Species Diversity of Oak Stands and Its Significance for Drought Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetation Indices Change Threshold
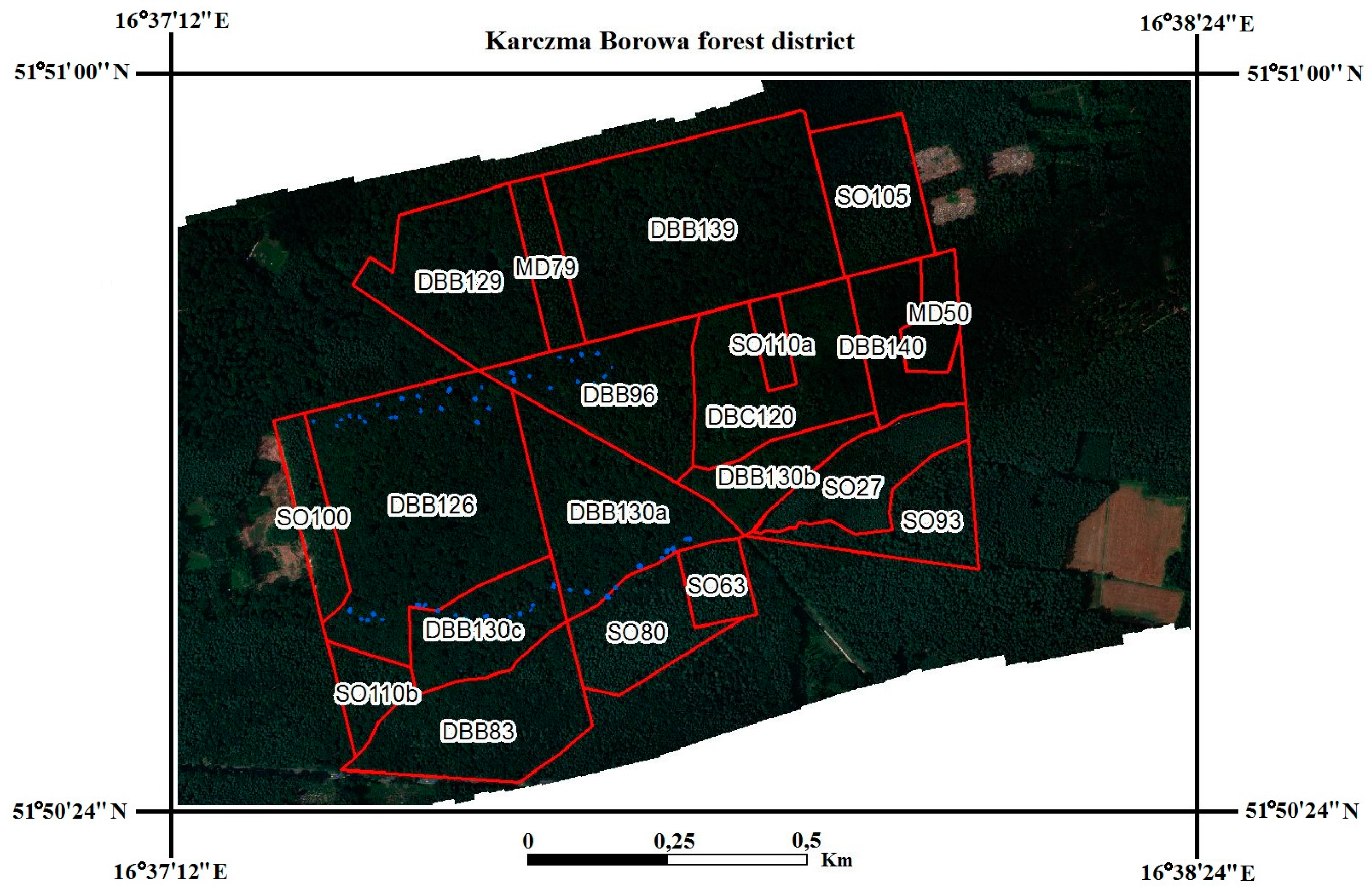
2.2. Determination of Regions of Interest (ROIs)
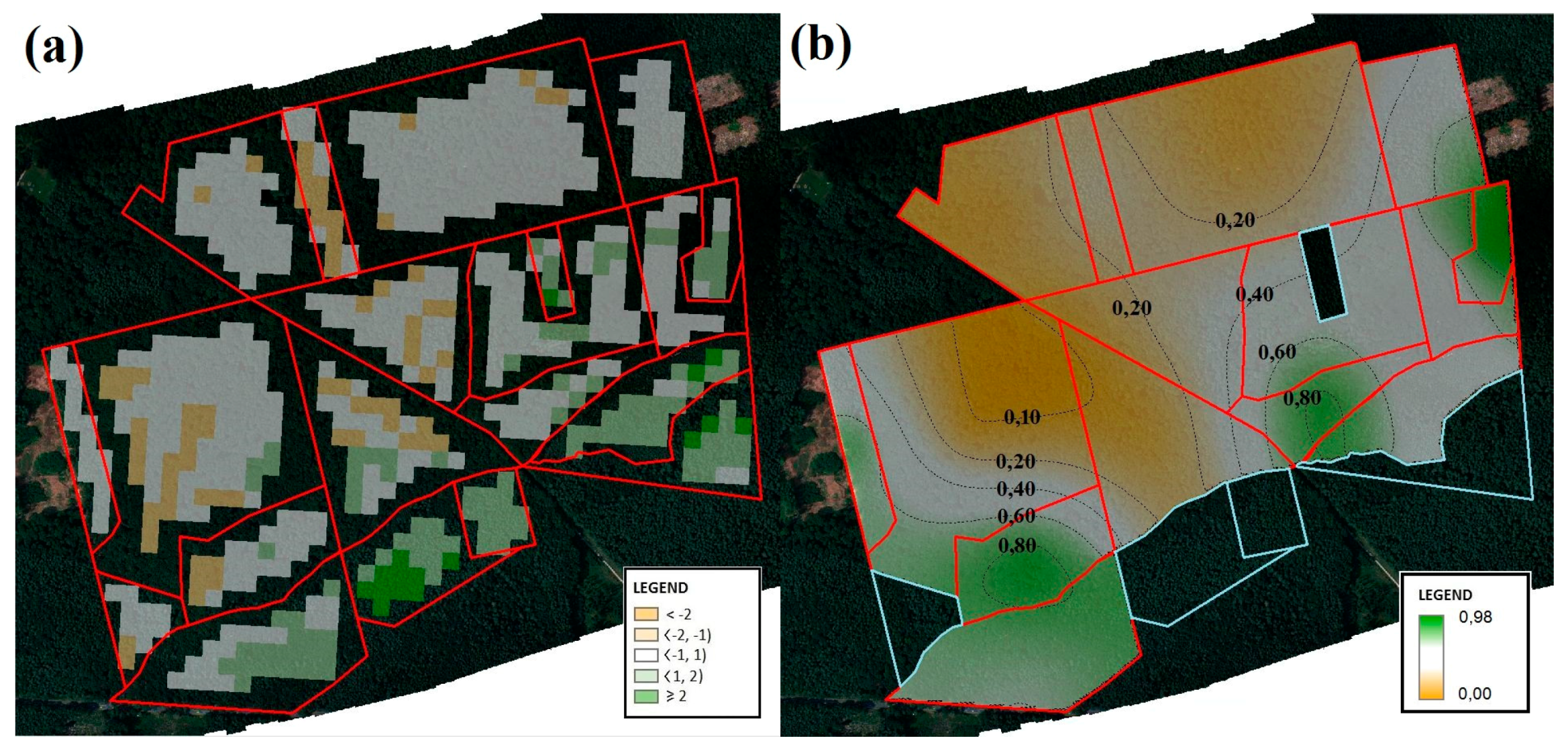
2.3. Estimation of Changes in the Condition of Forest Complexes between 2014 and 2015
2.4. Segmentation of Tree Crowns in Aerial Images
2.5. Estimation of the Species Diversity Index
2.6. A Comparison of Inventory-Based Stand Characteristics in ROIs and Their Species Diversity with the Changes Observed between 2014 and 2015
2.7. Different Forest Trees Species Features Related to Drought
2.7.1. Quercus robur
2.7.2. Quercus rubra
2.7.3. Pinus sylvestris
2.7.4. Betula pendula
3. Results
3.1. Habitat Characteristics
