2.1. Characteristics of Immobilized ZnO and the Synergic Effect of Visible Light/ZnO Photocatalyst
The characteristics of the immobilized ZnO photocatalyst on the steel mesh were determined by various instruments and reported to have a Brunauer-Emmett-Teller (BET) specific surface area of 7.14 m
2 g
−1, porosity of 5.69 Å, and an average particle size of 43 nm. By adjusting the weight ratio of ZnO and the steel mesh, i.e., 1.5 g ZnO was immobilized on 9.3 g steel mesh, the specific surface area contributed by ZnO particles was calculated to be 51.41 m
2 g
−1. From field emission scanning electron microscope (FESEM) imagery (
Figure 1a), uniform distribution of ZnO particles on the steel mesh surface and space within the mesh eyes can be observed. From the figure, the shape of the ZnO particles is observed to be cylindrical, and the particle size of ZnO is about 30–50 nm for the cylindrical diameter. The FESEM image after the photocatalytic reaction is presented in
Figure 1b and demonstrates a similar size and shape of ZnO particles on the steel mesh. The changes are slightly larger in size and slightly curvier in shape. EDS scanning gives 76.25% of Zn and 23.75% of oxygen by weight percentage (
Figure 1c). A thin-film X-ray diffractometer (XRD), X-RAY/TF, was utilized to obtain the XRD spectra before and after the photocatalytic reaction. Both spectra in
Figure 1d show a similarity to the standard ZnO spectrum with only 44 degree higher absorbance, as shown in
Figure 1d, before and after the photocatalytic reaction. The XRD spectra of immobilized ZnO on the steel mesh are perfectly matched with the standard ZnO spectrum and show the stability of the immobilized ZnO catalyst. Furthermore, the stability of the immobilized ZnO catalyst was tested in our previous work under UV 365 irradiation for 10 cycles and found that OG removal efficiency at the end of the reaction for 10 runs can all reach 100% in 120 min [
28]. However, the TOC removal efficiency dropped from 90% to 78% after repeating 10 runs. Meanwhile, there was almost no ZnO catalyst loss (0.04%) after 10 runs, as verified by Atomic Absorption Spectroscopy (AA) measurement of the Zn solution concentration. Thus, the loss of the TOC-mineralization capability may be caused by surface poisoning of the ZnO catalyst after 10 runs.
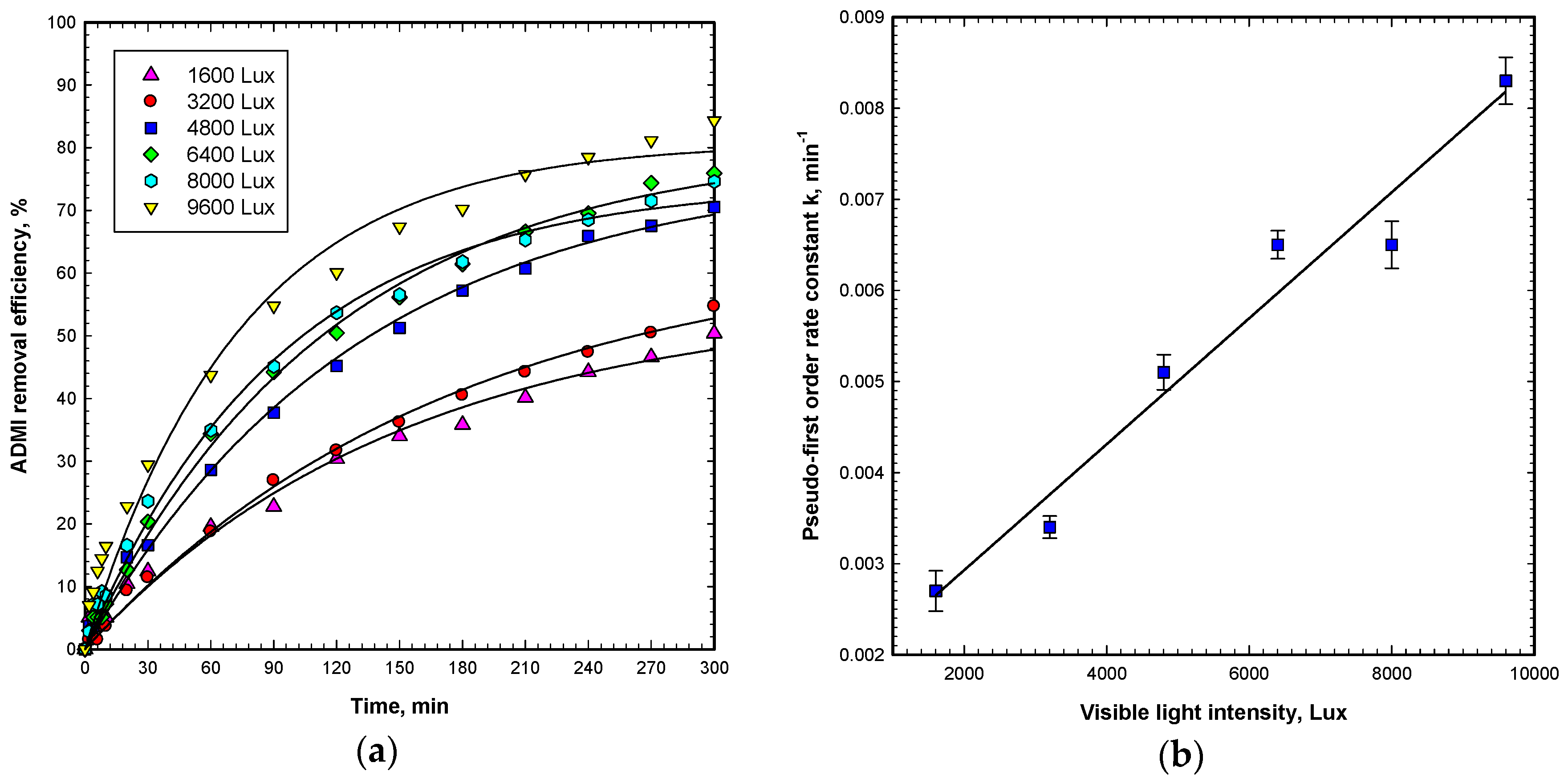
To differentiate the decolorization effects from visible light irradiation, ZnO adsorption, and photocatalytic degradation, a set of control experiments were performed by using visible light irradiation alone, ZnO catalyst-coated steel mesh alone, and combining both visible light and the ZnO catalyst for degrading OG in wastewater (data not shown). The operating conditions were preset at an initial OG concentration of 50 mg L−1, ZnO dosage of 60 g m−2, and six cold cathode fluorescent lamps (CCFL) with measured light intensity of 9600 Lux in the visible region, with a 300 min reaction duration. The decolorization and degradation of OG was evaluated through the change in American Dye Manufacturers’ Institute (ADMI) color units, because ADMI color units represent the overall absorbance of various color regions. It is not a surprise that the control set of visible light irradiation alone and ZnO catalyst alone experiments are not able to even decolorize OG at observable levels. Accordingly, visible light irradiation on the ZnO catalyst surface generates electron-hole pairs and further produces hydroxyl radicals, superoxide radicals, hydrogen peroxide, and singlet oxygen for the synergic degradation of OG in aqueous solution. Thus, the combined Vis/ZnO system demonstrated effective degradation of OG and obtained 84.3% ADMI removal efficiency with a 300 min reaction time.
