Meat and Seafood Consumption in Relation to Plasma Metabolic Profiles in a Chinese Population: A Combined Untargeted and Targeted Metabolomics Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Diet Groups
2.2. Dietary Assessment
2.3. Plasma Collection and Pretreatment
2.4. Untargeted Analysis
2.5. Targeted Analysis
2.6. Statistical Analysis
3. Results
3.1. Participant and Blood Sample Characteristics
3.2. Differences of Plasma Metabolites among Four Dietary Groups
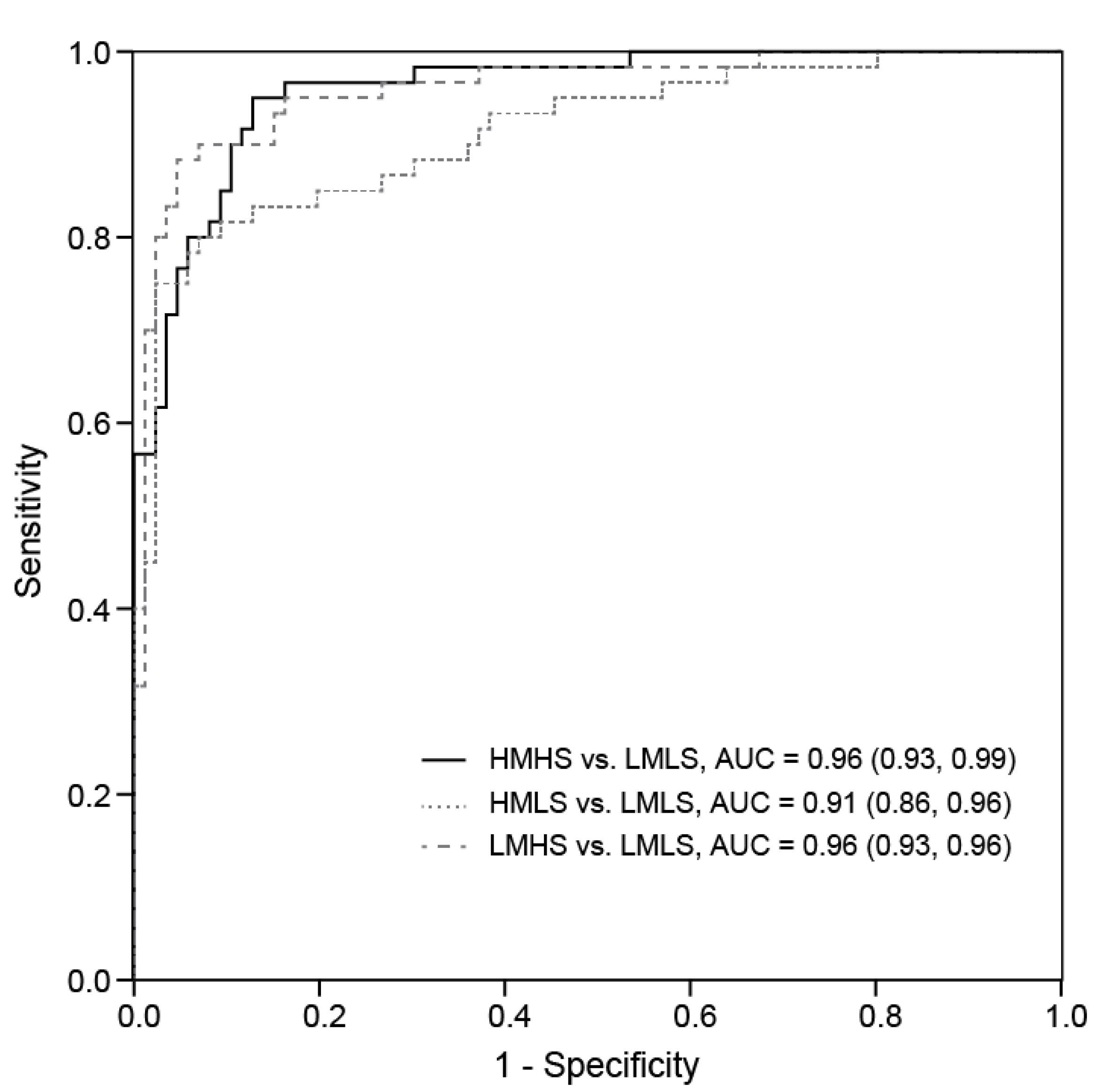
3.3. Associations between Dietary Consumption and Plasma Metabolites
3.4. Associations of Plasma Metabolites with Clinical Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maire, B.; Lioret, S.; Gartner, A.; Delpeuch, F. Nutritional transition and non-communicable diet-related chronic diseases in developing countries. Sante 2002, 12, 45–55. [Google Scholar] [PubMed]
- Burggraf, C.; Kuhn, L.; Zhao, Q.-R.; Teuber, R.; Glauben, T. Economic growth and nutrition transition: An empirical analysis comparing demand elasticities for foods in China and Russia. J. Integr. Agric. 2015, 14, 1008–1022. [Google Scholar] [CrossRef]
- Kim, S.; Moon, S.; Popkin, B.M. The nutrition transition in South Korea. Am. J. Clin. Nutr. 2000, 71, 44–53. [Google Scholar] [PubMed]
- Zhai, F.; Wang, H.; Du, S.; He, Y.; Wang, Z.; Ge, K.; Popkin, B.M. Prospective study on nutrition transition in China. Nutr. Rev. 2009, 67 (Suppl. 1), S56–S61. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.M.; Remais, J.; Fung, M.C.; Xu, L.; Sun, S.S. Food supply and food safety issues in China. Lancet 2013, 381, 2044–2053. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Sanchis-Gomar, F. Red meat consumption and ischemic heart disease. A systematic literature review. Meat. Sci. 2015, 108, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Abid, Z.; Cross, A.J.; Sinha, R. Meat, dairy, and cancer. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 386S–393S. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Bronner, L.; Willett, W.C.; Stampfer, M.J.; Rexrode, K.M.; Albert, C.M.; Hunter, D.; Manson, J.E. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 2002, 287, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Raatz, S.K.; Silverstein, J.T.; Jahns, L.; Picklo, M.J. Issues of fish consumption for cardiovascular disease risk reduction. Nutrients 2013, 5, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.S.; Sharp, S.J.; Luben, R.N.; Khaw, K.T.; Bingham, S.A.; Wareham, N.J.; Forouhi, N.G. Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes: The European prospective investigation of cancer (EPIC)-Norfolk cohort study. Diabetes Care 2009, 32, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Nkondjock, A.; Receveur, O. Fish-seafood consumption, obesity, and risk of type 2 diabetes: An ecological study. Diabetes Metab. 2003, 29, 635–642. [Google Scholar] [CrossRef]
- Van Woudenbergh, G.J.; van Ballegooijen, A.J.; Kuijsten, A.; Sijbrands, E.J.; van Rooij, F.J.; Geleijnse, J.M.; Hofman, A.; Witteman, J.C.; Feskens, E.J. Eating fish and risk of type 2 diabetes: A population-based, prospective follow-up study. Diabetes Care 2009, 32, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.J.; Mannan, N. Eating fish and risk of type 2 diabetes: A population-based, prospective follow-up study: Comment on van Woudenbergh et Al. Diabetes Care 2010, 33, e125. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Major, J.M.; Sinha, R. Urinary biomarkers of meat consumption. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.J.; Fave, G.; Beckmann, M.; Lin, W.; Tailliart, K.; Xie, L.; Mathers, J.C.; Draper, J. Use of mass spectrometry fingerprinting to identify urinary metabolites after consumption of specific foods. Am. J. Clin. Nutr. 2011, 94, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Krogholm, K.S.; Frederiksen, H.; Bugel, S.H.; Rasmussen, S.E. Urinary excretion of total isothiocyanates from cruciferous vegetables shows high dose-response relationship and may be a useful biomarker for isothiocyanate exposure. Eur. J. Nutr. 2007, 46, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.J.; Beckmann, M.; Fave, G.; Mathers, J.C.; Draper, J. Proline betaine and its biotransformation products in fasting urine samples are potential biomarkers of habitual citrus fruit consumption. Br. J. Nutr. 2011, 106, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Chan, S.Y.; Puddey, I.B.; Devine, A.; Wattanapenpaiboon, N.; Wahlqvist, M.L.; Lukito, W.; Burke, V.; Ward, N.C.; Prince, R.L.; et al. Phenolic acid metabolites as biomarkers for tea- and coffee-derived polyphenol exposure in human subjects. Br. J. Nutr. 2004, 91, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Rinaldi, S.; Ferrari, P.; Carayol, M.; Achaintre, D.; Scalbert, A.; Cross, A.J.; Gunter, M.J.; Fensom, G.K.; Appleby, P.N.; et al. Metabolic profiles of male meat eaters, fish eaters, vegetarians, and vegans from the EPIC-Oxford cohort. Am. J. Clin. Nutr. 2015, 102, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017, 105, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, S.; Cai, S.; Dong, J.; Li, X.; Chen, Z. Identification of biochemical changes in lactovegetarian urine using 1H NMR spectroscopy and pattern recognition. Anal. Bioanal. Chem. 2010, 396, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Hathwar, S.C.; Rai, A.K.; Modi, V.K.; Narayan, B. Characteristics and consumer acceptance of healthier meat and meat product formulations—A review. J. Food Sci. Technol. 2012, 49, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Mahanty, A.; Ganguly, S.; Sankar, T.V.; Chakraborty, K.; Rangasamy, A.; Paul, B.; Sarma, D.; Mathew, S.; Asha, K.K.; et al. Amino Acid compositions of 27 food fishes and their importance in clinical nutrition. J. Amino Acids 2014, 2014, 269797. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tai, E.S.; Heng, D.; Tan, C.E.; Low, L.P.; Lee, J. Risk factors associated with hypertension awareness, treatment, and control in a multi-ethnic Asian population. J. Hypertens. 2009, 27, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.P.; Lamoureux, E.L.; Cheung, C.Y.; Sabanayagam, C.; Wong, W.; Tai, E.S.; Lee, J.; Wong, T.Y. Racial differences in the prevalence of diabetes but not diabetic retinopathy in a multi-ethnic Asian population. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7586–7592. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, J.; van Dam, R.M.; Prem, K.; Hoong, J.Y.; Zou, L.; Lu, Y.; Ong, C.N. Dietary predictors and plasma concentrations of perfluorinated alkyl acids in a Singapore population. Chemosphere 2016, 171, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Report of the National Nutrition Survey. 2004. Available online: https://www.hpb.gov.sg/docs/default-source/default-document-library/national-nutrition-survey-2004.pdf?sfvrsn=82daeb72_0 (accessed on 1 January 2004).
