Neonatal Nutrition Predicts Energy Balance in Young Adults Born Preterm at Very Low Birth Weight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Analyses
3. Results
3.1. Regression Analysis
3.2. Path Analysis
3.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix A1. Statistical Methods—Description of the Path Analysis
Appendix A2. Missing Data
Appendix A3. Model Fit Indices
References
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization. Obesity and Overweight. Version Current 1.1.2015. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 3 July 2016).
- Bouchard, C.; Tremblay, A.; Despres, J.P.; Nadeau, A.; Lupien, P.J.; Theriault, G.; Dussault, J.; Moorjani, S.; Pinault, S.; Fournier, G. The response to long-term overfeeding in identical twins. N. Engl. J. Med. 1990, 322, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, M.; Thearle, M.S.; Ibrahim, M.; Hohenadel, M.G.; Bogardus, C.; Krakoff, J.; Votruba, S.B. A Human Thrifty Phenotype Associated with Less Weight Loss During Caloric Restriction. Diabetes 2015, 64, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.M.; Gray, C.; Li, M.; Segovia, S.A.; Vickers, M.H. Early Life Nutrition and Energy Balance Disorders in Offspring in Later Life. Nutrients 2015, 7, 8090–8111. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A. Programming by early nutrition: An experimental approach. J. Nutr. 1998, 128, 401S–406S. [Google Scholar] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Hovi, P.; Andersson, S.; Eriksson, J.G.; Järvenpää, A.L.; Strang-Karlsson, S.; Mäkitie, O.; Kajantie, E. Glucose regulation in young adults with very low birth weight. N. Engl. J. Med. 2007, 356, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Kajantie, E.; Hovi, P. Is very preterm birth a risk factor for adult cardiometabolic disease? Semin. Fetal Neonatal Med. 2014, 19, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Sipola-Leppanen, M.; Vaarasmaki, M.; Tikanmaki, M.; Matinolli, H.M.; Miettola, S.; Hovi, P.; Wehkalampi, K.; Ruokonen, A.; Sundvall, J.; Pouta, A.; et al. Cardiometabolic risk factors in young adults who were born preterm. Am. J. Epidemiol. 2015, 181, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Hovi, P.; Vohr, B.; Ment, L.; Doyle, L.W.; McGarvey, L.; Morrison, K.M.; Evensen, K.A.; van der Pal, S.; Grunau, R.E.; APIC Adults Born Preterm International Collaboration; et al. Blood pressure in young adults born at very low birth weight: Adults born preterm international collaboration. Hypertension 2016, 68, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.; Ramsingh, L.; Gunn, E.; Streiner, D.; Van Lieshout, R.; Boyle, M.; Gerstein, H.; Schmidt, L.; Saigal, S. Cardiometabolic health in adults born premature with extremely low birth weight. Pediatrics 2016, 138, e20160515. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, L.; Ong, K.; Dunger, D.B.; de Zegher, F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J. Clin. Endocrinol. Metab. 2006, 91, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Fabricius-Bjerre, S.; Jensen, R.B.; Faerch, K.; Larsen, T.; Molgaard, C.; Michaelsen, K.F.; Vaag, A.; Greisen, G. Impact of birth weight and early infant weight gain on insulin resistance and associated cardiovascular risk factors in adolescence. PLoS ONE 2011, 6, e20595. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, G.F.; Willemsen, R.H.; Leunissen, R.W.; Breukhoven, P.E.; Hokken-Koelega, A.C. Health profile of young adults born preterm: Negative effects of rapid weight gain in early life. J. Clin. Endocrinol. Metab. 2012, 97, 4498–4506. [Google Scholar] [CrossRef] [PubMed]
- Adair, L.S.; Cole, T.J. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension 2003, 41, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Dalle Molle, R.; Bischoff, A.R.; Portella, A.K.; Silveira, P.P. The fetal programming of food preferences: Current clinical and experimental evidence. J. Dev. Orig. Health Dis. 2015, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Valverde, D.; Rodriguez-Rodriguez, P.; Gutierrez-Arzapalo, P.Y.; Lopez de Pablo, A.L.; Carmen Gonzalez, M.; Lopez-Gimenez, R.; Somoza, B.; Arribas, S.M. Effect of fetal undernutrition and postnatal overfeeding on rat adipose tissue and organ growth at early stages of postnatal development. Physiol. Res. 2015, 64, 547–559. [Google Scholar] [PubMed]