The pseudo-first-order kinetics are widely adapted for AOP studies. The kinetic equations can be expressed as follows:
where C
OG,0 designates the initial concentration of OG and C
OG is the concentration of OG at time t in mg L
−1.
k1 denotes the pseudo-first-order reaction rate constant in min
−1 and t is the reaction time in min. In this work, ADMI color units were used to represent the OG concentration.
Furthermore, through first-order kinetics treatment of the experimental data, the pseudo-first order rate constant of the Vis/ZnO process is obtained as 0.0105 min
−1, which is 2.23 times higher than that of Vis/TiO
2 (utilizing P25 TiO
2) at 0.0047 min
−1. A similar observation was revealed by Sakthivel et al. [
24]; they concluded that the higher photo-activity of ZnO is caused by the large fraction of the solar spectrum and more light quanta absorption by ZnO than TiO
2. At the same time, Akyol et al. [
25] also reported that the ZnO is more active than TiO
2 for the decolorization of Remazol Red RR dye in a suspension system, and the reasons are due to the beneficial effects on the band gap energy, the charge carrier density, and the crystal structure of ZnO to reflect its great quantum efficiency.
2.2. Effect of ZnO Dosage
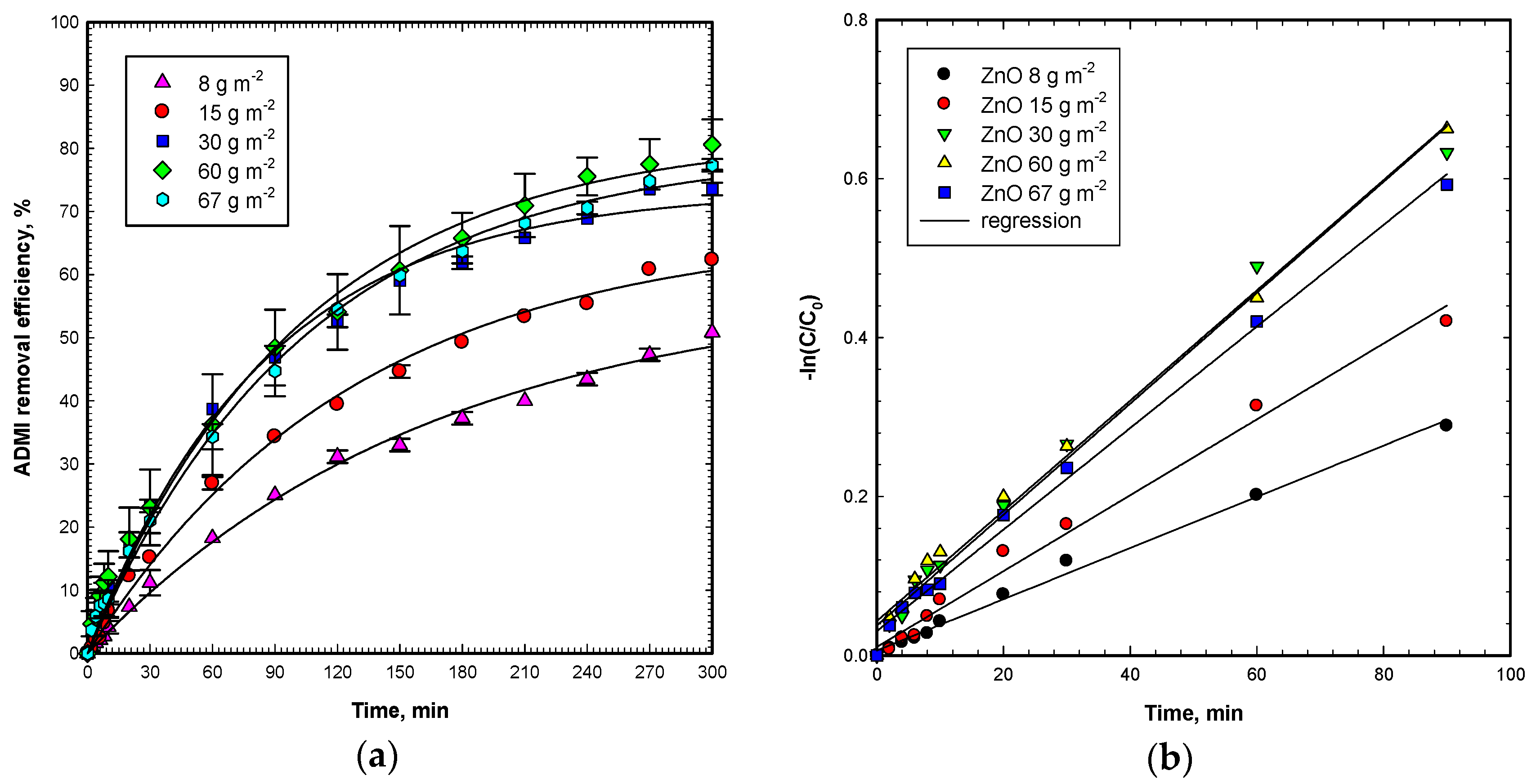
The ADMI color removal was tested under various ZnO catalyst dosages of 8–67 g m
−2, initial OG concentration of 50 mg L
−2, and visible light intensity 9600 Lux for a period of 300 min. The results in
Figure 2a present that the ADMI removal improves from 50.8% to 80.6% in 300 min when the surface loading of ZnO particles increases from 8 to 60 g m
−2. Moreover, the ADMI removal then reduces from 80.6% to 77.3% when the surface loading of ZnO further increases from 60 to 67 g m
−2, respectively. Thus, an optimal ZnO dosage for OG degradation is observed to occur at 60 g m
−2. For surface loading of ZnO higher than 60 g m
−2, the ZnO catalyst on the top layers will shield the visible light from the ZnO catalyst at the lower layer to reduce the photocatalytic activity, thus moderating the ADMI removal efficiency and reaction rate. For the suspension system, Sakthivel et al. [
24] reported an optimum ZnO loading of 2.5 g L
−1 for solar irradiation on the ZnO suspension. They further explained that the increase in turbidity reduces the light transmission through the solution, once over this optimum ZnO loading, and it moderates the photocatalytic activity of ZnO. Akyol et al. [
25] also obtained an optimum ZnO loading of 1.0 g L