3.2. Species Diversity Indices of Tree Crowns in the Particular Forest Divisions
3.3. Coefficients of Correlation between the Characteristics of ROIs and Their Responses to Drought
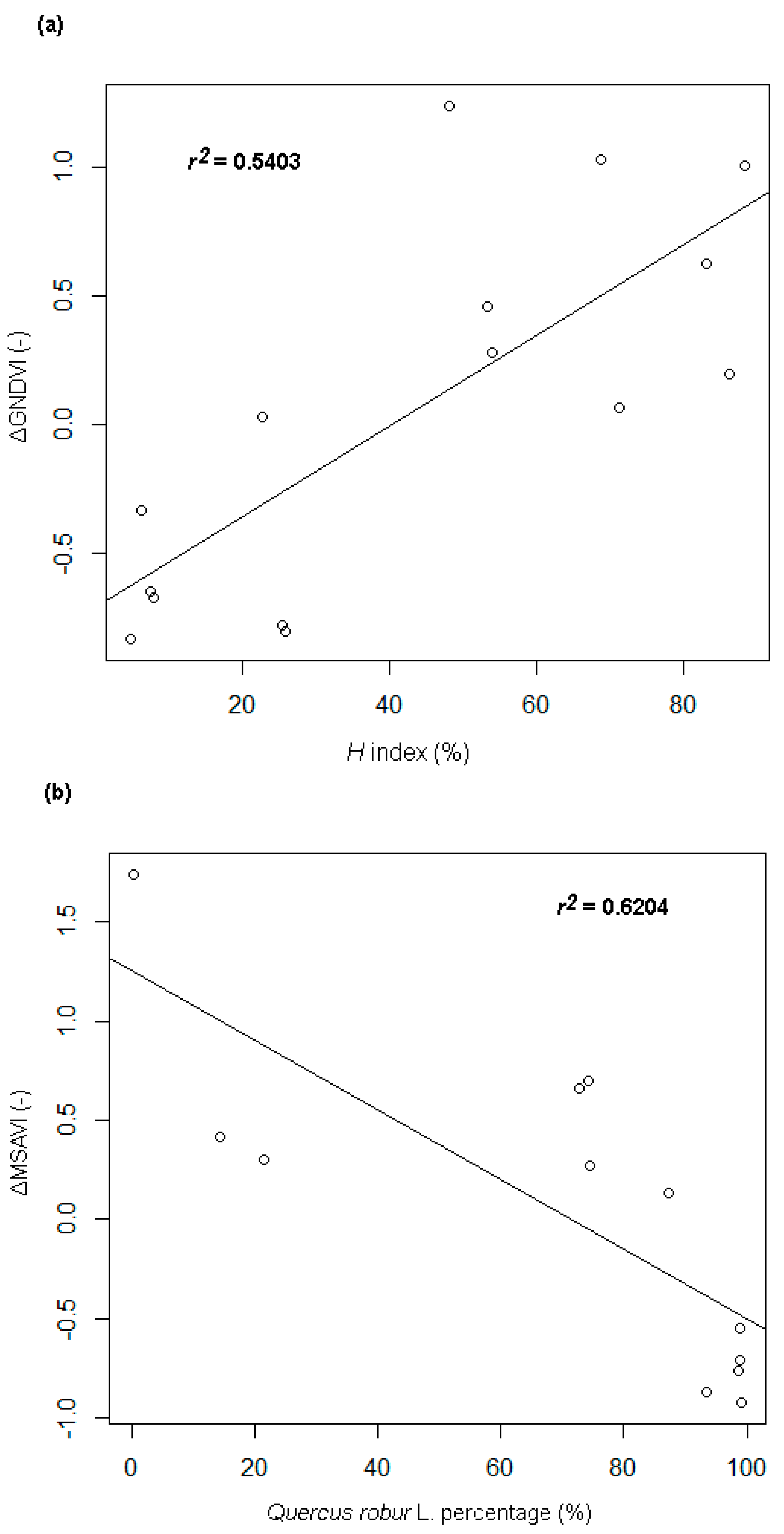
- A strong positive correlation between the Shannon-Wiener species diversity of tree crowns and the particular vegetation indices differences (ΔVIs); the mean value of the correlation is 0.66 (±0.083); the strongest correlation (+0.73, r² = 0.54; p-value < 0.005) occurred for the ΔGNDVI differential index (Figure 4a), while the lowest one could be observed for ΔNDMI (+0.57).
- A strong negative correlation between the percentage share of the tree crowns of the Q. robur species and the particular ΔVIs. The strongest relationship (with a correlation coefficient of −0.69, r² = 0.48) was found for the correlation with the ΔMSAVI index (Figure 4b).