- Deurenberg-Yap, M.; Li, T.; Tan, W.L.; van Staveren, W.A.; Deurenberg, P. Validation of a semiquantitative food frequency questionnaire for estimation of intakes of energy, fats and cholesterol among Singaporeans. Asia Pac. J. Clin. Nutr. 2000, 9, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zou, L.; Lin, Q.; Ong, C.N. Use of liquid chromatography/tandem mass spectrometry and online databases for identification of phosphocholines and lysophosphatidylcholines in human red blood cells. Rapid Commun. Mass Spectrom. 2009, 23, 3243–3254. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Tavintharan, S.; Sum, C.F.; Woon, K.; Lim, S.C.; Ong, C.N. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J. Clin. Endocrinol. Metab. 2013, 98, E1060–E1065. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Huang, C.; Gao, L.; Xu, Y.J.; Chia, S.E.; Chen, S.S.; Li, N.; Yu, K.K.; Ling, Q.X.; Cheng, Q.; et al. Identification of serum biomarkers associated with hepatitis B virus-related hepatocellular carcinoma and liver cirrhosis using mass-spectrometry-based metabolomics. Metabolomics 2015, 11, 1526–1538. [Google Scholar] [CrossRef]
- Lu, Y.; Li, N.; Gao, L.; Xu, Y.J.; Huang, C.; Yu, K.; Ling, Q.; Cheng, Q.; Chen, S.; Zhu, M.; et al. Acetylcarnitine Is a Candidate Diagnostic and Prognostic Biomarker of Hepatocellular Carcinoma. Cancer Res. 2016, 76, 2912–2920. [Google Scholar] [CrossRef] [PubMed]
- Gika, H.G.; Theodoridis, G.A.; Wingate, J.E.; Wilson, I.D. Within-day reproducibility of an HPLC-MS-based method for metabonomic analysis: Application to human urine. J. Proteome Res. 2007, 6, 3291–3303. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, J.; Li, X.; Chen, J.; Bai, G. Determination of free amino acids in isatidis radix by HILIC-UPLC-MS/MS. Bull. Korean Chem. Soc. 2014, 35, 197–203. [Google Scholar] [CrossRef]
- Xu, Y.J.; Ho, W.E.; Xu, F.; Wen, T.; Ong, C.N. Exploratory investigation reveals parallel alteration of plasma fatty acids and eicosanoids in coronary artery disease patients. Prostaglandins Other Lipid Mediat. 2013, 106, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, S.; Bobeldijk, I.; Verheij, E.R.; Ramaker, R.; Kochhar, S.; Macdonald, I.A.; van Ommen, B.; Smilde, A.K. Large-scale human metabolomics studies: A strategy for data (pre-) processing and validation. Anal. Chem. 2006, 78, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Smilde, A.K.; van der Werf, M.J.; Bijlsma, S.; van der Werff-van der Vat, B.J.; Jellema, R.H. Fusion of mass spectrometry-based metabolomics data. Anal. Chem. 2005, 77, 6729–6736. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar]
- Guertin, K.A.; Moore, S.C.; Sampson, J.N.; Huang, W.Y.; Xiao, Q.; Stolzenberg-Solomon, R.Z.; Sinha, R.; Cross, A.J. Metabolomics in nutritional epidemiology: Identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am. J. Clin. Nutr. 2014, 100, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Astorg, P.; Arnault, N.; Czernichow, S.; Noisette, N.; Galan, P.; Hercberg, S. Dietary intakes and food sources of n-6 and n-3 PUFA in French adult men and women. Lipids 2004, 39, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Poitout, V. CMPF: A Biomarker for Type 2 Diabetes Mellitus Progression? Trends Endocrinol. Metab. 2016, 27, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, F.; Smith, T.K. The Kennedy pathway—De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 2010, 62, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Farine, L.; Niemann, M.; Schneider, A.; Butikofer, P. Phosphatidylethanolamine and phosphatidylcholine biosynthesis by the Kennedy pathway occurs at different sites in Trypanosoma brucei. Sci. Rep. 2015, 5, 16787. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F. A comparative study on the composition and importance of free amino acids in semen of gilthead sea bream, Sparus aurata, and perch, Perca fluviatilis. Fish Physiol. Biochem. 2010, 36, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Rossary, A.; Farges, M.C.; Lamas, B.; Miles, E.A.; Noakes, P.S.; Kremmyda, L.S.; Vlachava, M.; Diaper, N.D.; Robinson, S.M.; et al. Increased consumption of salmon during pregnancy partly prevents the decline of some plasma essential amino acid concentrations in pregnant women. Clin. Nutr. 2014, 33, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Sun, Q.; Willett, W.C.; Eliassen, A.H.; Wu, K.; Pan, A.; Grodstein, F.; Hu, F.B. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am. J. Clin. Nutr. 2014, 99, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Zeimbekis, A.; Boutziouka, V.; Economou, M.; Kourlaba, G.; Toutouzas, P.; Polychronopoulos, E. Long-term fish intake is associated with better lipid profile, arterial blood pressure, and blood glucose levels in elderly people from Mediterranean islands (MEDIS epidemiological study). Med. Sci. Monit. 2007, 13, CR307–CR312. [Google Scholar] [PubMed]
- Wu, J.H.; Micha, R.; Imamura, F.; Pan, A.; Biggs, M.L.; Ajaz, O.; Djousse, L.; Hu, F.B.; Mozaffarian, D. Omega-3 fatty acids and incident type 2 diabetes: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107 (Suppl. 2), S214–S227. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, J.L.; Tuininga, A.R.; Ericsson, L.H. Identification of molecular species of glycerophospholipids and sphingomyelin using electrospray mass spectrometry. J. Lipid Res. 1994, 35, 1102–1114. [Google Scholar] [PubMed]
- Godzien, J.; Ciborowski, M.; Martinez-Alcazar, M.P.; Samczuk, P.; Kretowski, A.; Barbas, C. Rapid and Reliable Identification of Phospholipids for Untargeted Metabolomics with LC-ESI-QTOF-MS/MS. J. Proteome Res. 2015, 14, 3204–3216. [Google Scholar] [CrossRef] [PubMed]

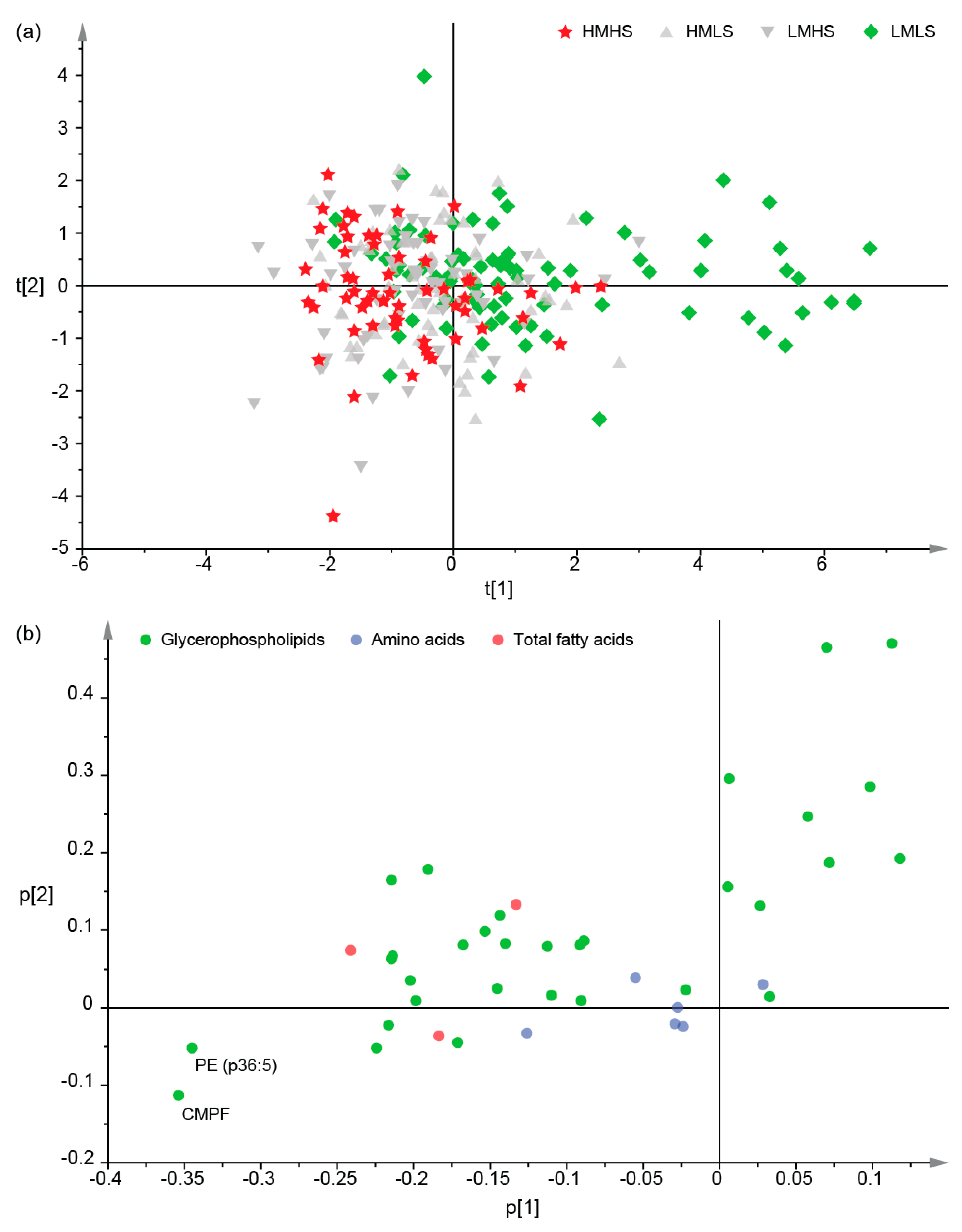
| Characteristics a | HMHS Consumers (n = 60) | HMLS Consumers (n = 64) | LMHS Consumers (n = 60) | LMLS Consumers (n = 86) | p b |
|---|---|---|---|---|---|
| Age (year) | 50.18 ± 12.43 c | 50.19 ± 13.37 | 53.03 ± 11.64 | 49.28 ± 11.29 | 0.310 |
| Sex n (%) | 0.735 | ||||
| Male | 33 (55.0%) | 29 (45.3%) | 31 (51.7%) | 42 (48.8%) | |
| Female | 27 (45.0%) | 35 (54.7%) | 29 (48.3%) | 44 (51.2%) | |
| Alcohol consumption status n (%) | 0.051 | ||||
| Abstainer | 40 (66.7%) | 47 (73.4%) | 30 (50.0%) | 53 (61.6%) | |
| Consumer | 20 (33.3%) | 17 (26.6%) | 30 (50.0%) | 33 (38.4%) | |
| BMI (kg/m2) | 23.41 ± 3.88 | 23.17 ± 3.62 | 23.83 ± 4.15 | 22.57 ± 3.82 | 0.255 |
| Fasting glucose (mmol/L) | 5.14 ± 1.32 * | 5.07 ± 1.48 | 5.27 ± 1.36 * | 4.71 ± 0.58 | 0.036 |
| Fasting insulin (mU/L) | 7.55 ± 4.10 | 7.51 ± 6.15 | 8.11 ± 7.16 | 7.65 ± 13.64 | 0.982 |
| Creatinine (µmol/L) | 78.62 ± 19.64 | 75.36 ±15.21 | 79.67 ± 20.21 | 79.30 ± 16.82 | 0.503 |
| Total cholesterol (mmol/L) | 5.21 ± 0.98 | 5.23 ± 1.07 | 5.14 ± 0.94 | 5.02 ± 0.88 | 0.541 |
| TG (mmol/L) | 1.38 ± 0.76 | 1.19 ± 0.61 | 1.20 ± 0.65 | 1.39 ± 1.32 | 0.401 |
| HDL (mmol/L) | 1.33 ± 0.32 | 1.45 ± 0.35 | 1.40 ± 0.34 | 1.44 ± 0.38 | 0.211 |
| LDL (mmol/L) | 3.25 ± 0.86 | 3.24 ± 0.92 | 3.20 ± 0.79 | 2.97 ± 0.67 | 0.107 |
| C-reactive protein (mg/L) | 1.79 ± 2.62 | 3.84 ± 10.69 | 1.33 ± 1.34 | 1.98 ± 4.18 | 0.092 |
| Metabolite | Candidate | Fold Differences | Area under the Curve (AUC) (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| HMHS/LMLS | HMLS/LMLS | LMHS/LMLS | HMHS vs. LMLS | HMLS vs. LMLS | LMHS vs. LMLS | ||
| Untargeted analysis | |||||||
| AA-content glycerophospholipids | |||||||
| PC (36:4) | PC (20:4/16:0) | 1.08 * | 1.10 ** | 1.07 | 0.60 (0.51, 0.69) | 0.62 (0.53, 0.71) | 0.57 (0.47, 0.66) |