−1 for methyl orange degradation. Similarly, Akyol et al. concluded that the photon absorption rate is affected by the scattering of the photocatalyst particles to reduce the photocatalytic activity. However, few investigations discuss the effect of photocatalyst loading for immobilized systems. Zhu et al. immobilized SnO
2/ZnO in a chitosan matrix and revealed an optimum dosage of 0.7 g L
−1 [
22]. In this case, the immobilized catalyst was operated as a traditional suspension system. For various supported ZnO reactors, the ZnO loadings were reported by Akyol and Bayramoglu [
26] between 0.19 to 0.27 mg cm
−2 depending on the geometry and type of support. Therefore, no investigation on ZnO optimum loading for the immobilized form was reported.
The rate constants of OG degradation through the Vis/ZnO process for various ZnO loadings follow a similar trend as the ADMI removal. By applying pseudo-first-order reaction kinetics, the rate constants are calculated through linear regression for the first 90 min period. As shown in
Figure 2b, the rate constants are calculated to be 3.2, 4.8, 7.0, 6.9, and 6.4 (×10
−3 min
−1) at the surface ZnO loading dosages of 8, 15, 30, 60, and 67 g m
−2, respectively. The regressions are with good agreement (
r2 = 0.987–0.995). It is worth noting that the maximum rate constant occurred at a ZnO loading of 30 g m
−2, but it is almost the same with that at 60 g m
−2. In conclusion, the higher surface loading of ZnO reaches higher ADMI removal efficiencies and rate constants. Moreover, the rate constants for the Vis/ZnO process are 0.0032 and 0.0070 min
−1 at an OG initial concentration of 50 mg L
−1 and ZnO dosage of 8 and 60 g m
−2, respectively. It is about a two times increase of the first-order rate constant for a 7.5 times increase in the ZnO dosage. The rate constants are in the same order of magnitude as reported by Zhu et al. of 0.00559 min
−1 for 50 mg L
−1 methyl orange decolorization by visible light irradiation on SnO
2/ZnO-immobilized chitosan film [
22]. Likewise, the rate constants reported by Mittal et al. of 0.00306 to 0.00746 min
−1 for 10 mg L
−1 crystal violet decolorization by visible light irradiation on Cu-doped ZnO were in the same range [
20]. The results imply that the higher surface loading of ZnO under visible light irradiation can give more electron/hole pairs, as well as hydroxyl and superoxide radicals, singlet oxygen, and hydrogen peroxide, which contribute to the degradation of OG molecules and its chromophores. Therefore, the removal efficiency and degradation rate increase with the increase of ZnO surface loading before reaching the optimal surface loading of ZnO.