- Arenz, S.; Ruckerl, R.; Koletzko, B.; von Kries, R. Breast-feeding and childhood obesity—A systematic review. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Portella, A.K.; Silveira, P.P. Neurobehavioral determinants of nutritional security in fetal growth-restricted individuals. Ann. N. Y. Acad. Sci. 2014, 1331, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: Follow-up of a randomized trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Lussana, F.; Painter, R.C.; Ocke, M.C.; Buller, H.R.; Bossuyt, P.M.; Roseboom, T.J. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. Am. J. Clin. Nutr. 2008, 88, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Perälä, M.M.; Männistö, S.; Kaartinen, N.E.; Kajantie, E.; Osmond, C.; Barker, D.J.; Valsta, L.M.; Eriksson, J.G. Body size at birth is associated with food and nutrient intake in adulthood. PLoS ONE 2012, 7, e46139. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.A.; Portella, A.K.; Silveira, P.P.; Bettiol, H.; Agranonik, M.; Silva, A.A.; Goldani, M.Z. Severe intrauterine growth restriction is associated with higher spontaneous carbohydrate intake in young women. Pediatr. Res. 2009, 65, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Kennedy, K.; Lanigan, J.; Fewtrell, M.; Cole, T.J.; Stephenson, T.; Elias-Jones, A.; Weaver, L.T.; Ibhanesebhor, S.; MacDonald, P.D.; et al. Nutrition in infancy and long-term risk of obesity: Evidence from 2 randomized controlled trials. Am. J. Clin. Nutr. 2010, 92, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Auser, H.; Sherar, L.B.; Erlandson, M.C.; Baxter-Jones, A.D.; Jackowski, S.A.; Arnold, C.; Sankaran, K. Influence of nutrition provision during the first two weeks of life in premature infants on adolescent body composition and blood pressure. Chin. J. Contemp. Pediatr. 2013, 15, 161–170. [Google Scholar]
- Regan, F.M.; Cutfield, W.S.; Jefferies, C.; Robinson, E.; Hofman, P.L. The impact of early nutrition in premature infants on later childhood insulin sensitivity and growth. Pediatrics 2006, 118, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Matinolli, H.M.; Hovi, P.; Männistö, S.; Sipola-Leppänen, M.; Eriksson, J.G.; Mäkitie, O.; Järvenpää, A.; Andersson, S.; Kajantie, E. Early Protein Intake Is Associated with Body Composition and Resting Energy Expenditure in Young Adults Born with Very Low Birth Weight. J. Nutr. 2015, 145, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Pihkala, J.; Hakala, T.; Voutilainen, P.; Raivio, K. Characteristic of recent fetal growth curves in Finland. Duodecim 1989, 105, 1540–1546. (In Finnish) [Google Scholar] [PubMed]
- Kaseva, N.; Wehkalampi, K.; Hemio, K.; Hovi, P.; Järvenpää, A.L.; Andersson, S.; Eriksson, J.G.; Lindström, J.; Kajantie, E. Diet and nutrient intake in young adults born preterm at very low birth weight. J. Pediatr. 2013, 163, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [PubMed]
- Haapa, E.; Toponen, T.; Pietinen, P.; Räsänen, L. Picture Booklet of Food Portions (Annoskuvakirja); National Public Health Institute: Helsinki, Finland, 1985. [Google Scholar]
- Reinivuo, H.; Hirvonen, T.; Ovaskainen, M.L.; Korhonen, T.; Valsta, L.M. Dietary survey methodology of FINDIET 2007 with a risk assessment perspective. Public Health Nutr. 2010, 13, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Hovi, P.; Andersson, S.; Järvenpää, A.L.; Eriksson, J.G.; Strang-Karlsson, S.; Kajantie, E.; Mäkitie, O. Decreased bone mineral density in adults born with very low birth weight: A cohort study. PLoS Med. 2009, 6, e1000135. [Google Scholar] [CrossRef] [PubMed]
- Sipola-Leppänen, M.; Hovi, P.; Andersson, S.; Wehkalampi, K.; Vääräsmäki, M.; Strang-Karlsson, S.; Järvenpää, A.L.; Mäkitie, O.; Eriksson, J.G.; Kajantie, E. Resting energy expenditure in young adults born preterm--the Helsinki study of very low birth weight adults. PLoS ONE 2011, 6, e17700. [Google Scholar] [CrossRef] [PubMed]
- Kajantie, E.; Strang-Karlsson, S.; Hovi, P.; Räikkönen, K.; Pesonen, A.K.; Heinonen, K.; Järvenpää, A.L.; Eriksson, J.G.; Andersson, S. Adults born at very low birth weight exercise less than their peers born at term. J. Pediatr. 2010, 157, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am. J. Obstet. Gynecol. 2000, 183, S1–S22.
