Energy Efficient and Intermittently Variable Ammonia Synthesis over Mesoporous Carbon-Supported Cs-Ru Nanocatalysts
Abstract
:1. Introduction
2. Results and Discussion
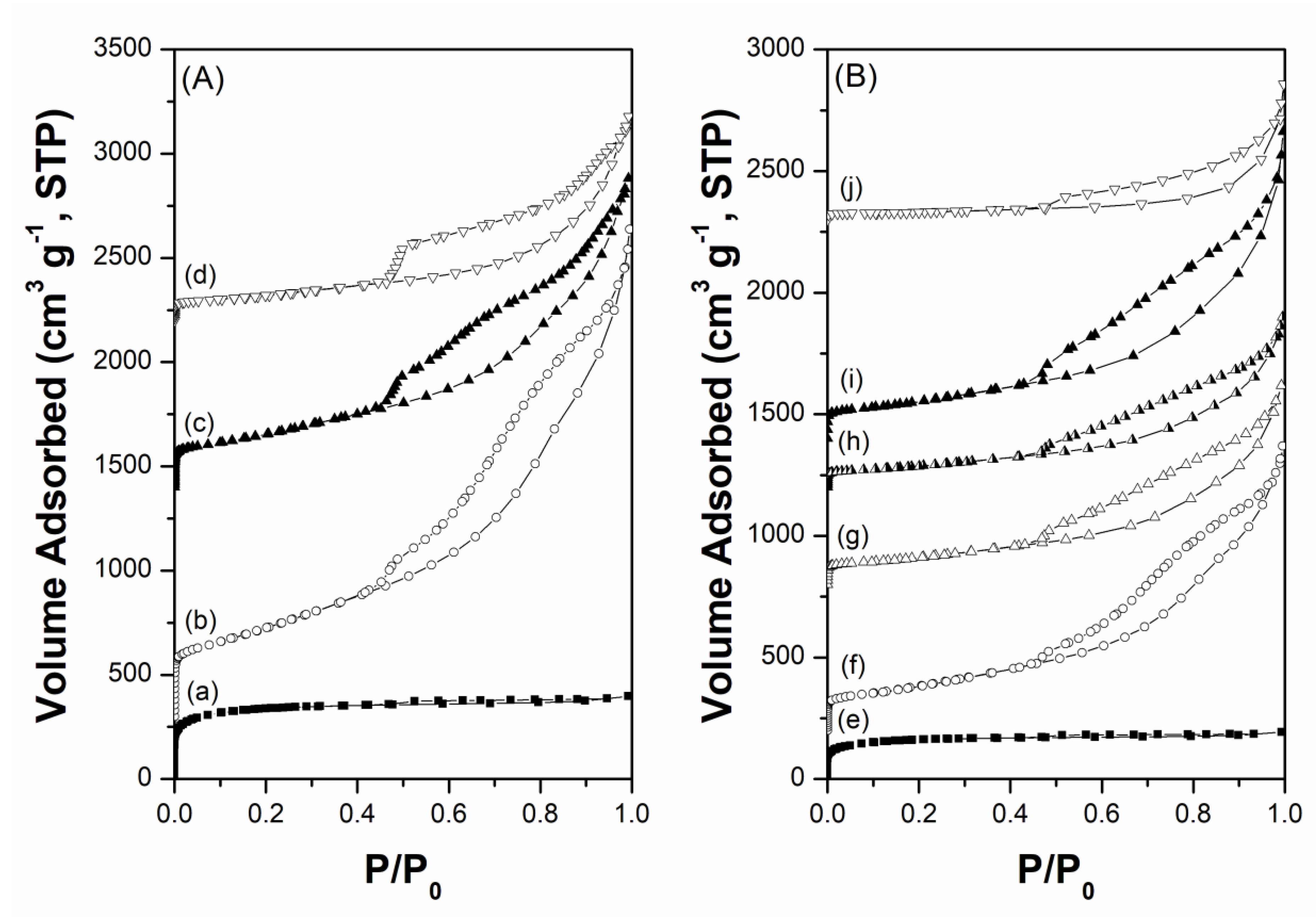
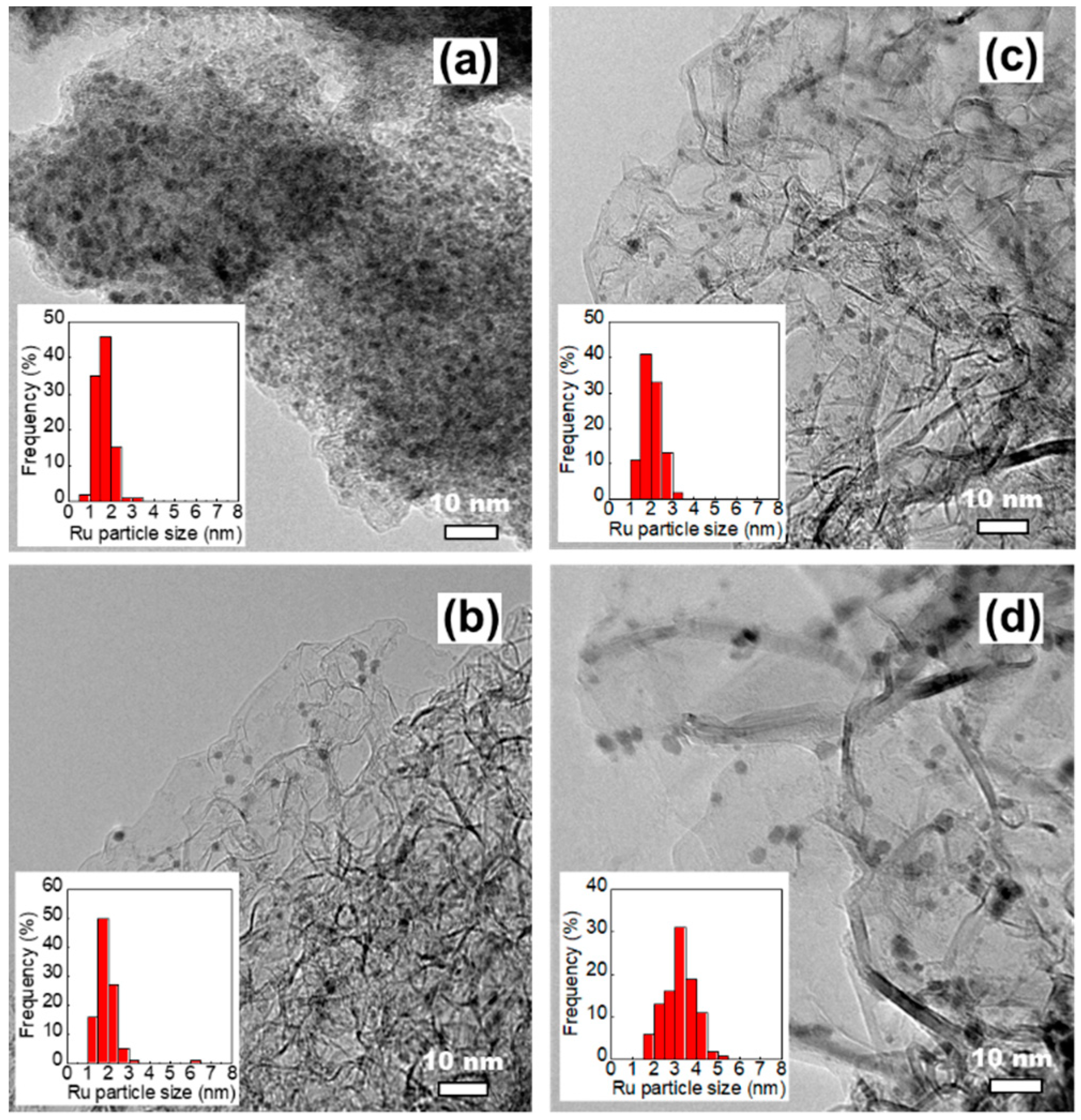
2.1. Characterizations
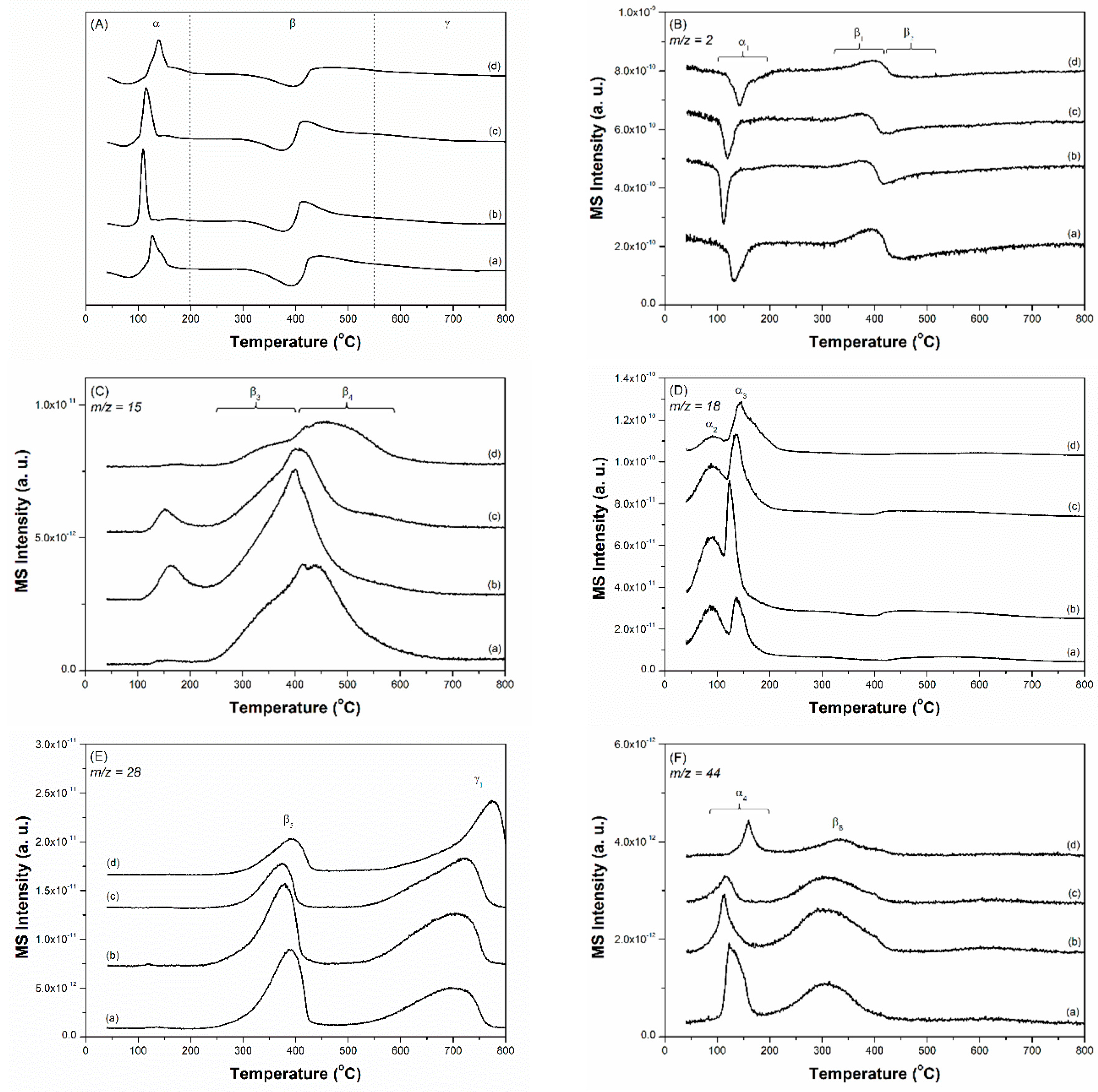
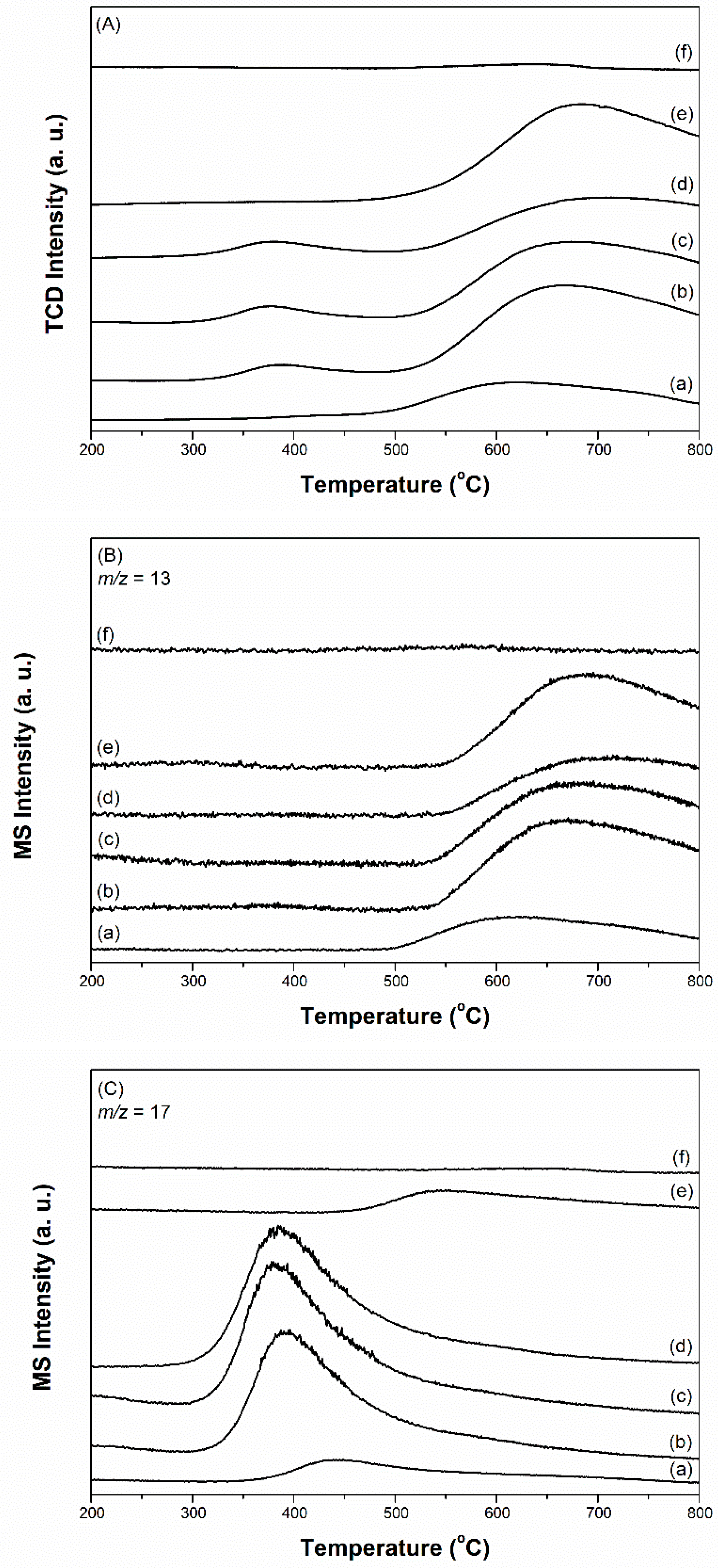