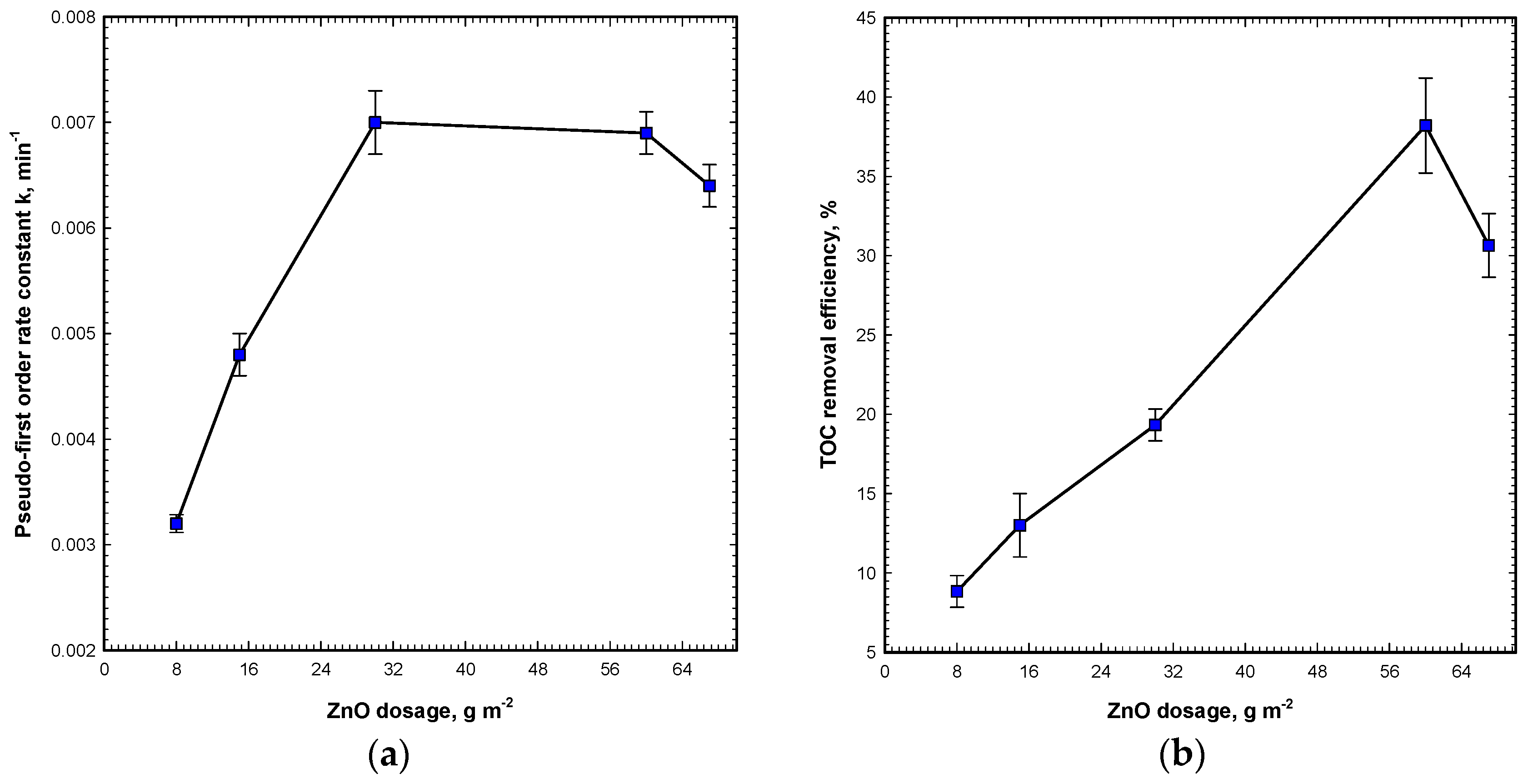
The TOC value represents the total organic carbons concentration in the solution. It is an important indicator of mineralization. At the beginning of the oxidation reaction by the oxidation species, such as hydroxyl radicals, superoxide radicals, hydrogen peroxide, and singlet oxygen, the solution TOC is hardly diminished. In the time region of less than 10 min, OG molecules are destroyed to lower molecular weight intermediates with a contribution to the TOC measurement. Therefore, in this region, TOC removal efficiency may persist slightly changed. After this lag phase (10 min), TOC can be diminished to the end of the reaction. In
Figure 2c, under a low ZnO dosage as 8 g m
−2, the results show that TOC is hardly mineralized by the Vis/ZnO process. For a higher ZnO dosage of 60 g m
−2, TOC removal efficiency increases to about 38.2% in 300 min. For the highest dosage of 67 g m
−1, the TOC mineralization efficiency drops from 38.2% to 30.6% for a reaction time of 300 min. It is correlated to the ADMI removal, and the optimal ZnO dosage is at 60 g m
−2. In general, AOPs with long reaction times can mineralize the TOC from pollutants effectively. However, for the case of the visible light photocatalytic process, the Vis/ZnO process shows weakness in mineralizing OG. It is concluded that TOC mineralization is more difficult than that of OG decolorization by the Vis/ZnO process. In order to decolorize OG, as well as mineralize TOC, higher light intensity and longer irradiation time are required.
The results in
Figure 3a show that the rate constant increases proportionally to the ZnO dosage for OG degradation by Vis/ZnO. The rate constant increases with the increase of the ZnO dosage up to 30 g m
−2, and from the ZnO dosage of 30–60 g m
−2, the rate constant remains almost unchanged. For the highest ZnO dosage, the rate constant dropped from 0.069 to 0.064 min
−1, slightly. The comparison of TOC removal efficiencies for various ZnO dosages on the Vis/ZnO process is illustrated in
Figure 3b. The figure illustrates that TOC removal efficiency increases linearly with the ZnO dosage for OG mineralization by the Vis/ZnO process. The TOC removal rises with the increase of the ZnO dosage up to 60 g m
−2. In contrast, for the ZnO dosages of 60–67 g m
−2, the TOC removal efficiency drops from 38.3% to 30.6%, significantly. The decolorization rate is observed to be higher than that of demineralization rate. This implies that the bonding of the OG dye chromophore is easier to destroy than that the organic carbons.