| PC (38:4) | PC (18:0/20:4) | 1.11 * | 1.14 ** | 1.07 | 0.61 (0.51, 0.70) | 0.62 (0.53, 0.71) | 0.56 (0.46, 0.65) |
| PC (p36:4) | PC (20:4/P-16:0) | 1.15 ** | 1.18 ** | 1.08 | 0.66 (0.57, 0.75) | 0.69 (0.60, 0.77) | 0.58 (0.49, 0.67) |
| PC (o36:4) | PC (O-16:0/20:4) | 1.14 ** | 1.15 ** | 1.10 * | 0.64 (0.55, 0.73) | 0.65 (0.57, 0.74) | 0.60 (0.51, 0.70) |
| PC (o38:5) | PC (O-18:1/20:4) | 1.08 * | 1.11 ** | 1.04 | 0.59 (0.50, 0.68) | 0.63 (0.54, 0.72) | 0.55 (0.46, 0.65) |
| PE (p36:4) | PE (P-16:0/20:4) | 1.31 ** | 1.26 ** | 1.17 * | 0.72 (0.64, 0.81) | 0.69 (0.61, 0.78) | 0.64 (0.55, 0.73) |
| PE (p38:4) | PE (P-18:0/20:4) | 1.29 ** | 1.29 ** | 1.21 * | 0.69 (0.60, 0.78) | 0.69 (0.61, 0.78) | 0.65 (0.57, 0.74) |
| PE (p38:5) | PE (P-18:1/20:4) | 1.25 ** | 1.24 ** | 1.17 * | 0.68 (0.59, 0.77) | 0.67 (0.59, 0.76) | 0.64 (0.55, 0.73) |
| EPA-content glycerophospholipids | |||||||
| PC (36:5) | PC (16:0/20:5) | 1.64 ** | 1.31 * | 1.83 ** | 0.74 (0.66, 0.82) | 0.61 (0.53, 0.70) | 0.78 (0.71, 0.86) |
| PE (p36:5) | PE (P-16:0/20:5) | 1.92 ** | 1.52 ** | 2.16 ** | 0.76 (0.68, 0.84) | 0.66 (0.57, 0.74) | 0.77 (0.70, 0.85) |
| DHA-content glycerophospholipids | |||||||
| LPC (22:6) | 1.45 ** | 1.29 ** | 1.42 ** | 0.69 (0.60, 0.77) | 0.65 (0.56, 0.74) | 0.71 (0.63, 0.80) | |
| PC (38:6) | PC (22:6/16:0) | 1.36 ** | 1.26 ** | 1.35 ** | 0.77 (0.69, 0.84) | 0.70 (0.62, 0.78) | 0.75 (0.68, 0.83) |
| PC (40:6) | PC (22:6/18:0) | 1.41 ** | 1.27 ** | 1.38 ** | 0.76 (0.68, 0.84) | 0.67 (0.59, 0.76) | 0.73 (0.65, 0.81) |
| PC (40:7) | PC (18:1/22:6) | 1.16 * | 1.18 ** | 1.23 ** | 0.61 (0.52, 0.70) | 0.63 (0.55, 0.72) | 0.65 (0.56, 0.74) |
| PC (o38:6) | PC (O-16:0/22:6) | 1.32 ** | 1.28 ** | 1.36 ** | 0.74 (0.65, 0.82) | 0.73 (0.65, 0.81) | 0.73 (0.65, 0.81) |
| PC (p40:6) | PC (P-18:0/22:6) | 1.36 ** | 1.30 ** | 1.42 ** | 0.73 (0.65, 0.81) | 0.70 (0.62, 0.78) | 0.74 (0.66, 0.82) |
| LPE (22:6) | 1.27 ** | 1.24 ** | 1.26 ** | 0.70 (0.62, 0.79) | 0.68 (0.59, 0.76) | 0.70 (0.62, 0.79) | |
| PE (38:6) | PE (22:6/16:0) | 1.40 ** | 1.31 ** | 1.38 ** | 0.69 (0.60, 0.77) | 0.68 (0.60, 0.77) | 0.72 (0.63, 0.80) |
| PE (40:6) | PE (22:6/18:0) | 1.53 ** | 1.34 ** | 1.42 ** | 0.73 (0.65, 0.81) | 0.69 (0.60, 0.77) | 0.71 (0.63, 0.79) |
| PE (40:9) | PE (22:6/18:3) | 1.40 ** | 1.28 ** | 1.35 ** | 0.68 (0.59, 0.76) | 0.66 (0.57, 0.74) | 0.68 (0.59, 0.76) |
| PE (p38:6) | PE (P-16:0/22:6) | 1.44 ** | 1.30 ** | 1.41 ** | 0.78 (0.70, 0.85) | 0.70 (0.62, 0.79) | 0.75 (0.67, 0.83) |
| Non-AA/EPA/DHA-content Glycerophospholipids | |||||||
| PC (34:2) | PC (18:2/16:0) | 0.94* | 0.92** | 0.96 | 0.62 (0.53, 0.71) | 0.63 (0.54, 0.72) | 0.57 (0.47, 0.66) |
| PC (36:2) | PC (18:1/18:1) | 0.81 ** | 0.87 | 0.82 ** | 0.64 (0.54, 0.73) | 0.59 (0.49, 0.68) | 0.62 (0.52, 0.71) |
| PC (36:4) | PC (18:2/18:2) | 0.90 ** | 0.96 | 0.91 ** | 0.66 (0.57, 0.75) | 0.58 (0.49, 0.67) | 0.62 (0.53, 0.72) |
| PC (o34:1) | PC (O-16:0/18:1) | 0.93 ** | 0.93 ** | 0.95 * | 0.64 (0.55, 0.73) | 0.64 (0.55, 0.73) | 0.59 (0.50, 0.69) |
| LPE (18:1) | 0.82 * | 0.82 * | 0.87 | 0.63 (0.53, 0.72) | 0.61 (0.52, 0.70) | 0.58 (0.48, 0.67) | |
| LPE (18:2) | 0.70 ** | 0.71 ** | 0.75 ** | 0.67 (0.58, 0.76) | 0.66 (0.58, 0.75) | 0.63 (0.54, 0.73) | |
| PE (36:3) | PE (18:2/18:1) | 0.56 ** | 0.65 ** | 0.65 ** | 0.66 (0.57, 0.75) | 0.63 (0.54, 0.72) | 0.61 (0.52, 0.70) |
| PI (34:2) | PI (18:2/16:0) | 0.78 ** | 0.81 ** | 0.87 * | 0.69 (0.60, 0.78) | 0.65 (0.56, 0.74) | 0.59 (0.50, 0.69) |
| PI (36:2) | PI (18:2/18:0) | 0.80 * | 0.84 * | 0.91 | 0.62 (0.53, 0.71) | 0.59 (0.50, 0.68) | 0.51 (0.41, 0.60) |
| PI (38:3) | PI (20:3/18:0) | 0.72 ** | 0.81 * | 0.78 ** | 0.66 (0.57, 0.75) | 0.58 (0.49, 0.68) | 0.60 (0.51, 0.70) |
| Other | |||||||