2.2. Temperature-Programmed Studies
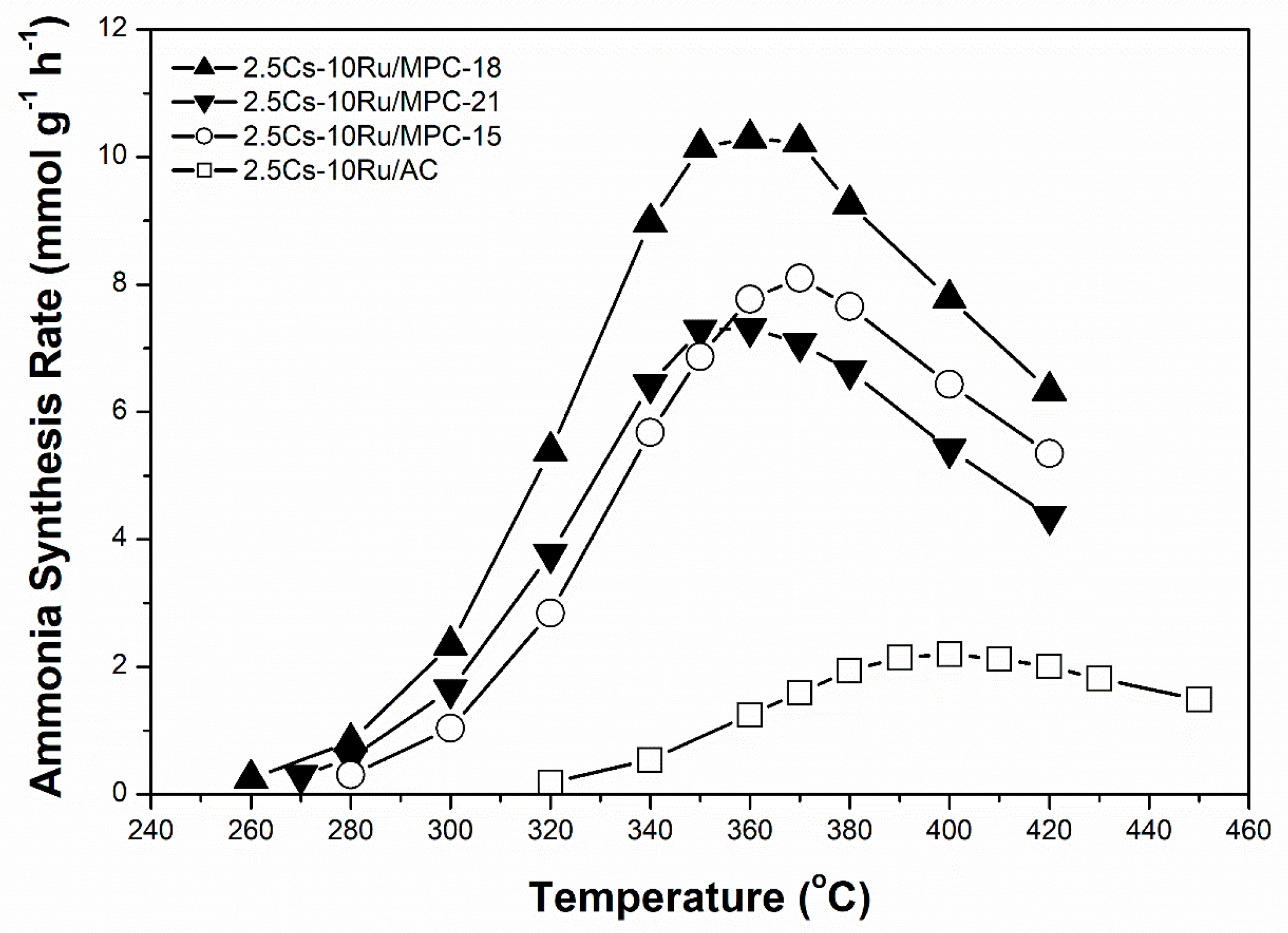
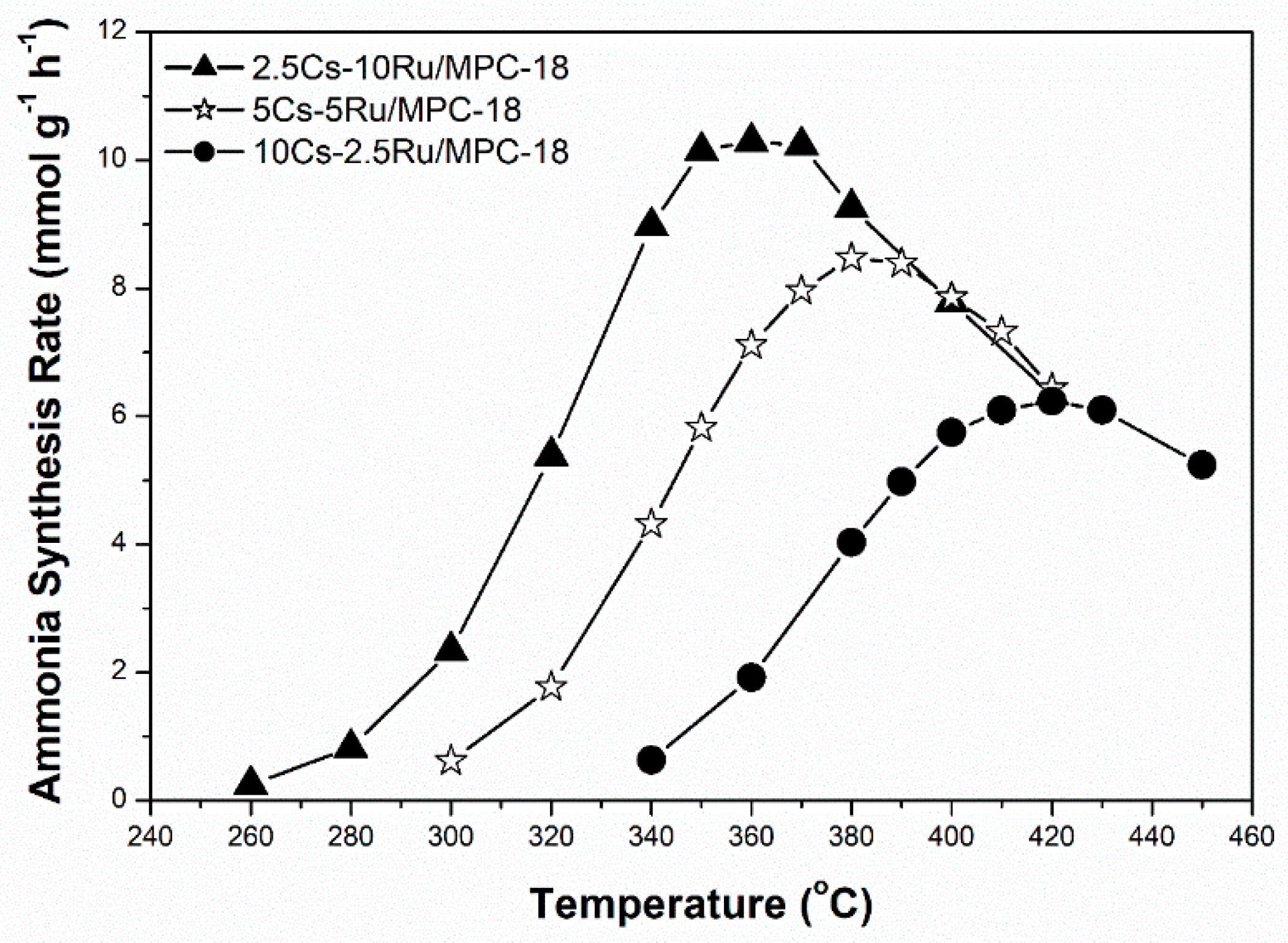
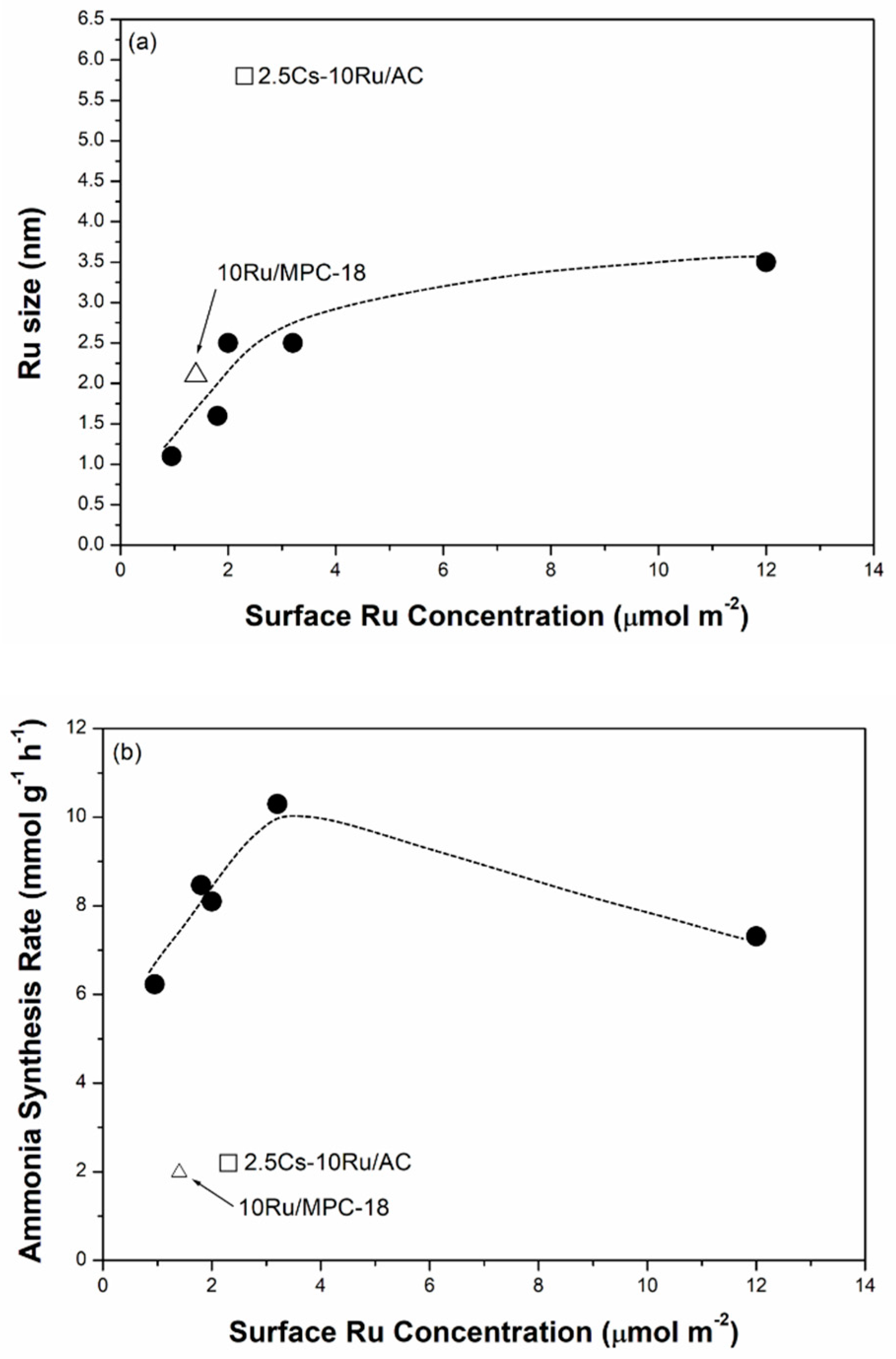