- Northway, W.H., Jr.; Rosan, R.C.; Porter, D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease—Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, D.P.; Lockwood, C.M.; William, S.J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivar. Behav. Res. 2004, 39, 99–128. [Google Scholar] [CrossRef] [PubMed]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide; Muthén & Muthén: Los Angeles, CA, USA, 2007. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 14; StataCorp LP: College Station, TX, USA, 2015. [Google Scholar]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral Nutrient Supply for Preterm Infants: Commentary from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.G.; Osmond, C.; Kajantie, E.; Forsen, T.J.; Barker, D.J. Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia 2006, 49, 2853–2858. [Google Scholar] [CrossRef] [PubMed]
- Fall, C.H.; Sachdev, H.S.; Osmond, C.; Lakshmy, R.; Biswas, S.D.; Prabhakaran, D.; Tandon, N.; Ramji, S.; Reddy, K.S.; Barker, D.J.; et al. Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of BMI gain during infancy: Data from the New Delhi Birth Cohort. Diabetes Care 2008, 31, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Sachdev, H.; Fall, C.; Osmond, C.; Lakshmy, R.; Barker, D.J.; Biswas, S.K.; Ramji, S.; Prabhakaran, D.; Reddy, K.S. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N. Engl. J. Med. 2004, 350, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Tzoulaki, I.; Sovio, U.; Pillas, D.; Hartikainen, A.L.; Pouta, A.; Laitinen, J.; Tammelin, T.H.; Jarvelin, M.R.; Elliott, P. Relation of immediate postnatal growth with obesity and related metabolic risk factors in adulthood: The northern finland birth cohort 1966 study. Am. J. Epidemiol. 2010, 171, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Mericq, V.; Ong, K.K.; Bazaes, R.; Peña, V.; Avila, A.; Salazar, T.; Soto, N.; Iñiguez, G.; Dunger, D.B. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia 2005, 48, 2609–2614. [Google Scholar] [CrossRef] [PubMed]
- Sammallahti, S.; Pyhälä, R.; Lahti, M.; Lahti, J.; Pesonen, A.K.; Heinonen, K.; Hovi, P.; Eriksson, J.G.; Strang-Karlsson, S.; Andersson, S.; et al. Infant growth after preterm birth and neurocognitive abilities in young adulthood. J. Pediatr. 