| d-Glucose | 1.09 ** | 1.04 | 1.10 ** | 0.66 (0.56, 0.75) | 0.58 (0.49, 0.68) | 0.70 (0.61, 0.79) | |
| CMPF | 2.10 ** | 1.32 | 2.11 ** | 0.77 (0.69, 0.84) | 0.64 (0.55, 0.72) | 0.77 (0.70, 0.85) | |
| Targeted analysis | |||||||
| Amino acids | |||||||
| Glycine | 0.89 * | 0.98 | 0.98 | 0.64 (0.55, 0.73) | 0.51 (0.42, 0.61) | 0.52 (0.42, 0.61) | |
| Hydroxyproline | 1.43 ** | 1.19 | 1.46 ** | 0.71 (0.63, 0.80) | 0.64 (0.55, 0.73) | 0.73 (0.65, 0.81) | |
| Lysine | 1.16 ** | 1.10** | 1.15 ** | 0.64 (0.54, 0.73) | 0.62 (0.53, 0.71) | 0.65 (0.57, 0.74) | |
| Threonine | 1.08 * | 0.97 | 1.01 | 0.63 (0.53, 0.72) | 0.52 (0.43, 0.61) | 0.51 (0.42, 0.61) | |
| Tryptophan | 1.07 * | 0.97 | 1.04 | 0.63 (0.54, 0.72) | 0.52 (0.43, 0.61) | 0.58 (0.48, 0.67) | |
| Valine | 1.07 * | 1.05 | 1.10 ** | 0.60 (0.51, 0.69) | 0.57 (0.48, 0.66) | 0.66 (0.57, 0.75) | |
| Total fatty acids | |||||||
| AA | 1.26 ** | 1.22 ** | 1.10 | 0.67 (0.58, 0.76) | 0.64 (0.55, 0.73) | 0.59 (0.5, 0.68) | |
| EPA | 1.50 ** | 1.24 | 1.60 ** | 0.74 (0.66, 0.82) | 0.61 (0.52, 0.70) | 0.78 (0.7, 0.85) | |
| DHA | 1.78 ** | 1.40 ** | 1.63 ** | 0.79 (0.72, 0.86) | 0.71 (0.63, 0.79) | 0.77 (0.7, 0.85) | |
| Food Groups | Metabolites | Class | Correlation (r) | p |
|---|---|---|---|---|
| Meat a | PC (p36:4) | AA-content GP | 0.254 | 2.43 × 10−05 |
| PE (p36:4) | AA-content GP | 0.235 | 9.42 × 10−05 | |
| PC (o36:4) | AA-content GP | 0.215 | 3.73 × 10−04 | |
| PC (38:6) | DHA-content GP | 0.263 | 1.22 × 10−05 | |
| PC (40:6) | DHA-content GP | 0.223 | 2.24 × 10−04 | |
| PC (o38:6) | DHA-content GP | 0.216 | 3.49 × 10−04 | |
| LPE (18:2) | Non-AA/EPA/DHA-content GP | −0.280 | 3.01 × 10−06 | |
| PI (34:2) | Non-AA/EPA/DHA-content GP | −0.246 | 4.44 × 10−05 | |
| PE (36:3) | Non-AA/EPA/DHA-content GP | −0.241 | 6.13 × 10−05 | |
| Seafood a | Hydroxyproline | Amino acids | 0.321 | 6.91 × 10−08 |
| Valine | Amino acids | 0.247 | 3.97 × 10−05 | |
| Lysine | Amino acids | 0.240 | 6.92 × 10−05 | |
| d-Glucose | Carbohydrates | 0.252 | 2.70 × 10−05 | |
| CMPF | Fatty acids | 0.399 | 9.26 × 10−12 | |
| DHA | Fatty acids | 0.421 | 5.13 × 10−13 | |
| EPA | Fatty acids | 0.285 | 1.91 × 10−06 | |
| PC (38:6) | DHA-content GP | 0.442 | 2.62 × 10−14 | |
| PE (p38:6) | DHA-content GP | 0.421 | 5.37 × 10−13 | |
| PC (40:6) | DHA-content GP | 0.415 | 1.14 × 10−12 | |
| PC (p40:6) | DHA-content GP | 0.405 | 4.68 × 10−12 | |
| PC (o38:6) | DHA-content GP | 0.377 | 1.46 × 10−10 | |
| LPC (22:6) | DHA-content GP | 0.343 | 7.35 × 10−09 | |
| PE (40:6) | DHA-content GP | 0.304 | 3.64 × 10−07 | |
| LPE (22:6) | DHA-content GP | 0.293 | 9.40 × 10−07 | |
| PE (38:6) | DHA-content GP | 0.258 | 1.83 × 10−05 | |
| PE (40:9) | DHA-content GP | 0.255 | 2.27 × 10−05 | |
| PE (p36:5) | EPA-content GP | 0.387 | 4.65 × 10−11 | |
| PC (36:5) | EPA-content GP | 0.341 | 9.22 × 10−09 | |
| PI (38:3) | Non-AA/EPA/DHA-content GP | −0.238 | 7.84 × 10−05 | |
| LPE (18:2) | Non-AA/EPA/DHA-content GP | −0.224 | 2.05 × 10−04 | |
| Red meat b | LPE (18:2) | Non-AA/EPA/DHA-content GP | −0.235 | 9.84 × 10−05 |
| Poultry b | PC (p36:4) | AA-content GP | 0.262 | 1.30 × 10−05 |
| PC (o38:6) | DHA-content GP | 0.250 | 3.19 × 10−05 | |
| PC (38:6) | DHA-content GP | 0.235 | 9.36 × 10−05 | |
| Fish b | Hydroxyproline | Amino acids | 0.310 | 2.03 × 10−07 |
| d-Glucose | Carbohydrates | 0.252 | 2.83 × 10−05 | |
| CMPF | Fatty acids | 0.411 | 1.94 × 10−12 | |
| DHA | Fatty acids | 0.428 | 1.93 × 10−13 | |
| EPA | Fatty acids | 0.299 | 5.77 × 10−07 | |
| PC (38:6) | DHA-content GP | 0.448 | 9.82 × 10−15 | |
| PC (40:6) | DHA-content GP | 0.426 | 2.39 × 10−13 | |
| PC (p40:6) | DHA-content GP | 0.414 | 1.31 × 10−12 | |
| PE (p38:6) | DHA-content GP | 0.405 | 4.19 × 10−12 | |
| LPC (22:6) | DHA-content GP | 0.371 | 2.94 × 10−10 | |