2.3. Effect of Initial OG Concentration
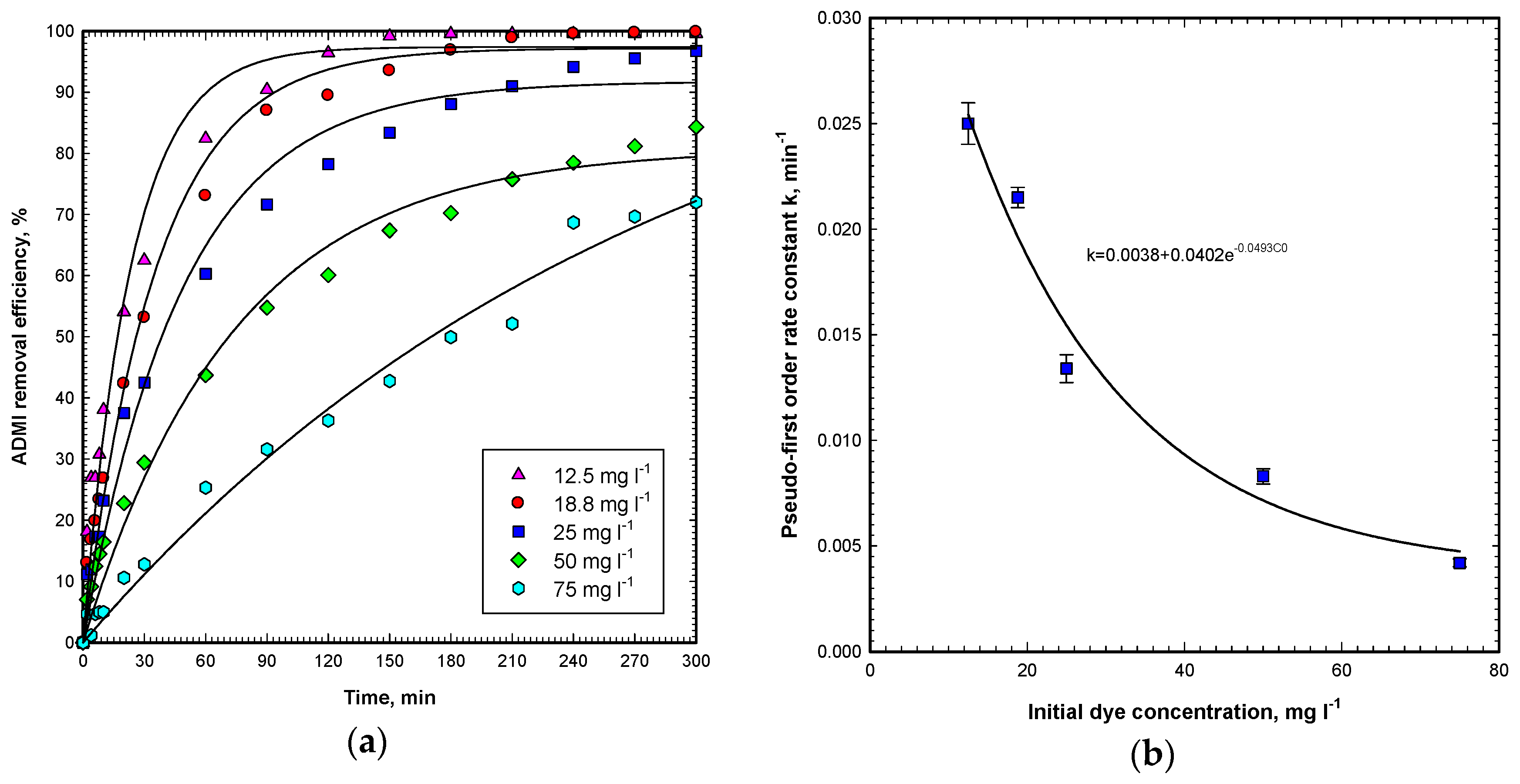
Experiments were implemented at OG initial concentrations of 12.5–75 mg L
−1, ZnO dosage of 60 g m
−2, and 9600 Lux visible light intensity for a period of 300 min. Results in
Figure 4a demonstrate that the degradation of OG is fast and complete in OG concentrations less than 25 mg L
−1. For OG concentrations of 50 and 75 mg L
−1, The ADMI removal efficiencies are 84.3% and 72.0%, respectively. The decolorization capability of the Vis/ZnO process is fairly good for initial OG concentration ranges from 12.5 to 75 mg L
−1.
As summarized in
Figure 4b, the pseudo-first-order rate constant declines with the increase of the initial OG concentration exponentially. Furthermore, the correlation of the rate constant and initial OG concentration can be expressed as follows (C
OG,0 denotes the OG initial concentration):
In
Figure 5b, the first-order rate constants for all initial OG concentrations by the Vis/ZnO process are summarized. In the figure, the first-order rate constant,
k1, declines exponentially by increasing the initial OG concentration from 12.5 to 75 mg L
−1. The results are consistent with the observations by previous work that the pseudo-first-order rate constant declines with an increase in the initial dye concentration reported by Zhu et al. [
22]. The observation can be explained that when irradiating under visible light, the limited numbers of active sites on the ZnO catalyst surface controls OG decolorization, while increasing the OG initial concentration, the path length of photons entering the reaction solution declines, which also reduces the formation of hydroxyl radicals, superoxide radicals, hydrogen peroxide and singlet oxygen at the same time.