2014, 165, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Pyhälä, R.; Hovi, P.; Lahti, M.; Sammallahti, S.; Lahti, J.; Heinonen, K.; Pesonen, A.K.; Strang-Karlsson, S.; Eriksson, J.G.; Andersson, S.; et al. Very low birth weight, infant growth, and autism-spectrum traits in adulthood. Pediatrics 2014, 134, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, M.; Duffy, V.B.; Miller, R.J.; Winchester, S.B.; Huedo-Medina, T.B.; Sullivan, M.C. Dietary behaviors of adults born prematurely may explain future risk for cardiovascular disease. Appetite 2016, 99, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.P.; Raubenheimer, D.; Badaloo, A.V.; Gluckman, P.D.; Martinez, C.; Gosby, A.; Simpson, S.J.; Osmond, C.; Boyne, M.S.; Forrester, T.E. Developmental contributions to macronutrient selection: A randomized controlled trial in adult survivors of malnutrition. Evol. Med. Public Health 2016, 1, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.G.; Desai, M. Developmental programming of appetite/satiety. Ann. Nutr. Metab. 2014, 64 (Suppl. 1), 36–44. [Google Scholar] [CrossRef] [PubMed]
- Fulton, S.; Pissios, P.; Manchon, R.P.; Stiles, L.; Frank, L.; Pothos, E.N.; Maratos-Flier, E.; Flier, J.S. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 2006, 51, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.B.; Dalle Molle, R.; Desai, M.; Ross, M.G.; Silveira, P.P. Increased palatable food intake and response to food cues in intrauterine growth-restricted rats are related to tyrosine hydroxylase content in the orbitofrontal cortex and nucleus accumbens. Behav. Brain Res. 2015, 28, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Dalle Molle, R.; Laureano, D.P.; Alves, M.B.; Reis, T.M.; Desai, M.; Ross, M.G.; Silveira, P.P. Intrauterine growth restriction increases the preference for palatable foods and affects sensitivity to food rewards in male and female adult rats. Brain Res. 2015, 1618, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Beeler, J.A.; Faust, R.P.; Turkson, S.; Ye, H.; Zhuang, X. Low Dopamine D2 Receptor Increases Vulnerability to Obesity Via Reduced Physical Activity, Not Increased Appetitive Motivation. Biol. Psychiatry 2016, 79, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Ovaskainen, M.; Paturi, M.; Reinivuo, H.; Hannila, M.; Sinkko, H.; Lehtisalo, J.; Pynnönen-Polari, O.; Männistö, S. Accuracy in the Estimation of Food Servings Against the Portions in Food Photographs. Eur. J. Clin. Nutr. 2008, 62, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Royston, P. Multiple imputation of missing values. STATA J. 2004, 4, 227–241. [Google Scholar]
- Hu, L.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. 1999, 6, 1–55. [Google Scholar] [CrossRef]
| Characteristic | n (%)/Mean ± SD/Mean (25th, 75th Percentiles) | n | |
|---|---|---|---|