| PC (o38:6) | DHA-content GP | 0.369 | 3.98 × 10−10 | |
| LPE (22:6) | DHA-content GP | 0.301 | 4.56 × 10−07 | |
| PE (40:6) | DHA-content GP | 0.297 | 6.67 × 10−07 | |
| PE (40:9) | DHA-content GP | 0.251 | 2.94 × 10−05 | |
| PE (38:6) | DHA-content GP | 0.251 | 3.10 × 10−05 | |
| PE (p36:5) | EPA-content GP | 0.382 | 8.75 × 10−11 | |
| PC (36:5) | EPA-content GP | 0.356 | 1.72 × 10−09 | |
| Shellfish b | PE (p36:4) | AA-content GP | 0.243 | 5.58 × 10−05 |
| Vegetables b | - | |||
| Fruits b | - | |||
| Whole grains b | - | |||
| Refined grains b | - | |||
| Soy products b | PC (o38:5) | AA-content GP | −0.247 | 4.06 × 10−05 |
| PC (38:6) | DHA-content GP | −0.308 | 2.51 × 10−07 | |
| PC (40:6) | DHA-content GP | −0.304 | 3.37 × 10−07 | |
| PC (p40:6) | DHA-content GP | −0.289 | 1.35 × 10−06 | |
| PC (o38:6) | DHA-content GP | −0.277 | 3.94 × 10−06 | |
| PE (p38:6) | DHA-content GP | −0.252 | 2.85 × 10−05 | |
| LPE (18:2) | DHA-content GP | 0.236 | 9.15 × 10−05 | |
| PE (p36:5) | EPA-content GP | −0.236 | 9.17 × 10−05 | |
| PI (38:3) | Non-AA/EPA/DHA-content GP | 0.279 | 3.11 × 10−06 | |
| Dairy b | Valine | Amino acids | −0.242 | 5.67 × 10−05 |
| Eggs b | - | |||
| Nuts and seeds b | - |
| Clinical Characteristics | Metabolites | Class | Correlation (r) | p |
|---|---|---|---|---|
| Fasting glucose | d-Glucose | Carbohydrates | 0.638 | 1.61 × 10−31 |
| Hydroxyproline | Amino acids | 0.248 | 4.60 × 10−05 | |
| Valine | Amino acids | 0.235 | 1.15 × 10−04 | |
| CMPF | Fatty acids | 0.401 | 1.28 × 10−11 | |
| DHA | Fatty acids | 0.284 | 2.72 × 10−06 | |
| EPA | Fatty acids | 0.236 | 1.08 × 10−04 | |
| Fasting insulin | d-Glucose | Carbohydrates | 0.301 | 4.79 × 10−07 |
| Valine | Amino acids | 0.267 | 8.99 × 10−06 | |
| Creatinine | Valine | Amino acids | 0.335 | 1.67 × 10−08 |
| Hydroxyproline | Amino acids | 0.332 | 2.18 × 10−08 | |
| Tryptophan | Amino acids | 0.247 | 4.00 × 10−05 | |
| Total cholesterol | AA | Fatty acids | 0.520 | 3.99 × 10−20 |
| DHA | Fatty acids | 0.431 | 1.22 × 10−13 | |
| PC (38:4) | AA-content GP | 0.416 | 9.77 × 10−13 | |
| PC (o36:4) | AA-content GP | 0.332 | 2.26 × 10−08 | |
| PC (o38:5) | AA-content GP | 0.529 | 7.50 × 10−21 | |
| PC (p36:4) | AA-content GP | 0.385 | 6.01 × 10−11 | |
| PE (p36:4) | AA-content GP | 0.298 | 6.32 × 10−07 | |
| PE (p38:4) | AA-content GP | 0.434 | 8.26 × 10−14 | |
| PE (p38:5) | AA-content GP | 0.476 | 1.08 × 10−16 | |
| LPE (22:6) | DHA-content GP | 0.250 | 3.34 × 10−05 | |
| PC (38:6) | DHA-content GP | 0.409 | 2.57 × 10−12 | |
| PC (40:6) | DHA-content GP | 0.436 | 5.88 × 10−14 | |
| PC (40:7) | DHA-content GP | 0.350 | 3.28 × 10−09 | |
| PC (o38:6) | DHA-content GP | 0.266 | 9.51 × 10−06 | |
| PC (p40:6) | DHA-content GP | 0.310 | 2.00 × 10−07 | |
| PE (38:6) | DHA-content GP | 0.360 | 1.09 × 10−09 | |
| PE (40:6) | DHA-content GP | 0.386 | 5.13 × 10−11 | |
| PE (40:9) | DHA-content GP | 0.294 | 9.11 × 10−07 | |
| PE (p38:6) | DHA-content GP | 0.561 | 7.99 × 10−24 | |
| PC (34:2) | Non-AA/EPA/DHA-content GP | 0.603 | 4.17 × 10−28 | |
| PC (36:2) | Non-AA/EPA/DHA-content GP | 0.376 | 1.62 × 10−10 | |
| PC (36:4) | Non-AA/EPA/DHA-content GP | 0.436 | 6.29 × 10−14 | |
| PC (o34:1) | Non-AA/EPA/DHA-content GP | 0.524 | 1.92 × 10−20 | |
| PE (36:3) | Non-AA/EPA/DHA-content GP | 0.274 | 4.97 × 10−06 | |
| PI (34:2) | Non-AA/EPA/DHA-content GP | 0.336 | 1.56 × 10−08 | |
| PI (36:2) | Non-AA/EPA/DHA-content GP | 0.572 | 7.78 × 10−25 | |
| PI (38:3) | Non-AA/EPA/DHA-content GP | 0.299 | 5.57 × 10−07 | |
| TG | Lysine | Amino acids | 0.256 | 2.06 × 10−05 |
| Valine | Amino acids | 0.323 | 5.81 × 10−08 | |
| AA | Fatty acids | 0.406 | 4.00 × 10−12 | |
| DHA | Fatty acids | 0.304 | 3.37 × 10−07 | |