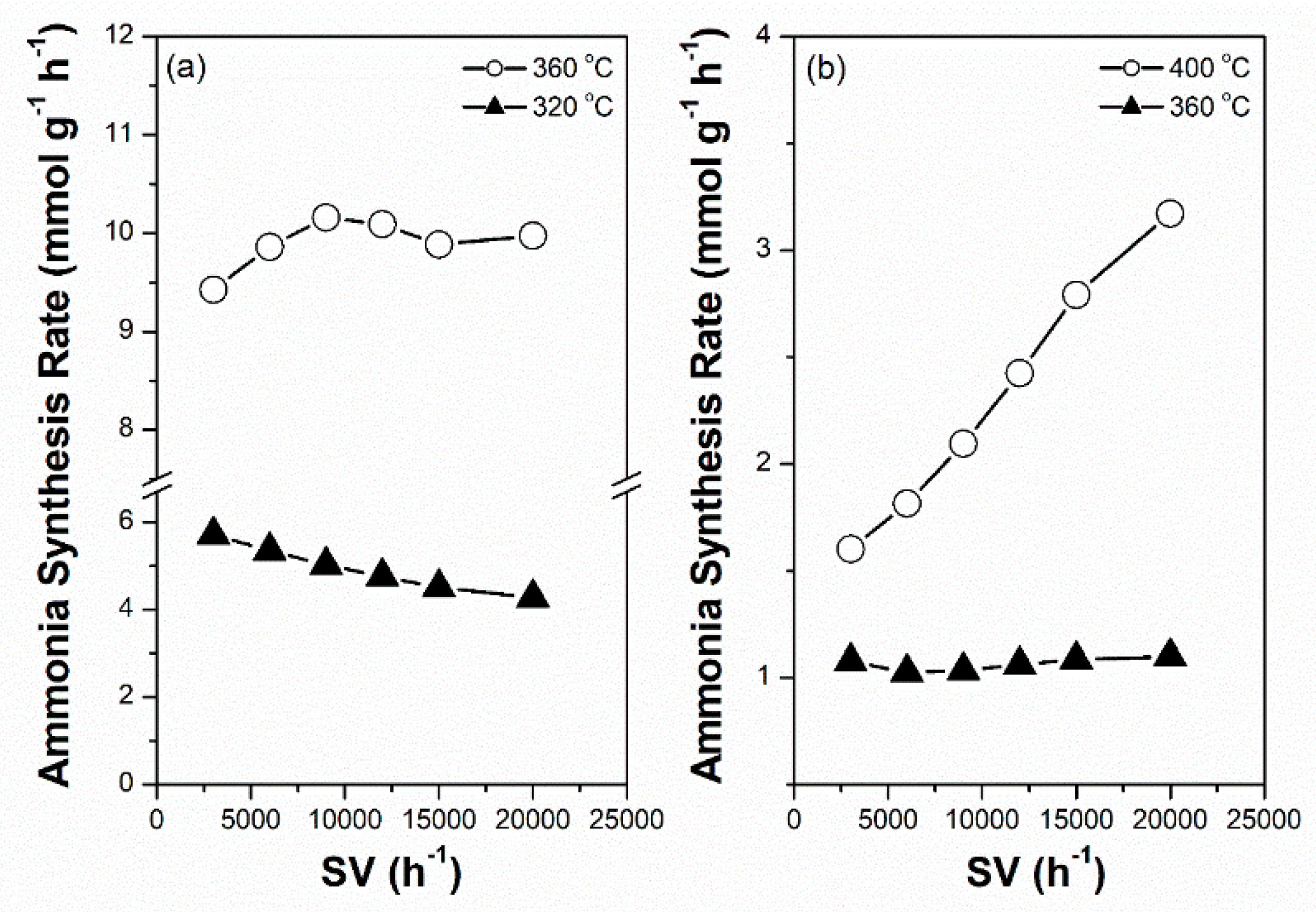
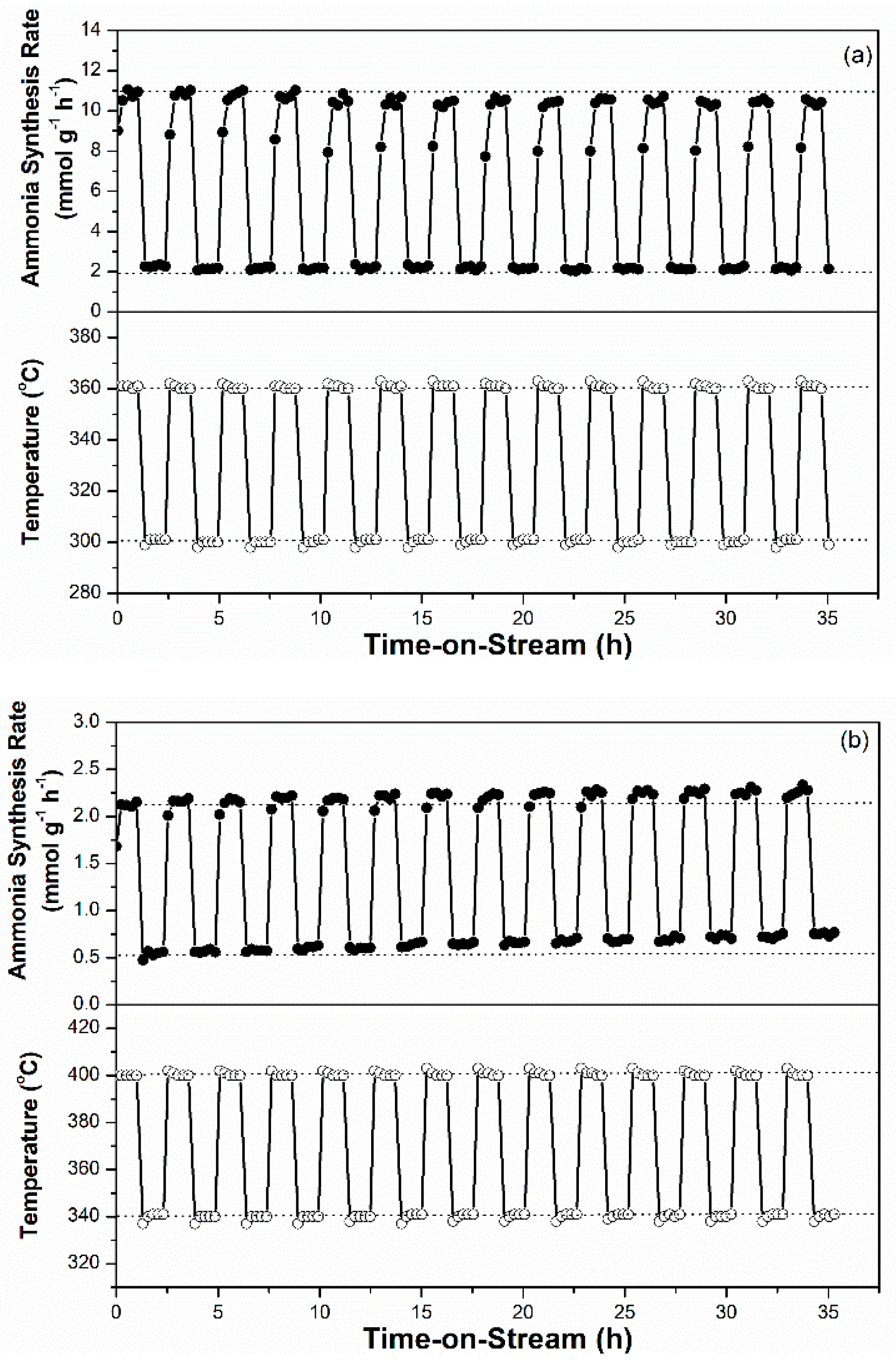
2.3. Mild Ammonia Synthesis
3. Materials and Methods
3.1. Synthesis of Mesoporous Carbon Material-Supported Cs-Ru Catalysts
3.2. Synthesis of Reference Catalysts
3.3. Characterization
3.4. Mild Ammonia Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Energy Information Administration (EIA). International Energy Outlook 2017. Available online: https://www.eia.gov/outlooks/ieo/pdf/0484(2017).pdf (accessed on 11 July 2018).