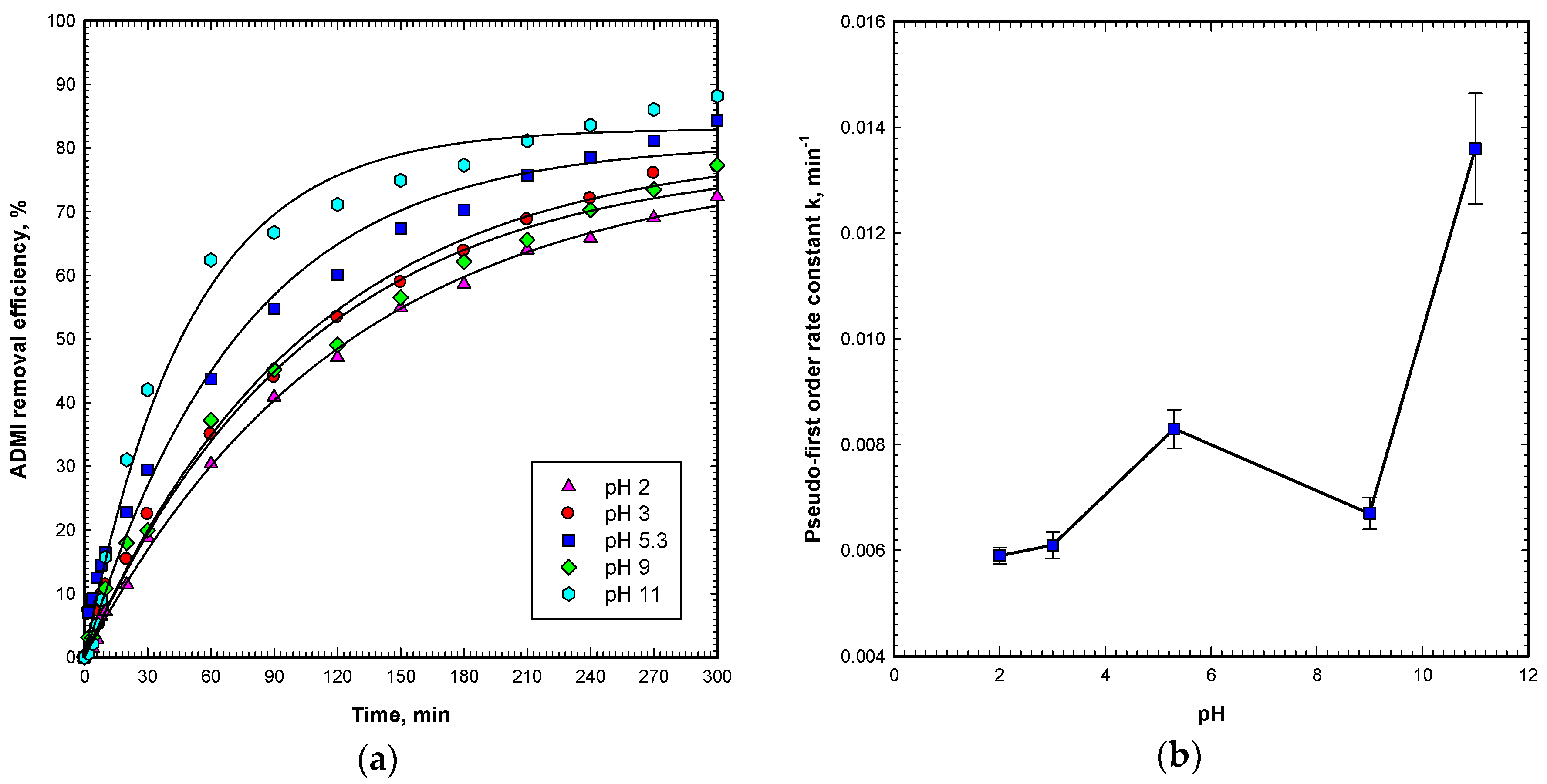
2.5. Effect of pH
pH is an important operating parameter which affects the photocatalytic degradation process. The effect of the pH value on the degradation of OG by the Vis/ZnO process was investigated in the pH range of 2–11 at a constant initial OG concentration (50 mg L
−1), ZnO dosage (60 g m
−2), and visible light intensity (9600 Lux). Results in
Figure 6a indicate that at an original pH of 5.3, the OG removal efficiency reaches 84.3% in 300 min of the Vis/ZnO reaction. At acidic pH of 2 and 3, and alkaline pH of 9, the OG degradation efficiencies reach 72.4%–77.3%, with lower reaction rates. This concludes that adjusting pH by either HCl or NaOH will result in hindering effects on OG degradation by the Vis/ZnO process. On the other hand, the pH 11 condition shows the highest OG degradation efficiency of 88.2% and the maximum rate of degradation.
Figure 6b summarizes the rate constants for all pH conditions and the rate constants are in the range of 0.0059–0.0136 min
−1. In this figure, the pH 11 condition presents the highest rate constant among all conditions. Sakthivel et al. also reported the maximum degradation rate at alkaline pH 10 [
24]. The pH condition shows two effects on ZnO catalytic degradation of OG. Firstly, the zero point of charge (zpc) of ZnO is 9.0 and the surface is positively charged in the acidic condition. In contrast, the surface of the ZnO catalyst is negatively charged in the alkaline condition when pH > pH
zpc. Thus, for OG as an anionic dye, the adsorption of OG on the ZnO surface is not favored in the alkaline pH 11 condition. Secondly, in the alkaline condition, the presence of a large quantity of hydroxyl ions on the ZnO surface in the alkaline solution favors the formation of hydroxyl radicals, which enhances the degradation of OG in the Vis/ZnO process [
28].
2.6. Effect of the Loaded Area of the Catalyst and Treatment Volume
Although ZnO dosage (in g m
−2) is a very important operating parameter of the Vis/ZnO process, the loaded area of the catalyst is also as important to provide sufficient area for receiving visible light irradiation. Therefore, the immobilized ZnO catalyst steel mesh was trimmed to various sizes (42–252 cm
2) to perform the OG degradation under visible light irradiation. As shown in
Figure 7a, the degradation of OG (in ADMI removal efficiency) by the Vis/ZnO process with various loaded areas of catalyst for the same ZnO dosage shows that the higher loaded area of the catalyst demonstrates higher OG removal efficiency during the reaction. The first-order rate constants for various loaded areas are summarized in
Figure 7b. It is reasonable that the rate constant increases as the loaded area of catalyst increases linearly. Furthermore, for up-scaling purposes, the same reactor with 252 cm
2 of loaded ZnO catalyst was conducted for treating a greater volume of wastewater, up to 400 mL.