| PC (38:4) | AA-content GP | 0.239 | 7.34 × 10−05 | |
| PE (38:6) | DHA-content GP | 0.338 | 1.28 × 10−08 | |
| PE (40:6) | DHA-content GP | 0.490 | 1.13 × 10−17 | |
| PE (40:9) | DHA-content GP | 0.309 | 2.17 × 10−07 | |
| LPE (18:1) | Non-AA/EPA/DHA-content GP | 0.247 | 4.15 × 10−05 | |
| PC (34:2) | Non-AA/EPA/DHA-content GP | 0.367 | 4.81 × 10−10 | |
| PC (36:2) | Non-AA/EPA/DHA-content GP | 0.585 | 3.64 × 10−26 | |
| PC (36:4) | Non-AA/EPA/DHA-content GP | 0.302 | 4.19 × 10−07 | |
| PC (o34:1) | Non-AA/EPA/DHA-content GP | 0.318 | 9.70 × 10−08 | |
| PE (36:3) | Non-AA/EPA/DHA-content GP | 0.566 | 2.79 × 10−24 | |
| PI (34:2) | Non-AA/EPA/DHA-content GP | 0.511 | 2.11 × 10−19 | |
| PI (36:2) | Non-AA/EPA/DHA-content GP | 0.642 | 1.04 × 10−32 | |
| PI (38:3) | Non-AA/EPA/DHA-content GP | 0.506 | 5.63 × 10−19 | |
| HDL | Lysine | Amino acids | −0.315 | 1.22 × 10−07 |
| Valine | Amino acids | −0.482 | 3.99 × 10−17 | |
| PC (o36:4) | AA-content GP | 0.233 | 1.09 × 10−04 | |
| PC (o38:5) | AA-content GP | 0.410 | 2.34 × 10−12 | |
| PC (p36:4) | AA-content GP | 0.298 | 6.01 × 10−07 | |
| PE (p38:4) | AA-content GP | 0.235 | 9.58 × 10−05 | |
| PE (p38:5) | AA-content GP | 0.261 | 1.35 × 10−05 | |
| PC (40:7) | DHA-content GP | 0.446 | 1.37 × 10−14 | |
| PC (o38:6) | DHA-content GP | 0.368 | 4.33 × 10−10 | |
| PC (p40:6) | DHA-content GP | 0.390 | 2.88 × 10−11 | |
| PE (p38:6) | DHA-content GP | 0.235 | 9.95 × 10−05 | |
| PC (34:2) | Non-AA/EPA/DHA-content GP | 0.378 | 1.31 × 10−10 | |
| PC (o34:1) | Non-AA/EPA/DHA-content GP | 0.343 | 7.09 × 10−09 | |
| PC (36:4) | Non-AA/EPA/DHA-content GP | 0.549 | 1.19 × 10−22 | |
| LDL | AA | Fatty acids | 0.423 | 5.06 × 10−13 |
| DHA | Fatty acids | 0.361 | 1.28 × 10−09 | |
| PC (38:4) | AA-content GP | 0.329 | 3.75 × 10−08 | |
| PC (o36:4) | AA-content GP | 0.315 | 1.48 × 10−07 | |
| PC (o38:5) | AA-content GP | 0.438 | 6.40 × 10−14 | |
| PC (p36:4) | AA-content GP | 0.355 | 2.28 × 10−09 | |
| PE (p36:4) | AA-content GP | 0.282 | 2.86 × 10−06 | |
| PE (p38:4) | AA-content GP | 0.344 | 7.91 × 10−09 | |
| PE (p38:5) | AA-content GP | 0.379 | 1.57 × 10−10 | |
| PC (38:6) | DHA-content GP | 0.350 | 4.32 × 10−09 | |
| PC (40:6) | DHA-content GP | 0.380 | 1.40 × 10−10 | |
| PC (o38:6) | DHA-content GP | 0.256 | 2.23 × 10−05 | |
| PC (p40:6) | DHA-content GP | 0.289 | 1.60 × 10−06 | |
| PE (40:6) | DHA-content GP | 0.269 | 8.35 × 10−06 | |
| PE (p38:6) | DHA-content GP | 0.485 | 3.52 × 10−17 | |
| PC (34:2) | Non-AA/EPA/DHA-content GP | 0.428 | 2.71 × 10−13 | |
| PC (36:2) | Non-AA/EPA/DHA-content GP | 0.252 | 3.08 × 10−05 | |
| PC (36:4) | Non-AA/EPA/DHA-content GP | 0.338 | 1.55 × 10−08 | |
| PC (o34:1) | Non-AA/EPA/DHA-content GP | 0.361 | 1.28 × 10−09 | |
| PI (36:2) | Non-AA/EPA/DHA-content GP | 0.520 | 6.75 × 10−20 | |
| C-reactive protein | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zou, L.; Su, J.; Tai, E.S.; Whitton, C.; Van Dam, R.M.; Ong, C.N. Meat and Seafood Consumption in Relation to Plasma Metabolic Profiles in a Chinese Population: A Combined Untargeted and Targeted Metabolomics Study. Nutrients 2017, 9, 683. https://doi.org/10.3390/nu9070683
Lu Y, Zou L, Su J, Tai ES, Whitton C, Van Dam RM, Ong CN. Meat and Seafood Consumption in Relation to Plasma Metabolic Profiles in a Chinese Population: A Combined Untargeted and Targeted Metabolomics Study. Nutrients. 2017; 9(7):683. https://doi.org/10.3390/nu9070683
Chicago/Turabian StyleLu, Yonghai, Li Zou, Jin Su, E. Shyong Tai, Clare Whitton, Rob M. Van Dam, and Choon Nam Ong. 2017. "Meat and Seafood Consumption in Relation to Plasma Metabolic Profiles in a Chinese Population: A Combined Untargeted and Targeted Metabolomics Study" Nutrients 9, no. 7: 683. https://doi.org/10.3390/nu9070683