- United Nations Framework Convention on Climate Change (UNFCCC). Paris agreement. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 8 April 2019).
- Ministry of the Environment, Outline of Long-term Low-carbon Vision. Available online: http://www.env.go.jp/press/103822/713.pdf (accessed on 11 July 2018).
- Ministry of Economy, Trade and Industry, Basic Hydrogen Strategy. Available online: http://www.meti.go.jp/english/press/2017/pdf/1226_003a.pdf (accessed on 6 August 2018).
- Gandía, L.M.; Oroz, R.; Ursúa, A.; Sanchis, P.; Diéguez, P.M. Renewable hydrogen production: performance of an alkaline water electrolyzer working under emulated wind conditions. Energy Fuels 2007, 21, 1699–1706. [Google Scholar] [CrossRef]
- Felice, L.D.; Courson, C.; Jand, N.; Gallucci, K.; Foscolo, P.U.; Kiennemann, A. Catalytic biomass gasification: Simultaneous hydrocarbons steam reforming and CO2 capture in a fluidised bed reactor. Chem. Eng. J. 2009, 154, 375–383. [Google Scholar] [CrossRef]
- Mukherjee, S.; Devaguptapu, S.V.; Sviripa, A.; Lund, C.R.F.; Wu, G. Low-temperature ammonia decomposition catalysts for hydrogen generation. Appl. Catal. B Environ. 2018, 226, 162–181. [Google Scholar] [CrossRef]
- Ju, X.; Liu, L.; Yu, P.; Guo, J.; Zhang, X.; He, T.; Wu, G.; Chen, P. Mesoporous Ru/MgO prepared by a deposition-precipitation method as highly active catalyst for producing COx-free hydrogen from ammonia decomposition. Appl. Catal. B Environ. 2017, 211, 167–175. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Ng, C.F.; Au, C.T. Nano Ru/CNTs: a highly active and stable catalyst for the generation of COx-free hydrogen in ammonia decomposition. Appl. Catal. B Environ. 2004, 48, 237–241. [Google Scholar] [CrossRef]
- Wang, S.J.; Yin, S.F.; Li, L.; Xu, B.Q.; Ng, C.F.; Au, C.T. Investigation on modification of Ru/CNTs catalyst for the generation of COx-free hydrogen from ammonia. Appl. Catal. B Environ. 2004, 52, 287–299. [Google Scholar] [CrossRef]
- US Geological Survey, Nitrogen (Fixed)–Ammonia. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/nitrogen/mcs-2019-nitro.pdf (accessed on 1 February 2019).