| Sex, women | 68 (59.1%) | 115 | |
| BIRTH CHARACTERISTICS | |||
| Birth weight, g | 1113 ± 217 | 115 | |
| Gestational age, week | 29.0 ± 2.1 | 115 | |
| Birth weight SD score | −1.17 ± 1.53 | 115 | |
| Maternal smoking during pregnancy | 22 (19.1) | 115 | |
| Maternal pre-eclampsia | 21 (18.3) | 115 | |
| NEONATAL CHARACTERISTICS | |||
| Neonatal sepsis | 8 (7.1) | 112 | |
| Bronchopulmonary dysplasia | 22 (19.1) | 115 | |
| Indomethasin treatment or PDA operation | 33 (29.2) | 113 | |
| Mechanical ventilation, days | 12.5 (0,17) | 113 | |
| NEONATAL NUTRITION AND GROWTH | |||
| Weight SD score at 3 weeks | −3.22 ± 0.86 | 111 | |
| Weight SD score at 6 weeks | −3.42 ± 0.85 | 103 | |
| Weight SD score at 9 weeks | −3.37 ± 0.87 | 74 | |
| Mean energy intake 1st to 3rd weeks, kcal/kg/day | 90.4 ± 10.6 | 115 | |
| Mean energy intake 4th to 6th weeks, kcal/kg/day | 118.8 ± 15.0 | 112 | |
| Mean energy intake 7th to 9th weeks, kcal/kg/day | 124 ± 13 | 102 | |
| Mean protein intake 1st to 3rd weeks, g/kg/day | 1.4 ± 0.4 | 115 | |
| Mean protein intake 4th to 6th weeks, g/kg/day | 1.9 ± 0.4 | 112 | |
| Mean protein intake 7th to 9th weeks, g/kg/day | 2.1 ± 0.5 | 102 | |
| Mean fat intake 1st to 3rd weeks, g/kg/day | 4.3 ± 1.1 | 115 | |
| Mean fat intake 4th to 6th weeks, g/kg/day | 5.9 ± 1.0 | 112 | |
| Mean fat intake 7th to 9th weeks, g/kg/day | 6.1 ± 0.9 | 102 | |
| Mean carbohydrate intake 1st to 3rd weeks, g/kg/day | 11.1 ± 1.3 | 115 | |
| Mean carbohydrate intake 4th to 6th weeks, g/kg/day | 12.4 ± 1.3 | 112 | |
| Mean carbohydrate intake 7th to 9th weeks, g/kg/day | 12.9 ± 1.3 | 102 | |
| Term weight SD score | −2.93 ± 0.93 | 104 | |
| CURRENT CHARACTERISTICS | |||
| Highest parental education | |||
| Elementary | 11 (9.7) | ||
| High School | 29 (25.7) | ||
| Intermediate | 41 (36.3) | ||
| University | 32 (28.3) | ||
| Age at clinical examination, year | 22.5 (2.1) | ||
| Daily smoking | 29 (25.7) | ||
| Height at clinical examination, cm | Women | 163.1 (7.1) | 68 |
| Men | 174.9 (7.4) | 47 | |
| Weight at clinical examination, kg | Women | 59.1 (12.0) | 68 |
| Men | 65.9 (11.7) | 47 | |
| BMI, kg/m2 | Women | 22.2 (3.7) | 68 |
| Men | 21.5 (3.1) | 47 | |
| Lean body mass, kg | Women | 39.9 (5.5) | 68 |
| Men | 53.3 (7.7) | 47 | |
| Resting energy expenditure, kcal/24 h | Women | 1464 (214) | 56 |
| Men | 1817 (218) | 36 | |
| Total caloric intake, kcal | Women | 1597 (447) | 67 |
| Men | 2170 (555) | 42 | |
| Protein, E% | Women | 15.9 (4.0) | 67 |
| Men | 16.2 (4.1) | 42 | |
| Fat, E% | Women | 33.6 (7.0) | 67 |
