | B4= 0.04887 × (D2H)0.63246 |
| Thinning Treatment (%) | 2019 Total Carbon (t ha−1) | 2023 Total Carbon (t ha−1) | Annual Increment Carbon (t ha−1y−1) | Relative Increment Carbon (%) |
|---|---|---|---|---|
| 0 | 97.3 | 134.45 | 9.29 | 9.5 |
| 10 | 79.00 | 116.99 | 9.50 | 12.0 |
| 11 | 76.14 | 117.25 | 10.28 | 13.5 |
| 16 | 76.03 | 117.50 | 10.37 | 13.6 |
| 18 | 75.75 | 120.27 | 11.13 | 14.7 |
| 22 | 79.19 | 111.16 | 7.99 | 10.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakib, N.; Poudel, T.R.; Hao, Y.; Roberts, N.J.; Iddrisu, A.-Q.; Adhikari, S.; Zhang, P. Effects of Different Thinning Intensities on Carbon Storage in Pinus koraiensis Middle-Aged Plantations in Northeast China. Forests 2024, 15, 738. https://doi.org/10.3390/f15050738
Sakib N, Poudel TR, Hao Y, Roberts NJ, Iddrisu A-Q, Adhikari S, Zhang P. Effects of Different Thinning Intensities on Carbon Storage in Pinus koraiensis Middle-Aged Plantations in Northeast China. Forests. 2024; 15(5):738. https://doi.org/10.3390/f15050738
Chicago/Turabian StyleSakib, Nazmus, Tika Ram Poudel, Yuanqin Hao, Nathan James Roberts, Abdul-Qadir Iddrisu, Saraswoti Adhikari, and Peng Zhang. 2024. "Effects of Different Thinning Intensities on Carbon Storage in Pinus koraiensis Middle-Aged Plantations in Northeast China" Forests 15, no. 5: 738. https://doi.org/10.3390/f15050738