Figure 8a shows OG degradation by the Vis/ZnO process at various treatment volumes. Results reveal that the OG removal efficiencies reach 29.1%–80.6% in 300 min for 200–400 mL treatment volumes. The results present that the higher treatment volume represents lower OG removal efficiency during the photocatalytic reaction. The rate constants for various treatment volumes are summarized in
Figure 8b and shows that rate constant exponentially decays with an increase of the treatment volume, and an empirical equation can be derived as follows:
where,
k1 denotes the pseudo first-order rate constant of Vis/ZnO in min
−1 for various treatment volumes.
V denotes the specific treatment volume in L m
−2.
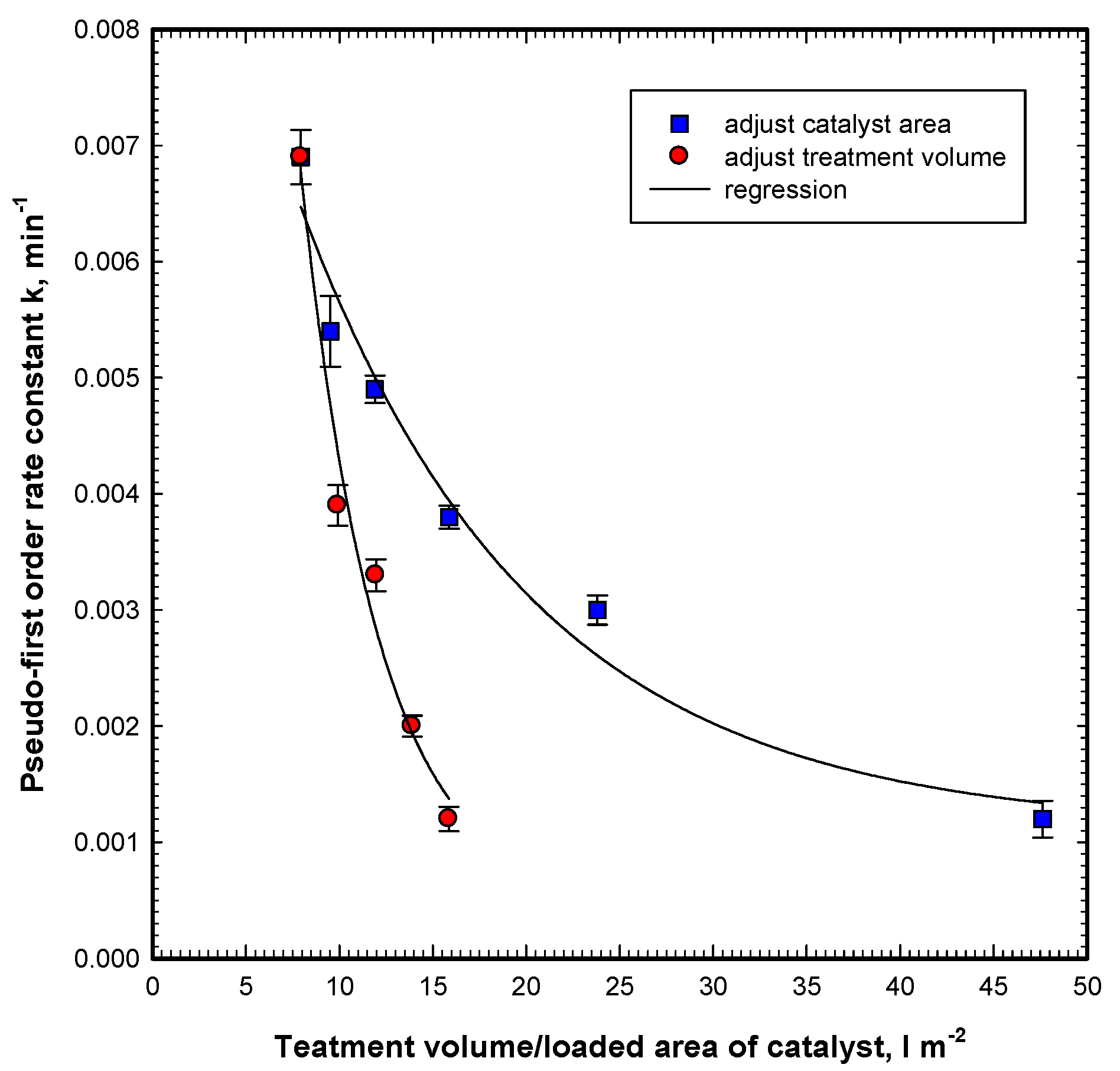
For the purpose of scaling up and real case application, it is important to evaluate the effects of the treatment volume per specific catalyst area, i.e., specific treatment volume (STV) (L m
−2), on the first-order rate constant. Under the same visible light irradiation and OG initial concentration, the experiments of adjusting the catalyst area from 42 to 252 cm
2 to treat 200 mL OG wastewater can obtain rate constants from 0.0012 to 0.0069 min
−1. Further calculating the specific treatment volume (STV) by deriving the treatment volume with the catalyst area obtains 47.6–7.9 L m
−2 for the catalyst area of 42–252 cm
2. This can be used to plot the decreasing trend of the first-order rate constant versus the specific treatment volume shown in
Figure 9. Similarly, the experiments of adjusting the treatment volume from 200 to 400 mL with a 252 cm
2 catalyst area to treat 200 mL of OG wastewater can also obtain rate constants from 0.0012 to 0.0069 min
−1. The specific treatment volume (STV), by deriving the treatment volume with the catalyst area can obtain 7.9–15.9 L m
−2 for the treatment volume of 200–400 mL. From
Figure 9, adjusting the catalyst area to the same STV can maintain the higher first-order rate constant than that of adjusting the treatment volume. For the same STV of 15.9 L m
−2, the rate constants are 0.0038 and 0.0012 min
−1 for adjusting the catalyst area and treatment volume, respectively. The difference is significant. This is due to the increase of the treatment volume at the same catalyst resulting in the increase of the water depth and the increase of the path length of photons entering the solution to activate the ZnO surface. Therefore, from this figure, it is suggested that the catalyst area be adjusted to enhance the degradation of OG in the Vis/ZnO process.