| Men | 37.3 (6.9) | 42 | |
| Carbohydrate, E% | Women | 47.9 (7.3) | 67 |
| Men | 42.7 (6.6) | 42 |
| Predictors | Outcomes | |||||
|---|---|---|---|---|---|---|
| Relative Energy Intake (n = 84–107) | Relative REE (n = 55–90) | Physical Activity, MET Hours (n = 78–122) | ||||
| Unstandardized Regression Coefficient (95% CI) | p | Unstandardized Regression Coefficient (95% CI) | p | Unstandardized Regression Coefficient (95% CI) | p | |
| Energy expenditure, 10 kcal/24 h | −0.06 (−0.15 to 0.02) | 0.19 | −0.02 (−0.05 to 0.01) | 0.10 | 0.12 (−0.00 to 0.24) | 0.29 |
| Birth weight SD score | −0.64 (−2.01 to 0.74) | 0.36 | −0.4 (−0.88 to 0.08) | 0.12 | −0.75 (−2.63 to 1.14) | 0.50 |
| Mean energy intake 1st to 3rd weeks, kcal | −1.34 (−2.62 to −0.06) | 0.04 | −0.51 (−0.96 to −0.05) | 0.02 | 0.67 (−1.16 to 2.51) | 0.96 |
| Mean protein intake 1st to 3rd weeks, g | −5.12 (−10.72 to 0.49) | 0.07 | −2.44 (−4.40 to −0.49) | 0.006 | 3.13 (−4.74 to 11.0) | 0.67 |
| Mean fat intake 1st to 3rd weeks, g | −1.75 (−3.55 to 0.05) | 0.06 | −0.67 (−1.33 to −0.02) | 0.04 | 0.97 (−1.61 to 3.56) | 0.99 |
| Mean carbohydrate intake 1st to 3rd weeks, g | −0.82 (−2.41 to 0.78) | 0.31 | −0.23 (−0.77 to 0.32) | 0.37 | 0.93 (−1.25 to 3.12) | 0.34 |
| Mean energy intake 4th to 6th weeks, kcal | −0.26 (−1.67 to 1.15) | 0.72 | −0.27 (−0.77 to 0.23) | 0.24 | −0.48 (−2.41 to 1.44) | 0.56 |
| Mean protein intake 4th to 6th weeks, g | −3.77 (−8.81 to 1.26) | 0.14 | −0.91 (−2.58 to 0.75) | 0.18 | −1.27 (−7.80 to 5.27) | 0.87 |
| Mean fat intake 4th to 6th weeks, g | −0.67 (−2.79 to 1.45) | 0.53 | −0.18 (−0.93 to 0.57) | 0.59 | −1.07 (−3.92 to 1.78) | 0.40 |
| Mean carbohydrate intake 4th to 6th weeks, g | 0.71 (−0.89 to 2.32) | 0.38 | −0.11 (−0.70 to 0.48) | 0.65 | −0.32 (−2.50 to 1.86) | 0.93 |
| Mean energy intake 7th to 9th weeks, kcal | 0.65 (−1.01 to 2.31) | 0.44 | 0.08 (−0.48 to 0.64) | 0.71 | −0.48 (−2.80 to 1.83) | 0.64 |
| Mean protein intake 7th to 9th weeks, g | −2.91 (−7.48 to 1.65) | 0.21 | −0.94 (−2.51 to 0.64) | 0.15 | 2.11 (−4.11 to 8.33) | 0.72 |
| Mean fat intake 7th to 9th weeks, g | 0.48 (−1.89 to 2.84) | 0.69 | 0.4 (−0.39 to 1.19) | 0.31 | −1.23 (−4.49 to 2.04) | 0.63 |
| Mean carbohydrate intake 7th to 9th weeks, g | 0.9 (−0.87 to 2.68) | 0.31 | −0.06 (−0.64 to 0.53) | 0.98 | 0.17 (−2.19 to 2.53) | 0.73 |
| Weight SD score at 3 weeks | −1.74 (−4.28 to 0.80) | 0.18 | −0.89 (−1.78 to 0.00) | 0.04 | −1.03 (−4.39 to 2.33) | 0.56 |
| Weight SD score at 6 weeks | −1.26 (−3.97 to 1.46) | 0.36 | −1.01 (−1.91 to −0.12) | 0.01 | −0.18 (−3.80 to 3.43) | 0.99 |