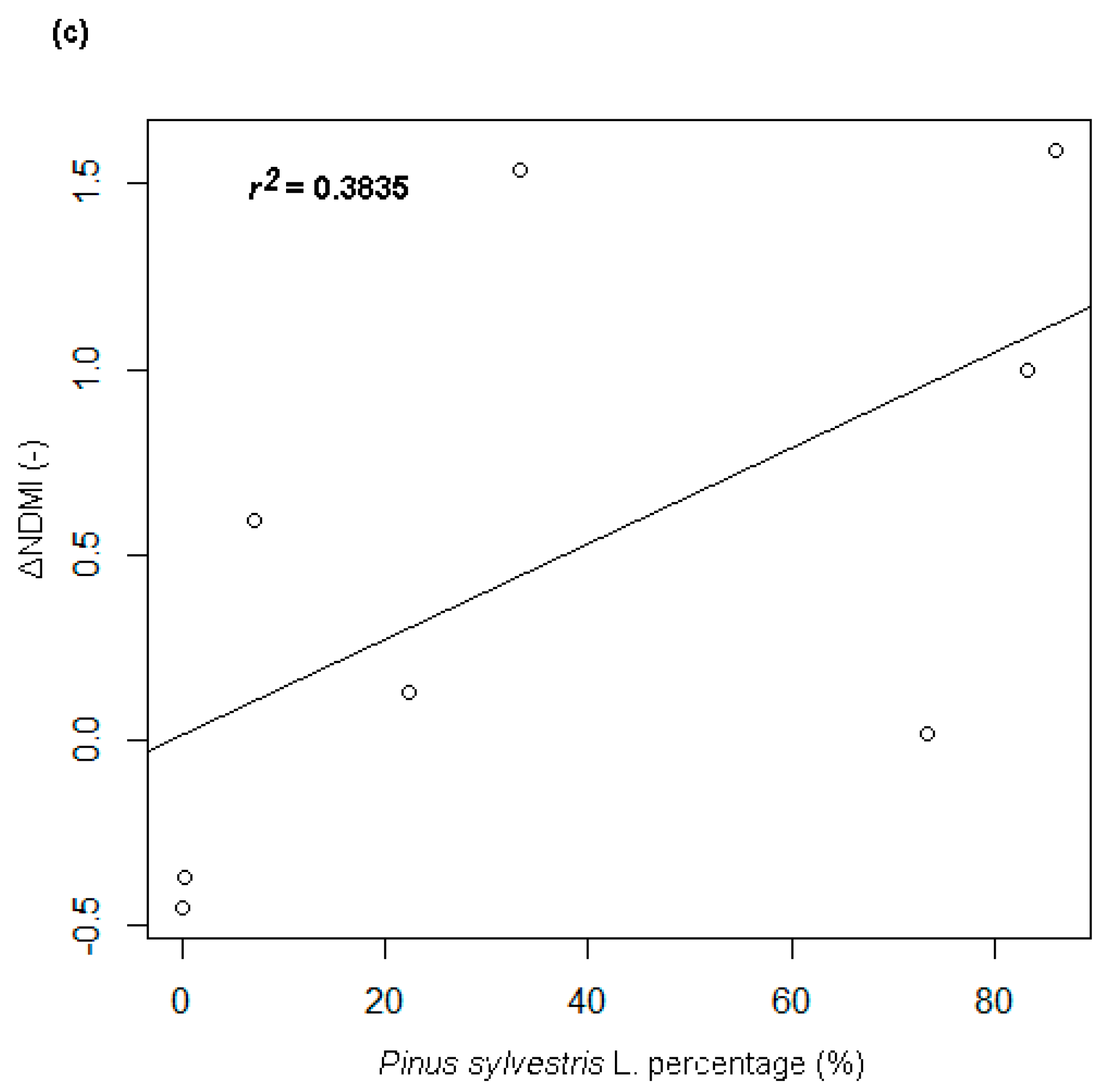
- A strong positive correlation between the percentage share of the tree crowns of the Pinus sp. and the particular ΔVIs. The strongest relationship (with the correlation coefficient of +0.72, r² = 0.53) was found for the correlation with the ΔNDMI index (Figure 4c).
4. Discussion
4.1. Species Diversity
4.2. Dominant Species
4.3. Monitoring Water Stress Using Differential Indices
4.3.1. Quercus sp. L.
4.3.2. Pinus sp. L.
5. Conclusions
- In assessing drought tolerance, remote sensing analysis showed the following order, beginning with the most tolerant monocultures of species: P. sylvestris > Q. robur > Q. rubra. Additionally, a negative impact of gley soil was observed on Q. robur monoculture.
- Mixed stands with Q. robur as the dominant species are more resistant to drought than Q. robur monocultures. Having a ~20% admixture of Q. robur and P. sylvestris significantly reduced deterioration of vegetation indices during short-term drought. In a single case, a ~10% admixture of Larix sp. contributed to the decline of vegetation indices.
- In a single case, a ~20% admixture of Q. robur in the stand with P. sylvestris as a dominant species contributed to the decline of vegetation indices. We suggest that this particular case may confirm the results of Urli et al. (2015) [82]. This particular ROI is the only pine stand with rusty brown soil, which may cause limited water availability and the threshold water transport variables observation [82]. This observation should be explained in future research.
- The particular differential indices have different degrees of usefulness, depending on the examined factor. In general, the sensitivity to green light is important for biodiversity research. The index MSAVI is most useful for investigating Q. robur, while NDMI is more adequate for P. sylvestris. It is important to note that such conclusions can only be drawn for species that dominate a given habitat. More detailed analyses require data with significantly higher spatial resolution than that of Landsat. It is therefore envisaged that further research will use data with a GSD of 0.25 m, acquired from the QUERCUS.6 aerial platform, which was used in the present study for image segmentation. This will allow the analysis of the spectral response of individual crowns, using six channels registered in the range from 400 to 1000 nm. An additional advantage of high-resolution data is the ability to eliminate shadows and clearances between trees, facilitating the analysis of tree properties.