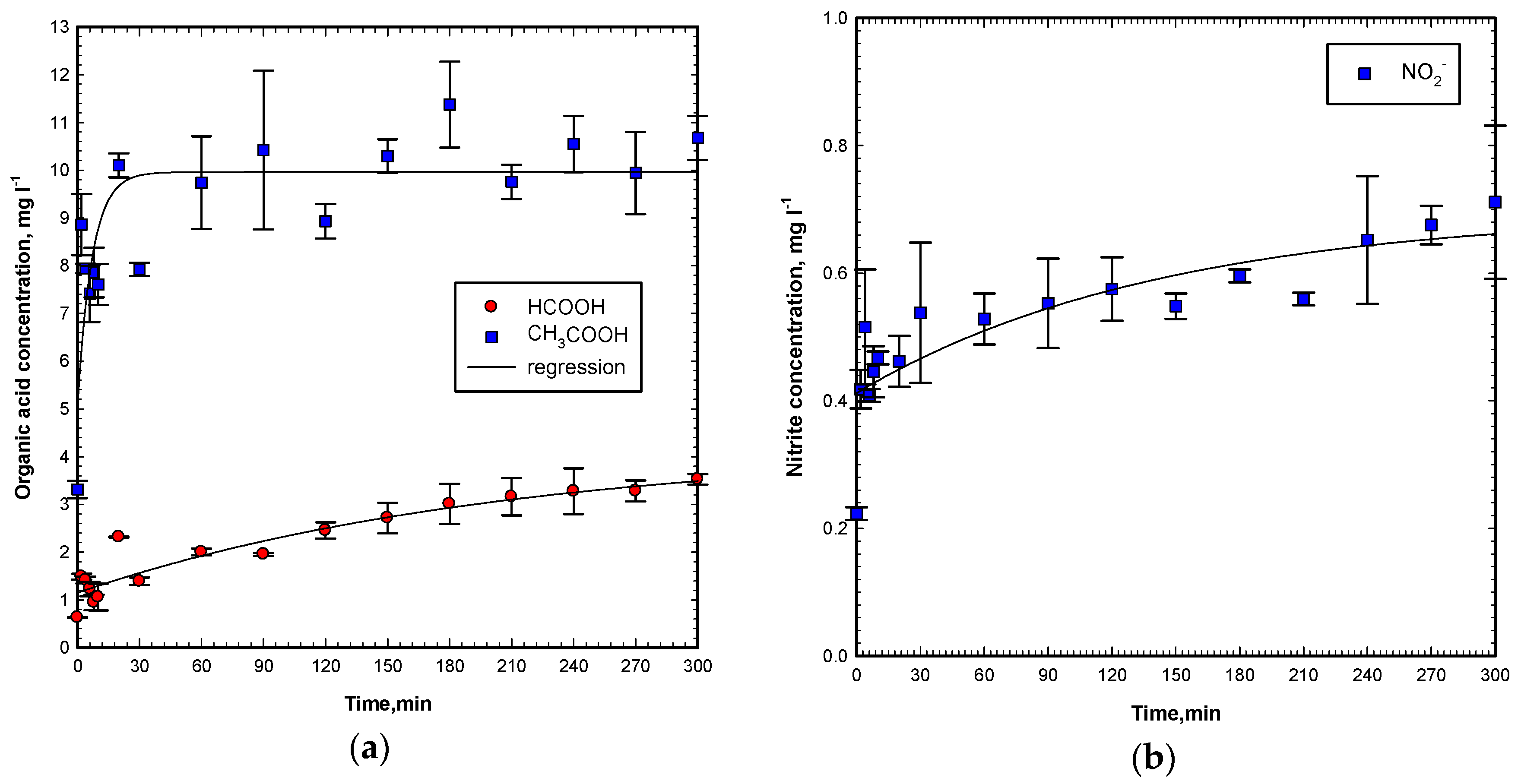
2.8. Product Analysis by FTIR and IC
After treatment by the Vis/ZnO process, the effluent was collected and freeze-dried by a freeze dryer. The dry solid was then mixed with KBr and pressed into a tablet for Fourier transform infrared spectrometry (FTIR) examination. The scan spectra (data not shown) showed the disappearance of the azo link (–N=N–) and the formation of the carboxyl group (–C=O). This is due to the hydroxyl radicals, superoxide radicals, hydrogen peroxide, and singlet oxygen attack that cleaves the azo double bond and oxidizes the carbon in organic molecules. While the OG molecule was degraded and decolorized, ion chromatography (IC) can determine the formation of nitrate, nitrite, and organic acids. From
Figure 11a, organic acids, such as formic acid and acetic acid, are formed during the reaction. The acetic acid concentration increases very fast to 10 mg L
−1 for a 20 min reaction and remains stable. The formic acid increases to about 3 mg L
−1 during a 300 min reaction, slowly. Nitrate is not found in this Vis/ZnO experiment. However, the nitrite concentration is very low, only 0.2 to 0.7 mg L
−1, as shown in
Figure 11b. From the above FTIR and IC results, the fate of the OG molecule in the Vis/ZnO process is proposed to be, firstly, the degradation of OG started from the cleavage of the azo bond and the loss of chromophores to diminish the color. Secondly, the oxidation of OG and intermediates by hydroxyl radicals, superoxide radicals, hydrogen peroxide, and singlet oxygen performs the degradation of benzene and naphthalene compounds, which contribute to TOC. Therefore, the TOC measurement starts to reduce. With certain conditions, the TOC can be totally mineralized. Furthermore, formic acid and acetic acid are formed and identified by IC. The inorganic ions, such as nitrite, also form during the reaction.