| Weight SD score at 9 weeks | −3.27 (−6.46 to −0.08) | 0.04 | −1.31 (−2.30 to −0.33) | 0.001 | −1.03 (−4.64 to 2.57) | 0.72 |
| Physical activity, MET/day | −0.07 (−0.21 to 0.07) | 0.47 | −0.01 (−0.05 to 0.04) | 0.94 | ||
| Relative MET | −2.67 (−8.6 to 3.26) | 0.74 | 1.55 (−0.51 to 6.61) | 0.06 | ||
| Relative REE | 0.29 (−0.35 to 0.94) | 0.41 | ||||
| Early Nutrition | Relative Energy Intake | Relative Resting Energy Expenditure | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 Path Coefficient (95% CI) | Model 2 Path Coefficient (95% CI) | Model 3 Path Coefficient (95% CI) | Model 4 Path Coefficient (95% CI) | Model 1 Path Coefficient (95% CI) | Model 2 Path Coefficient (95% CI) | Model 3 Path Coefficient (95% CI) | Model 4 Path Coefficient (95% CI) | |
| Mean energy intake (10 kcal/kg/day) | ||||||||
| birth to 3rd week | −1.4 (−2.9; −0.1) | −1.6 (−3.1; −0.2) | −1.7 (−3.1; −0.2) | −2.7 (−4.4; −1.0) | −0.50 (−0.94; −0.09) | −0.43 (−0.96; −0.02) | −0.40 (−0.92; −0.00) | −0.41 (−0.91; −0.00) |
| 4th to 6th week | −0.0 (−2.0; 2.0) | −0.2 (−2.3; 1.9) | −0.1 (−2.3; 1.9) | 0.1 (−2.1; 2.0) | −0.14 (−0.73; 0.46) | −0.11 (−0.72; 0.48) | −0.17 (−0.77; 0.44) | −0.16 (−0.77; 0.48) |
| 7th to 9th week | 0.8 (−1.5; 2.4) | 0.6 (−1.5; 2.4) | 0.6 (−1.6; 2.4) | 0.4 (−1.5; 2.2) | 0.06 (−0.49; 0.80) | 0.15 (−0.46; 0.87) | 0.25 (−0.40; 1.03) | 0.27 (−0.43; 1.05) |
| Mean protein intake (g/kg/day) | ||||||||
| birth to 3rd week | −4.7 (−12.4; 1.5) | −5.7 (−13.4; 1.1) | −5.7 (−13.5; 0.9) | −8.1 (−16.5; −0.3) | −2.81 (−4.73; −0.91) | −2.47 (−4.61; −0.60) | −2.48 (−4.59; −0.66) | −2.53 (−4.80; −0.73) |
| 4th to 6th week | 0.4 (−6.6; 8.9) | 1.0 (−6.2; 10.2) | 0.9 (−6.4; 10.2) | 1.6 (−6.1; 11.0) | 0.82 (−1.26; 2.83) | 0.60 (−1.50; 2.66) | 0.62 (−1.61; 2.74) | 0.73 (−1.40; 3.00) |
| 7th to 9th week | −2.9 (−8.2; 3.6) | −3.6 (−9.1; 3.0) | −3.5 (−9.1; 3.2) | −2.4 (−8.1; 4.3) | −0.49 (−2.29; 1.42) | −0.09 (−2.07; 1.92) | −0.13 (−2.19; 2.00) | −0.21 (−2.47; 1.99) |
| Mean fat intake (g/kg/day) | ||||||||
| birth to 3rd week | −1.8 (−4.1; 0.2) | −2.1 (−4.5; 0.0) | −2.1 (−4.5; 0.0) | −3.3 (−6.0; −0.8) | −0.85 (−1.52; −0.21) | −0.74 (−1.48; −0.15) | −0.71 (−1.46; −0.10) | −0.71 (−1.46; −0.08) |
| 4th to 6th week | −0.0 (−2.9; 3.4) | −0.1 (−3.0; 3.4) | −0.0 (−3.0; 3.4) | 0.3 (−2.9; 3.9) | 0.06 (−0.95; 0.99) | 0.09 (−0.92; 1.05) | −0.02 (−1.07; 0.96) | −0.02 (−1.12; 0.95) |
| 7th to 9th week | 0.6 (−2.4; 3.3) | 0.7 (−2.2; 3.5) | 0.6 (−2.2; 3.6) | 0.7 (−2.2; 4.0) | 0.22 (−0.63; 1.51) | 0.25 (−0.73; 1.42) | 0.39 (−0.59; 1.67) | 0.41 (−0.58; 1.62) |