- Despite all constraints, Landsat satellite images with a 30-m accuracy are suitable to obtain a quick and general overview of a situation. Their analysis is relatively rapid, and they are generally available for any time and place. The major constraints include cloudiness and correct radiometric adjustment. However, the present study demonstrates that, based on accurate knowledge of the quantitative shares of individual species, indirect conclusions can be drawn about their contribution to changes in the particular remote sensing indices. Their characteristics and interrelationships can also be assessed.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thompson, I.; Mackey, B.; McNulty, S.; Mosseler, A. Forest Resilience, Biodiversity, and Climate Change. In A Synthesis of the Biodiversity/Resilience/Stability Relationship in Forest Ecosystems; Technical Series; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2009; pp. 43–67. [Google Scholar]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Coder, K.D.; Daniel, B. Drought Damage to Trees; University of Georgia: Athens, GA, USA, 1999. [Google Scholar]
- Singh, A. Digital change detection techniques using remotely-sensed data. Int. J. Remote Sens. 1989, 10, 989–1003. [Google Scholar] [CrossRef]
- Jensen, J.R. Introductory Digital Image Processing: A Remote Sensing Perspective; University of South Carolina: Columbus, OH, USA, 1986. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; Dowell, N.M.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Delzon, S.; Urli, M.; Samalens, J.; Lamy, J.; Lischke, H.; Sin, F.; Zimmermann, N.E.; Porté, A.J. Field evidence of colonisation by Holm oak, at the northern margin of its distribution range, during the Anthropocene period. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Sohar, K.; Helama, S.; Läänelaid, A.; Raiso, J.; Tuomenvirta, H. Oak decline in a southern Finnish forest as affected by a drought sequence. Geochronometria 2014, 41, 92–103. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, J.E.; Reyer, C.; Delzon, S.; der Maaten, E.; Schelhaas, M.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Rummukainen, M. Changes in climate and weather extremes in the 21st century. Wiley Interdiscip. Rev. Clim. Chang. 2012, 3, 115–129. [Google Scholar] [CrossRef]
- Orlowsky, B.; Seneviratne, S.I. Global changes in extreme events: Regional and seasonal dimension. Clim. Chang. 2012, 110, 669–696. [Google Scholar] [CrossRef]
- Helama, S.; Meriläinen, J.; Tuomenvirta, H. Multicentennial megadrought in northern Europe coincided with a global El Niño–Southern Oscillation drought pattern during the Medieval Climate Anomaly. Geology 2009, 37, 175–178. [Google Scholar] [CrossRef]
- Bonacina, L.C.W. The European drought of 1921. Nature 1923, 112, 488–489. [Google Scholar] [CrossRef]
- Cook, E.R.; Seager, R.; Kushnir, Y.; Briffa, K.R.; Büntgen, U.; Frank, D.; Krusic, P.J.; Tegel, W.; van der Schrier, G.; Andreu-Hayles, L.; et al. Old World megadroughts and pluvials during the Common Era. Sci. Adv. 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Andres, E.; Seely, B.; Blanco, J.A. Increased complementarity in water-limited environments in Scots pine and European beech mixtures under climate change. Ecohydrology 2017, 10. [Google Scholar] [CrossRef]
- González de Andrés, E.; Camarero, J.J.; Blanco, J.A.; Imbert, J.B.; Lo, Y.H.; Sangüesa-Barreda, G.; Castillo, F.J. Tree-to-tree competition in mixed European beech—Scots pine forests has different impacts on growth and water-use efficiency depending on site conditions. J. Ecol. 2018, 106, 59–75. [Google Scholar] [CrossRef]
- Río, M.D.; Schütze, G.; Pretzsch, H. Temporal variation of competition and facilitation in mixed species forests in Central Europe. Plant Biol. 2014, 16, 166–176. [Google Scholar] [PubMed]
- Pretzsch, H.; Del Río, M.; Ammer, C.; Avdagic, A.; Barbeito, I.; Bielak, K.; Brazaitis, G.; Coll, L.; Dirnberger, G.; Drössler, L.; et al. Growth and yield of mixed versus pure stands of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) analysed along a productivity gradient through Europe. Eur. J. For. Res. 2015, 134, 927–947. [Google Scholar] [CrossRef]
- Kelty, M.J. Comparative productivity of monocultures and mixed-species stands. In The Ecology and Silviculture of Mixed-Species Forests; Springer: Dordrecht, The Netherlands, 1992; pp. 125–141. [Google Scholar]
- Pretzsch, H.; Dieler, J.; Seifert, T.; Rötzer, T. Climate effects on productivity and resource-use efficiency of Norway spruce (Picea abies L. Karst.) and European beech (Fagus sylvatica L.) in stands with different spatial mixing patterns. Trees 2012, 26, 1343–1360. [Google Scholar] [CrossRef]
- Richards, A.E.; Forrester, D.I.; Bauhus, J.; Scherer-Lorenzen, M. The influence of mixed tree plantations on the nutrition of individual species: A review. Tree Physiol. 2010, 30, 1192–1208. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, E. Gleichaltrige Fichten-Buchen-Mischbestand; FAO: Rome, Italy, 1942. [Google Scholar]
- Vandermeer, J. The Ecology of Intercropping; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Sass-Klaassen, U.; Chowdhury, Q.; Sterck, F.J.; Zweifel, R. Effects of water availability on the growth and tree morphology of Quercus pubescens Willd. and Pinus sylvestris L. in the Valais, 2006, Switzerland, Section 1 Cultural Heritage. In Proceedings of the TRACE-Tree Rings in Archaeology, Climatology and Ecology, Proceedings of the Dendrosymposium, Tervuren, Belgium, 20–22 April 2006. [Google Scholar]
- Raftoyannis, Y.; Radoglou, K. Physiological Responses of Beech and Sessile Oak in a Natural Mixed Stand during a Dry Summer. Ann. Bot. 2002, 89, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, G.; Jacquemyn, H.; Mergeay, J.; Cox, K.; Janssens, P.; Gielen, B.A.S.; Muys, B.; Honnay, O. The effect of drought stress on heterozygosity–fitness correlations in pedunculate oak (Quercus robur). Ann. Bot. 2014, 113, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Pietrzykowski, M.; Krzaklewski, W.; Likus, J.; Woś, B. Assessment of English oak (Quercus robur L.) growth in varied soil-substrate conditions of reclaimed Piaseczno sulfur mine dump. Folia For. Pol. 2015, 57, 28–32. [Google Scholar] [CrossRef][Green Version]
- Dzwonko, Z.; Loster, S. Vegetation differentiation and secondary succession on a limestone hill in southern Poland. J. Veg. Sci. 1990, 1, 615–622. [Google Scholar] [CrossRef]
- Thomas, F.M.; Gausling, T. Morphological and physiological responses of oak seedlings (Quercus petraea and Q. robur) to moderate drought. Ann. For. Sci. React-Text 2002, 57, 325–333. [Google Scholar] [CrossRef]
- Dickson, R.E.; Tomlinson, P.T. Oak growth, development and carbon metabolism in response to water stress. Ann. Sci. For. 1996, 53, 181–196. [Google Scholar] [CrossRef]
- Dat, J.F.; Parent, C. Differential responses in sympatric tree species exposed to waterlogging. Tree Physiol. 2012, 32, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Cochard, H. Summer and winter embolism in oak: Impact on water relations. Ann. Sci. For. 1996, 53, 173–180. [Google Scholar] [CrossRef]
- Kubiske, M.E.; Abrams, M.D. Photosynthesis, water relations, and leaf morphology of xeric versus mesic Quercus rubra ecotypes in central Pennsylvania in relation to moisture stress. Can. J. For. Res. 1992, 22, 1402–1407. [Google Scholar] [CrossRef]
- Sverdrup, H.; Stjernquist, I. Forest vitality and stress implications. In Developing Principles and Models for Sustainable Forestry in Sweden (Managing Forest Ecosystems), 2002nd ed.; Sverdrup, H., Stjernquist, I., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 197–271. ISBN 978-94-015-9888-0. [Google Scholar]
- Farrar, J.L. Trees in Canada, 1st ed.; Fitzhenry & Whiteside Limited: Markham, ON, Canada; The Canadian Forest Services: Ottawa, ON, Canada, 1995. [Google Scholar]
- Gu, M.; Rom, C.R.; Robbins, J.A. Effect of water deficit on gas exchange, osmotic solutes, leaf abscission, and growth of four birch genotypes (Betula L.) under a controlled environment. HortScience 2007, 42, 1383–1391. [Google Scholar]
- Robson, T.M.; Hartikainen, S.M.; Aphalo, P.J. How does solar ultraviolet-B radiation improve drought tolerance of silver birch (Betula pendula Roth.) seedlings? Plant Cell Environ. 2015, 38, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; Lobo, A.; Estevan, H.; Maisongrande, P.; Vayreda, J.; Terradas, J. Woody plant richness and NDVI response to drought events in Catalonian (northeastern Spain) forests. Ecology 2007, 88, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Kuźmiński, R.; Szewczyk, W.; Korczyński, I.; Łakomy, P. Assessment of leaf damage in oak stands in the flood-affected Wołów Forest Division. For. Res. Pap. 2015, 76, 297–303. [Google Scholar]
- Oszako, T. Oak declines in Europe’s forest—History, causes and hypothesis. In Procedings of the Recent Advances on Oak Health in Europe, Warsaw, Poland, 22–24 November 1999; Instytut Badawczy Leśnictwa. Forest Research Institute: Warsaw, Poland, 1999. [Google Scholar]
- Menkis, A.; Ostbrant, I.L.; Wagstrom, K.; Vasaitis, R. Dutch elm disease on the island of Gotland: Monitoring disease vector and combat measures. Scan. J. For. Res. 2015, 31, 237–241. [Google Scholar] [CrossRef]
- Menkis, A.; Ostbrant, I.L.; Davydenko, K.; Bakys, R.; Balalaikins, M.; Vasaitis, R. Scolytus multistriatus associated with Dutch elm disease on the island of Gotland: Phenology and communities of vectored Fungi. Mycol. Prog. 2016, 15, 55. [Google Scholar] [CrossRef]
- Boczoń, A.; Kowalska, A.; Dudzińska, M.; Wróbel, M. Drought in Polish forests in 2015. Pol. J. Environ. Stud. 2016, 25, 5. [Google Scholar] [CrossRef]
- Jensen, J.R. Remote Sensing of the Environment: An Earth Resource Perspective, 2nd ed.; Prentice Hall Series in Geographic Information Science; Pearson Education India: London, UK, 2000. [Google Scholar]
- Mas, J.F. Monitoring land-cover changes: A comparison of change detection techniques. Int. J. Remote Sens. 1999, 20, 139–152. [Google Scholar] [CrossRef]
- Hui, F.; Fu, X.; Zhang, Z.; Wu, Q. Phenology-based vegetation index differencing for mapping of rubber plantations using Landsat OLI data. Remote Sens. 2015, 7, 6041–6058. [Google Scholar]
- Coppin, P.R.; Bauer, M.E. Processing of multitemporal Landsat TM imagery to optimize extraction of forest cover change features. IEEE Trans. Geosci. Remote Sens. 1994, 32, 918–927. [Google Scholar] [CrossRef]
- Wang, C.; Lu, Z.; Haithcoat, T.L. Using Landsat images to detect oak decline in the Mark Twain National Forest, Ozark Highlands. For. Ecol. Manag. 2007, 240, 70–78. [Google Scholar] [CrossRef]
- Nasiłowska, S.A.; Kotlarz, J.; Rynkiewicz, A.; Kacprzak, M.; Rotchimmel, K. The impact of drought in 2015 on the forest condition determined using Landsat-8 OLI images. Leśne Prace Badawcze 2018. under review. [Google Scholar]
- Volcani, A.; Karnieli, A.; Svoray, T. The use of remote sensing and GIS for spatio-temporal analysis of the physiological state of a semi-arid forest with respect to drought years. For. Ecol. Manag. 2005, 215, 239–250. [Google Scholar] [CrossRef]
- Huete, A.R.; Liu, H.Q.; Batchily, K.; van Leeuwen, W. A comparison of vegetation indices over a global set of TM images for EOS-MODIS. Remote Sens. Environ. 1997, 59, 440–451. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Key, C.H.; Benson, N.C. FIREMON: Fire Effects Monitoring and Inventory System; Technical Report in press for USDA Forest Service; Rocky Mountains Research Station: Washington, DC, USA, 2006. [Google Scholar]
- Hardisky, M.; Klemas, V.; Smart, R. The influences of soil salinity, growth form, and leaf moisture on the spectral reflectance of Spartina alterniflora. Photogramm. Eng. Remote Sens. 1983, 49, 77–83. [Google Scholar]
- Rouse, J.W., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; Texas A & M University: College Station, TX, USA, 1974. [Google Scholar]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Gitelson, A.; Kaufman, A.; Yoram, J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Kaufman, Y.J.; Tanre, D. Atmospherically resistant vegetation index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sens. 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Hancock, D.W.; Dougherty, C.T. Relationships between blue- and red-based vegetation indices and leaf area and yield of alfalfa. Crop Sci. 2007, 47, 2547–2556. [Google Scholar] [CrossRef]
- Asner, G.P.; Nepstad, D.; Cardinot, G.; Ray, D. Drought stress and carbon uptake in an Amazon forest measured with spaceborne imaging spectroscopy. Proc. Natl. Acad. Sci. USA 2004, 101, 6039–6044. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.E.; Pinzon, J.E.; Didan, K.; Morisette, J.T.; Tucker, C.J. Evaluation of the consistency of long-term NDVI time series derived from AVHRR, SPOT-Vegetation, SeaEiFS, MODIS, and Landsat ETM+ Sensors. IEEE Trans. Geosci. Remote Sens. 2006, 44, 1787–1793. [Google Scholar] [CrossRef]
- Product Guide. Landsat 8 Surface Reflectance Code (LaSRC) Product. Available online: https://landsat.usgs.gov/sites/default/files/documents/lasrc_product_guide.pdf (accessed on 1 March 2018).