| Early Nutrition | Relative Energy Intake * | Relative Energy Expenditure * | |||
|---|---|---|---|---|---|
| Nested Model ** | χ2 (df) | p-Value | χ2 (df) | p-Value | |
| Mean energy intake (10 kcal/kg/day) | 1 | 3.474 (3) | 0.32 | 3.661 (3) | 0.30 |
| 2 | 0.387 (1) | 0.53 | 0.813 (1) | 0.37 | |
| 3 | 1.398 (1) | 0.24 | 0.926 (1) | 0.34 | |
| 4 | 3.503 (1) | 0.06 | 0.000 (1) | 1.00 | |
| 5 | 13.88 (1) | <0.001 | 5.889 (1) | 0.02 | |
| Mean protein intake (g/kg/day) | 1 | 1.371 (3) | 0.71 | 0.942 (3) | 0.82 |
| 2 | 0.358 (1) | 0.55 | 0.820 (1) | 0.37 | |
| 3 | 1.416 (1) | 0.23 | 1.096 (1) | 0.30 | |
| 4 | 5.697 (1) | 0.02 | 0.000 (1) | 1.00 | |
| 5 | 7.521 (1) | 0.006 | 7.938 (1) | 0.004 | |
| Mean fat intake (g/kg/day) | 1 | 3.641 (3) | 0.30 | 3.703 (3) | 0.30 |
| 2 | 0.329 (1) | 0.57 | 0.744 (1) | 0.39 | |
| 3 | 1.050 (1) | 0.31 | 0.702 (1) | 0.40 | |
| 4 | 4.527 (1) | 0.03 | 0.000 (1) | 1.00 | |
| 5 | 10.54 (1) | 0.001 | 6.163 (1) | 0.01 | |
| Early Nutrition | Relative Energy Intake | ||
|---|---|---|---|
| Direct Effect: Estimate (95% CI) | Indirect Effect: Estimate (95% CI) | Total Effect: Estimate (95% CI) | |
| Mean energy intake (10 kcal/kg/day) | −2.14 (−3.77; −0.62) | −0.37 (−1.02; 0.03) | −2.51 (−3.92; −1.06) |
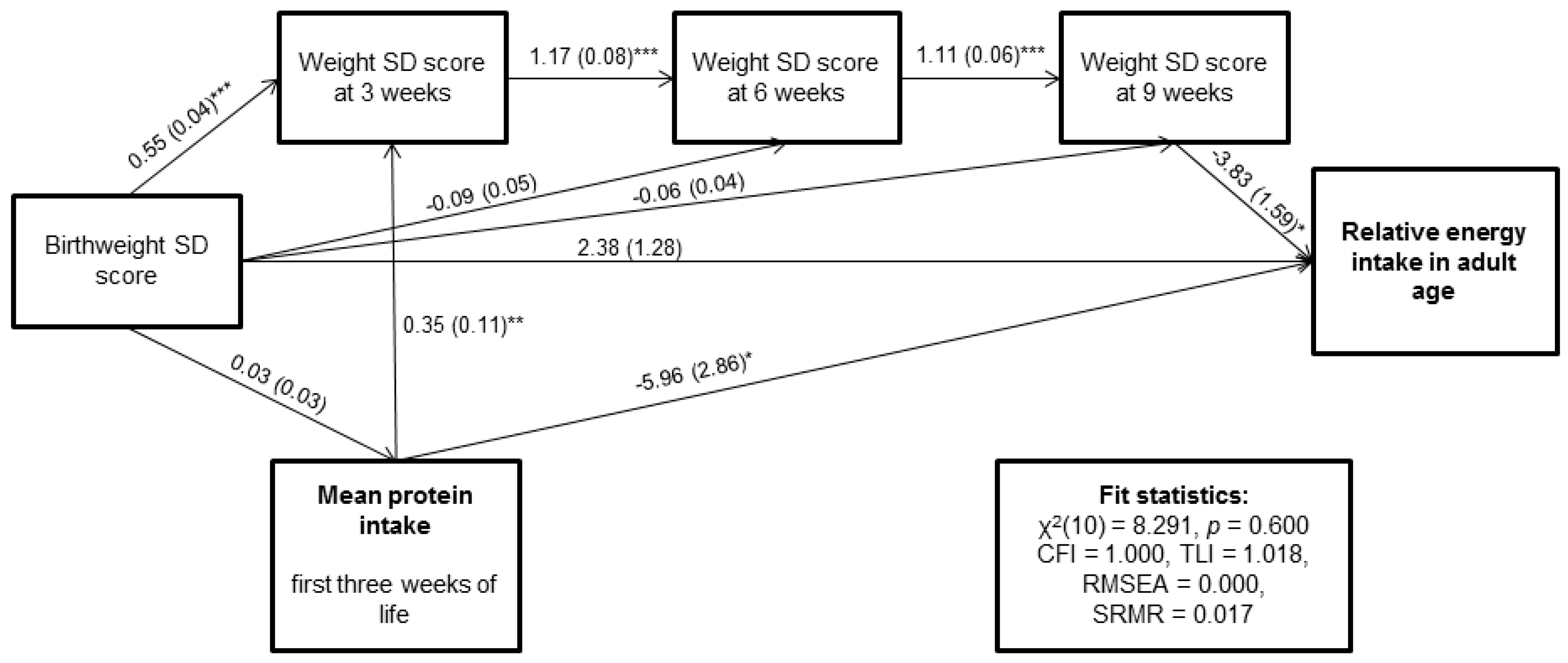
| Mean protein intake (g/kg/day) | −5.96 (−12.44; −0.59) | −1.73 ( −4.65; −0.09) | −7.68 (−13.60; −2.12) |
| Mean fat intake (g/kg/day) | −2.45 (−4.81; −0.33) | −0.54 (−1.36; 0.01) | −2.98 (−5.24; −0.81) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matinolli, H.-M.; Hovi, P.; Levälahti, E.; Kaseva, N.; Silveira, P.P.; Hemiö, K.; Järvenpää, A.-L.; Eriksson, J.G.; Andersson, S.; Lindström, J.; et al. Neonatal Nutrition Predicts Energy Balance in Young Adults Born Preterm at Very Low Birth Weight. Nutrients 2017, 9, 1282. https://doi.org/10.3390/nu9121282
Matinolli H-M, Hovi P, Levälahti E, Kaseva N, Silveira PP, Hemiö K, Järvenpää A-L, Eriksson JG, Andersson S, Lindström J, et al. Neonatal Nutrition Predicts Energy Balance in Young Adults Born Preterm at Very Low Birth Weight. Nutrients. 2017; 9(12):1282. https://doi.org/10.3390/nu9121282
Chicago/Turabian StyleMatinolli, Hanna-Maria, Petteri Hovi, Esko Levälahti, Nina Kaseva, Patricia P. Silveira, Katri Hemiö, Anna-Liisa Järvenpää, Johan G. Eriksson, Sture Andersson, Jaana Lindström, and et al. 2017. "Neonatal Nutrition Predicts Energy Balance in Young Adults Born Preterm at Very Low Birth Weight" Nutrients 9, no. 12: 1282. https://doi.org/10.3390/nu9121282