- Czapski, P.; Kacprzak, M.; Kotlarz, J.; Mrowiec, K.; Kubiak, K.; Tkaczyk, M. Preliminary analysis of the forest health state based on multispectral images acquired by Unmanned Aerial Vehicle. Folia For. Pol. 2015, 57, 138–144. [Google Scholar] [CrossRef]
- Kacprzak, M.; Rotchimmel, K. Creating photogrammetry products with photos acquired by array of non-metric cameras. Prace Instytutu Lotnictwa 2016, 243, 120–129. [Google Scholar]
- Pitkänen, J.; Maltamo, M.; Hyyppä, J.; Yu, X. Adaptive methods for individual tree detection on airborne laser based canopy height model. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2004, 36, 187–191. [Google Scholar]
- Stereńczak, K. Single tree detection based on airborne LIDAR (ALS) data. Roczniki Geomatyki 2009, 7, 121–126. [Google Scholar]
- Wężyk, P.; Sroga, R.; Szwed, P. Postex precision positioning of tree trunks on circular inventory plots using the postex device. Roczniki Geomatyki 2008, 8, 109–118. [Google Scholar]
- Myszkowski, M.; Ksepko, M.; Gajko, K. Tree number detection based on airborne laser scanning data. Archiwum Instytutu Inżynierii Lądowej 2009, 6, 63–72. [Google Scholar]
- Falińska, K. Ekologia Roślin; PWN: Warszawa, Poland, 2004; p. 511. [Google Scholar]
- Bai, Y.; Zhuang, C.; Ouyang, Z.; Zheng, H.; Jiang, B. Spatial characteristics between biodiversity and ecosystem services in a human-dominated watershed. Ecol. Complex. 2011, 8, 177–183. [Google Scholar] [CrossRef]
- Mendenhall, C.D.; Shields-Estrada, A.; Krishnaswami, A.J.; Daily, G.C. Quantifying and sustaining biodiversity in tropical agricultural landscapes. Proc. Natl. Acad. Sci. USA 2016, 113, 14544–14551. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.M.; Walter, J. Remotely sensed high-resolution global cloud dynamics for predicting ecosystem and biodiversity distributions. PLoS Biol. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Geller, G.N.; Halpin, P.N.; Helmuth, B.; Hestir, E.L.; Skidmore, A.; Abrams, M.J.; Aguirre, N.; Blair, M.; Botha, E.; Colloff, M.; et al. Remote sensing for biodiversity. In The GEO Handbook on Biodiversity Observation Networks; Springer International Publishing: New York, NY, USA, 2017; pp. 187–210. [Google Scholar]
- Wang, R.; Gamon, J.A.; Montgomery, R.A.; Townsend, P.A.; Zygielbaum, A.I.; Bitan, K.; Cavender-Bares, J. Seasonal variation in the NDVI—Species richness relationship in a prairie grassland experiment (Cedar Creek). Remote Sens. 2016, 8, 128. [Google Scholar] [CrossRef]
- Czapski, P.; Kotlarz, J.; Kubiak, K.; Tkaczyk, M. Analiza czynnikowa zdjęć wielospektralnych. Prace Instytutu Lotnictwa 2014, 234, 143–150. (In Polish) [Google Scholar] [CrossRef]
- Kacprzak, M.; Wodziński, K. Execution of photo mission by manned aircraft and unmanned aerial vehicle. Prace Instytutu Lotnictwa 2016, 243, 130–141. [Google Scholar]
- Kotlarz, J.; Kubiak, K.; Kacprzak, M.; Czapski, P. Estimation of tree species diversity of forest stands based on their spectral reflectance. Sylwan 2016, 160, 1036–1045. [Google Scholar]
- Kotlarz, J.; Kacprzak, M. Algorytm automatycznego oszacowania zróżnicowania gatunkowego drzewostanu z wykorzystaniem zdjęć RGB koron drzew. Pomiary Automatyka Robotyka 2017, 21, 63–70. (In Polish) [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology; Harper & Row: New York, NY, USA, 1989. [Google Scholar]
- Épron, D.; Dreyer, E. Starch and soluble carbohydrates in leaves of water-stressed oak saplings. Ann. Sci. For. 1996, 53, 263–268. [Google Scholar] [CrossRef][Green Version]
- Osonubi, O.; Davies, W.J. Solute accumulation in leaves and roots of woody plants subjected to water stress. Oecologia 1978, 32, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Urli, M.; Lamy, J.B.; SinRégis, F.; Delzon, B.S. The high vulnerability of Quercus robur to drought at its southern margin paves the way for Quercus ilex. Plant Ecol. 2015, 216, 177–187. [Google Scholar] [CrossRef]
- Ashton, P.; Berlyn, G.P. A Comparison of leaf physiology and anatomy of Quercus (Section Er Ythrobalanus-Fagaceae) species in different light environments. Am. J. Bot. 1994, 8, 589–597. [Google Scholar] [CrossRef]
- Abrams, M.D. Adaptations and responses to drought in Quercus species of North America. Tree Physiol. 1990, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Głowacki, D.; Sławska, M.; Sławski, M. The dynamics of northern red oak (Quercus rubra L.) in managed forests of central Poland. Leśne Prace Badawcze 2016, 77, 32–41. [Google Scholar]
- Parker, W.C.; Pallardy, S.G.; Hinckley, T.M.; Teskey, R.O. Seasonal changes in tissue water relations of three woody species of the Quercus-Carya forest type. Ecology 1982, 63, 1259–1267. [Google Scholar] [CrossRef]
- Hinckley, T.M.; Teskey, R.O.; Waring, R.H.; Morikawa, Y. The water relations of true firs. In True Fir, Proceedings of the Biology and Management of True Fir in the Pacific Northwest Symposium; University of Washington: Seattle, WA, USA, 1982; pp. 85–92. [Google Scholar]
- Roberts, S.W.; Knoerr, K.R. Components of water potential estimated from xylem pressure measurements in five tree species. Oecologia 1877, 28, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Kurkela, T.; Jalkanen, R. Revealing past needle retention in Pinus spp. Scand. J. For. Res. 1990, 5, 481–485. [Google Scholar] [CrossRef]
- Ranney, R.; Skroch, W.A. Comparative drought resistance among six species of birch (Betula): Influence of mild water stress on water relations and leaf gas exchange. Tree Physiol. 1990, 8, 351–360. [Google Scholar] [CrossRef]
- Humphries, R.N.; Jordan, M.A.; Guarino, L. The effect of water stress on the mortality of Betula pendula Roth. and Buddleia davidii Franch seedlings. Plant Soil 1982, 64, 273–276. [Google Scholar] [CrossRef]
- Kowalkowski, A.; Hoffman, M.; Wecko, D. Root system development of cherries in gley soils. Roczniki Gleboznawcze XXIV 1973, 24, 299–315. [Google Scholar]
- Agyare, W.A. Soil Characterization and Modeling of Spatial Distribution of Saturated Hydraulic Conductivity at Two Sites in the Volta Basin of Ghana; Cuvillier Verlag: Göttingen, Germany, 2004. [Google Scholar]
- Danielewicz, W.; Wiatrowska, B. Inwazyjne gatunki drzew i krzewów w lasach Polski. Peckiana 2014, 9, 59–67. (In Polish) [Google Scholar]
- Vivin, P.; Aussenac, G.; Levy, G. Differences in drought resistance among 3 deciduous oak speciesgrown in large boxes. Ann. Sci. For. 1993, 50, 221–233. [Google Scholar] [CrossRef]
- Jump, A.S.; Ruiz-Benito, P.; Greenwood, S.; Allen, C.D.; Kitzberger, T.; Fensham, R.; Lloret, F. Structural overshoot of tree growth with climate variability and the global spectrum of drought-induced forest dieback. Glob. Chang. Boil. 2017, 23, 3742–3757. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, J.; Hickler, T. Is drought-induced forest dieback globally increasing? J. Ecol. 2015, 103, 31–43. [Google Scholar] [CrossRef]
- Cater, M. Osmotic adaptation of Quercus robur L. under water stress in stands with different tree density—Relation with groundwater table. Dendrobiology 2011, 65, 29–36. [Google Scholar]
- Carreiras, J.; Pereira, J.; Pereira, J.S. Estimation of the canopy cover in evergreen oak woodlands using remote sensing. For. Ecol. Manag. 2006, 223, 45–53. [Google Scholar] [CrossRef]
- Ismail, R.; Mutanga, O.; Bob, U. Forest health and vitality: The detection and monitoring of Pinus patula trees infected by Sirex noctilio using digital multispectral imagery. South For. 2007, 69, 39–47. [Google Scholar] [CrossRef]
- Schwantes, A.M.; Swenson, J.J.; Jackson, R.B. Quantifying drought-induced tree mortality in the open canopy woodlands of central Texas. Remote Sens. Environ. 2016, 181, 54–64. [Google Scholar] [CrossRef]
- Horler, D.; Ahern, F. Forestry information content of thematic mapper data. Int. J. Remote Sens. 1986, 7, 405–428. [Google Scholar] [CrossRef]
- Collins, J.B.; Woodcock, C.E. An assessment of several linear change detection techniques for mapping forest mortality using multitemporal Landsat TM data. Remote Sens. Environ. 1999, 56, 66–77. [Google Scholar] [CrossRef]
- Vogelmann, J.; Rock, B. Assessing forest damage in high-elevation coniferous forests in Vermont and New Hampshire using thematic mapper data. Remote Sens. Environ. 1988, 24, 227–246. [Google Scholar] [CrossRef]

| ROI | Area (ha) | Soil Type | Tree Coverage (%) | Understory Coverage (%) | Properties of Dominant Species | Age of Tree Species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tree DBH (cm) | Tree height (m) | Stock (m3/ha) | Quercus robur | Picea abies (L.) H. Karst (P. abies) | Fagus sylvatica L. (F. sylvatica) | Carpinus betulus L. (C. betulus) | Quercus rubra | Larix sp. | Pinus sylvestris | Betula pendula | |||||
| DBB 126 | 14.9 | Gw | 100 | 60 | 42 | 33 | 509 | 126 | 126 | - | - | - | - | - | - |
| DBB 129 | 6.8 | RDbr | 80 | 70 | 46 | 32 | 550 | 129 | - | 129 | 101 | - | - | - | - |
| DBB 130a | 7.1 | RDbr | 100 | 50 | 43 | 31 | 586 | 130 | - | - | 130 | - | - | - | - |
| DBB 130b | 2.7 | RDbr | 100 | 40 | 44 | 31 | 466 | 130 | 130 | - | - | 130 | 130 | 130 | - |
| DBB 130c | 4.2 | RDbr | 100 | 70 | 42 | 31 | 411 | 130 | 66 | 66 | - | 130 | 130 | 130 | - |
| DBB 139 | 15.0 | BRk | 90 | 60 | 49 | 32 | 554 | 139 | 139 | - | 139 | - | 81 | 139 | - |
| DBB 140 | 3.1 | RDbr | 100 | 40 | 45 | 33 | 118 | 140 | 0 | 140 | - | 140 | 140 | - | - |
| DBB 83 | 6.7 | BRk | 100 | 50 | 27 | 25 | 346 | 83 | - | - | - | - | 83 | 83 | 83 |
| DBB 96 | 5.9 | RDbr | 90 | 20 | 35 | 30 | 390 | 96 | 51 | 130 | - | - | - | - | - |
| DBC 120 | 7.0 | RDbr | 70 | 10 | 47 | 32 | 411 | - | - | - | - | 120 | 120 | - | - |
| MD 50 | 1.7 | RDbr | 110 | 40 | 25 | 26 | 194 | - | - | - | 36 | - | 50 | 50 | - |
| MD 79 | 2.0 | RDbr | 110 | 80 | 40 | 34 | 509 | - | - | - | 79 | - | 79 | - | - |
| SO 100 | 2.6 | RDbr | 110 | 10 | 34 | 28 | 309 | 100 | 26 | - | - | - | - | 100 | - |
| SO 105 | 4.5 | BRk | 90 | 30 | 39 | 28 | 386 | 105 | - | - | 105 | - | 105 | 105 | 105 |
| SO 27 | 3.7 | OGw | 110 | 20 | 11 | 14 | 152 | 27 | 27 | - | 27 | - | 27 | 27 | 27 |
| FDB Region | H (%) | Share of Particular Species in a Forest Division (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Quercus robur | Picea abies | Fagus sylvatica | Carpinus betulus | Quercus rubra | Larix sp. | Pinus sylvestris | Betula pendula | ||
| DBB 126 | 4.94 | 99.14 | 0.86 | - | - | - | - | - | - |
| DBB 129 | 8.06 | 98.64 | - | 0.91 | 0.45 | - | - | - | - |
| DBB 130a | 6.39 | 98.83 | - | - | 1.17 | - | - | - | - |
| DBB 130b | 82.94 | 72.86 | 1.18 | - | - | 17.70 | 1.18 | 7.08 | - |
| DBB 130c | 86.03 | 74.40 | 0.88 | 3.06 | - | 11.16 | 10.28 | 0.22 | - |
| DBB 139 | 7.61 | 98.89 | 0.27 | - | 0.37 | - | 0.32 | 0.16 | - |
| DBB 140 | 53.30 | 87.23 | 3.19 | 6.91 | - | 0.53 | 1.60 | - | - |
| DBB 83 | 68.61 | 74.33 | - | - | - | - | 2.42 | 22.40 | 0.85 |
| DBB 96 | 25.53 | 93.54 | 5.17 | 0.90 | - | - | - | - | - |
| DBC 120 | 22.78 | - | - | - | - | 93.97 | 6.03 | - | - |
| MD 50 | 88.24 | - | - | - | 8.06 | - | 58.60 | 33.33 | - |
| MD 79 | 25.89 | - | - | - | 7.20 | - | 92.80 | - | - |
| SO 100 | 71.25 | 21.65 | 5.15 | - | - | - | - | 73.20 | - |
| SO 105 | 53.93 | 14.47 | - | - | 1.28 | - | 0.18 | 83.15 | 0.92 |
| SO 27 | 48.13 | 0.42 | 0.72 | - | 0.55 | - | 0.12 | 85.95 | 12.23 |
| FDB id | ΔEVI | ΔMSAVI | ΔNBR | ΔNBR2 | ΔNDMI | ΔNDVI | ΔSAVI | ΔGNDVI | ΔARVI | ΔBNDVI | MEAN | Standard Deviation (σ) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBB 126 | −0.91 | −0.92 | −0.94 | −0.79 | −0.92 | −0.93 | −0.91 | −0.83 | −0.89 | −0.99 | −0.90 | 0.06 |
| DBB 129 | −0.74 | −0.76 | −0.76 | −0.73 | −0.73 | −0.81 | −0.77 | −0.67 | −0.80 | −0.78 | −0.76 | 0.04 |
| DBB 130a | −0.54 | −0.55 | −0.34 | −0.29 | −0.32 | −0.54 | −0.54 | −0.33 | −0.47 | −0.66 | −0.46 | 0.13 |
| DBB 130b | 0.67 | 0.66 | 0.72 | 0.80 | 0.59 | 0.36 | 0.62 | 0.63 | 0.42 | 0.28 | 0.58 | 0.17 |
| DBB 130c | 0.30 | 0.27 | −0.28 | −0.08 | −0.37 | −0.05 | 0.31 | 0.20 | −0.08 | 0.02 | 0.02 | 0.24 |
| DBB 139 | −0.69 | −0.71 | −0.46 | −0.58 | −0.45 | −0.39 | −0.65 | −0.65 | −0.44 | −0.30 | −0.53 | 0.14 |
| DBB 140 | 0.16 | 0.13 | 0.34 | 0.50 | 0.13 | 0.42 | 0.20 | 0.46 | 0.42 | 0.41 | 0.32 | 0.15 |
| DBB 83 | 0.70 | 0.70 | 0.22 | 0.60 | 0.13 | 0.53 | 0.72 | 1.03 | 0.52 | 0.57 | 0.57 | 0.26 |
| DBB 96 | −0.86 | −0.87 | −0.69 | −0.72 | −0.68 | −0.76 | −0.86 | −0.78 | −0.75 | −0.79 | −0.78 | 0.07 |
| DBC 120 | 0.70 | 0.72 | −0.10 | −0.06 | −0.15 | −0.23 | 0.62 | 0.03 | −0.25 | −0.12 | 0.11 | 0.40 |
| MD 50 | 1.29 | 1.28 | 1.64 | 1.45 | 1.54 | 1.52 | 1.31 | 1.01 | 1.52 | 1.42 | 1.40 | 0.18 |
| MD 79 | −0.44 | −0.36 | −0.20 | −0.43 | −0.25 | −0.20 | −0.48 | −0.80 | −0.23 | −0.25 | −0.36 | 0.18 |
| SO 100 | 0.20 | 0.30 | −0.10 | −0.44 | 0.02 | 0.01 | 0.20 | 0.07 | −0.08 | 0.21 | 0.04 | 0.22 |
| SO 105 | 0.42 | 0.42 | 0.98 | 0.64 | 1.00 | 0.86 | 0.41 | 0.28 | 0.89 | 0.70 | 0.66 | 0.26 |
| SO 27 | 1.70 | 1.74 | 1.46 | 1.41 | 1.59 | 1.46 | 1.69 | 1.24 | 1.45 | 1.49 | 1.52 | 0.16 |
| MEAN | 0.13 | 0.14 | 0.10 | 0.09 | 0.08 | 0.08 | 0.12 | 0.06 | 0.08 | 0.08 | - | - |
| σ | 0.81 | 0.81 | 0.79 | 0.76 | 0.78 | 0.77 | 0.81 | 0.72 | 0.77 | 0.76 | - | - |
| ΔEVI | ΔMSAVI | ΔNBR | ΔNBR2 | ΔNDMI | ΔNDVI | ΔSAVI | ΔGNDVI | ΔARVI | ΔBNDVI | MEAN | σ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | 0.67 * | 0.66 * | 0.61 * | 0.64 * | 0.57 * | 0.66 * | 0.68 * | 0.73 ** | 0.65 * | 0.68 * | 0.66 | 0.042 |
| Q. robur | −0.67 * | −0.69 ** | −0.46 * | −0.15 | −0.66 * | −0.63 * | −0.65 * | −0.42 | −0.61 * | −0.64 * | −0.61 | 0.083 |
| P. abies | −0.1 | −0.1 | −0.1 | −0.2 | −0.1 | −0.1 | −0.1 | −0.1 | −0.1 | −0.1 | −0.17 | 0.034 |
| F. sylvatica | −0.0 | −0.0 | −0.0 | 0.04 | −0.1 | 0.01 | −0.0 | 0.09 | 0.01 | 0.02 | −0.01 | 0.061 |
| C. betulus | 0.17 | 0.18 | 0.38 | 0.27 | 0.36 | 0.35 | 0.17 | 0.05 | 0.35 | 0.31 | 0.26 | 0.112 |
| Q. rubra | 0.23 | 0.23 | −0.0 | −0.0 | −0.0 | −0.0 | 0.20 | 0.03 | −0.1 | −0.0 | 0.03 | 0.140 |
| Larix sp. | 0.07 | 0.08 | 0.19 | 0.11 | 0.17 | 0.19 | 0.06 | −0.0 | 0.18 | 0.16 | 0.12 | 0.083 |
| P. sylvestris | 0.60 * | 0.62 * | 0.65 * | 0.53 * | 0.72 ** | 0.67 * | 0.61 * | 0.54 * | 0.66 * | 0.69 ** | 0.63 | 0.061 |
| Tilia sp. | 0.01 | −0.0 | 0.08 | 0.14 | 0.01 | 0.12 | 0.02 | 0.15 | 0.12 | 0.12 | 0.08 | 0.061 |
| B. pendula | 0.55 * | 0.56 * | 0.50 | 0.50 | 0.56 * | 0.52 * | 0.56 * | 0.48 | 0.52 * | 0.54 * | 0.53 | 0.029 |
| Pseudotsuga menziesii Carrière | −0.34 | −0.34 | −0.28 | −0.29 | −0.27 | −0.30 | −0.34 | −0.32 | −0.30 | −0.32 | −0.31 | 0.027 |
| Tree coverage (%) | 0.29 | 0.30 | 0.40 | 0.37 | 0.41 | 0.46 | 0.31 | 0.35 | 0.45 | 0.44 | 0.38 | 0.064 |
| Understory coverage (%) | −0.46 | −0.47 | −0.37 | −0.31 | −0.41 | −0.33 | −0.45 | −0.39 | −0.33 | −0.37 | −0.39 | 0.056 |
| Age (years) | −0.62 * | −0.63 * | −0.64 * | −0.58 * | −0.68 ** | −0.67 * | −0.62 * | −0.51 * | −0.66 * | −0.67 * | −0.63 | 0.052 |
| Tree DBH (cm) | −0.66 * | −0.67 * | −0.64 * | −0.63 * | −0.68 ** | −0.7 ** | −0.67 * | −0.65 * | −0.69 ** | −0.7 ** | −0.67 | 0.025 |
| Tree height (m) | −0.72 ** | −0.73 ** | −0.67 * | −0.67 * | −0.73 ** | −0.72 ** | −0.73 ** | −0.71 ** | −0.71 ** | −0.73 ** | −0.71 | 0.023 |
| Tree stock (m3/ha) | −0.71 ** | −0.71 ** | −0.69 ** | −0.70 ** | −0.67 * | −0.76 ** | −0.72 ** | −0.74 ** | −0.74 ** | −0.78 ** | −0.72 | 0.033 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotlarz, J.; Nasiłowska, S.A.; Rotchimmel, K.; Kubiak, K.; Kacprzak, M. Species Diversity of Oak Stands and Its Significance for Drought Resistance. Forests 2018, 9, 126. https://doi.org/10.3390/f9030126
Kotlarz J, Nasiłowska SA, Rotchimmel K, Kubiak K, Kacprzak M. Species Diversity of Oak Stands and Its Significance for Drought Resistance. Forests. 2018; 9(3):126. https://doi.org/10.3390/f9030126
Chicago/Turabian StyleKotlarz, Jan, Sylwia A. Nasiłowska, Karol Rotchimmel, Katarzyna Kubiak, and Mariusz Kacprzak. 2018. "Species Diversity of Oak Stands and Its Significance for Drought Resistance" Forests 9, no. 3: 126. https://doi.org/10.3390/f9030126
APA StyleKotlarz, J., Nasiłowska, S. A., Rotchimmel, K., Kubiak, K., & Kacprzak, M. (2018). Species Diversity of Oak Stands and Its Significance for Drought Resistance. Forests, 9(3), 126. https://doi.org/10.3390/f9030126