- Smil, V. Detonator of the population explosion. Nature 1999, 400, 415. [Google Scholar] [CrossRef]
- Schrock, R.R. Reduction of dinitrogen. Proc. Natl. Acad. Sci. USA 2006, 103, 17087. [Google Scholar] [CrossRef] [PubMed]
- Farla, J.C.M.; Hendriks, C.A.; Blok, K. Carbon dioxide recovery from industrial processes. Energy Convers. Manag. 1995, 36, 827–830. [Google Scholar] [CrossRef]
- Ozaki, A.; Aika, K.; Hori, H. A new catalyst system for ammonia synthesis. Bull. Chem. Soc. Jpn. 1971, 44, 3216. [Google Scholar] [CrossRef]
- Aika, K.; Hori, H.; Ozaki, A. Activation of nitrogen by alkali metal promoted transition metal I. Ammonia synthesis over ruthenium promoted by alkali metal. J. Catal. 1972, 27, 424–431. [Google Scholar] [CrossRef]
- Truszkiewicz, E.; Raróg-Pilecka, W.; Schmidt-Szałowski, K.; Jodzis, S.; Wilczkowska, E.; Łomot, D.; Kaszkur, Z.; Karpiński, Z.; Kowalczyk, Z. Barium-promoted Ru/carbon catalyst for ammonia synthesis: State of the system when operating. J. Catal. 2009, 286, 181–190. [Google Scholar] [CrossRef]
- Rossetti, I.; Mangiarini, F.; Forni, L. Promoters state and catalyst activation during ammonia synthesis over Ru/C. Appl. Catal. A Gen. 2007, 323, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Qi, Y.; Guo, Y.; Lin, J.; Ni, J. Effect of potassium precursors on the thermal stability of K-promoted Ru/carbon catalysts for ammonia synthesis. Catal. Sci. Technol. 2015, 5, 2829–2838. [Google Scholar] [CrossRef]
- Fernández, C.; Sassoye, C.; Debecker, D.P.; Sanchez, C.; Ruiz, P. Effect of the size and distribution of supported Ru nanoparticles on their activity in ammonia synthesis under mild reaction conditions. Appl. Catal. A Gen. 2014, 474, 194–202. [Google Scholar]
- Hansen, T.W.; Hansen, P.L.; Dahl, S.; Jacobsen, C.J.H. Support effect and active sites on promoted ruthenium catalysts for ammonia synthesis. Catal. Lett. 2002, 84, 7–12. [Google Scholar] [CrossRef]
- Brown, D.E.; Edmonds, T.; Joyner, R.W.; McCarroll, J.J.; Tennison, S.R. The genesis and development of the commercial BP doubly promoted catalyst for ammonia synthesis. Catal. Lett. 2014, 144, 545–552. [Google Scholar] [CrossRef]
- Sato, K.; Imamura, K.; Kawano, Y.; Miyahara, S.; Yamamoto, T.; Matsumura, S.; Nagaoka, K. A low-crystalline ruthenium nano-layer supported on praseodymium oxide as an active catalyst for ammonia synthesis. Chem. Sci. 2017, 8, 674–679. [Google Scholar] [CrossRef]
- Ogura, Y.; Sato, K.; Miyahara, S.; Kawano, Y.; Toriyama, T.; Yamamoto, T.; Matsumura, S.; Hosokawa, S.; Nagaoka, K. Efficient ammonia synthesis over a Ru/La0.5Ce0.5O1.75 catalyst pre-reduced at high temperature. Chem. Sci. 2018, 9, 2230–2237. [Google Scholar] [CrossRef]
- Kitano, M.; Kanbara, S.; Inoue, Y.; Kuganathan, N.; Sushko, P.V.; Yokoyama, T.; Hara, M.; Hosono, H. Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis. Nat. Commun. 2015, 6, 6731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitano, M.; Inoue, Y.; Sasase, M.; Kishida, K.; Kobayashi, Y.; Nishiyama, K.; Tada, T.; Kawamura, S.; Yokoyama, T.; Hara, M.; et al. Self-organized ruthenium–barium core–shell nanoparticles on a mesoporous calcium amide matrix for efficient low-temperature ammonia synthesis. Angew. Chem. Int. Ed. 2018, 57, 2648–2652. [Google Scholar] [CrossRef]
- Mao, C.; Yu, L.; Li, J.; Zhao, J.; Zhang, L. Energy-confined solar thermal ammonia synthesis with K/Ru/TiO2−xHx. Appl. Catal. B Environ. 2018, 224, 612–620. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Miśkiewicz, E.; Szmigiel, D.; Kowalczyk, Z. Structure sensitivity of ammonia synthesis over promoted ruthenium catalysts supported on graphitised carbon. J. Catal. 2005, 231, 11–19. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Miśkiewicz, E.; Jodzis, S.; Petryk, J.; Łomot, D.; Kaszkur, Z.; Karpiński, Z.; Kowalczyk, Z. Carbon-supported ruthenium catalysts for NH3 synthesis doped with caesium nitrate: Activation process, working state of Cs–Ru/C. J. Catal. 2006, 239, 313–325. [Google Scholar] [CrossRef]
- Kowalczyk, Z.; Jodzis, S.; Raróg, W.; Zielinski, J.; Pielaszek, J.; Presz, A. Carbon-supported ruthenium catalyst for the synthesis of ammonia. The effect of the carbon support and barium promoter on the performance. Appl. Catal. A Gen. 1999, 184, 95–102. [Google Scholar] [CrossRef]
- Rossetti, I.; Pernicone, N.; Forni, L. Graphitised carbon as support for Ru/C ammonia synthesis catalyst. Catal. Today 2005, 102–103, 219–224. [Google Scholar] [CrossRef]
- Zeng, H.S.; Inazu, K.; Aika, K. The working state of the barium promoter in ammonia synthesis over an active-carbon-supported ruthenium catalyst using barium nitrate as the promoter precursor. J. Catal. 2002, 211, 33–41. [Google Scholar] [CrossRef]
- Nishi, M.; Chen, S.Y.; Takagi, H. A mesoporous carbon-supported and Cs-promoted Ru catalyst with enhanced activity and stability for sustainable ammonia synthesis. ChemCatChem 2018, 10, 3411–3414. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Orderd mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; Higgins, J.B.; Schlenker, J.L. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Fukuoka, A.; Kimura, J.; Oshio, T.; Sakamoto, Y.; Ichikawa, M. Preferential oxidation of carbon monoxide catalysed by platinum nanoparticles in mesoporous silica. J. Am. Chem. Soc. 2007, 129, 10120–10125. [Google Scholar] [CrossRef] [PubMed]
- Olkhovyk, O.; Jaroniec, M. Periodic mesoporous organosilica with large heterocyclic bridging groups. J. Am. Chem. Soc. 2004, 127, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, S.; Guan, S.; Fukushima, Y.; Ohsuna, T.; Terasaki, O. Novel mesoporous materials with a uniform distribution of organic groups and inorganic oxide in their frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614. [Google Scholar] [CrossRef]
- Liang, C.D.; Dai, S. Synthesis of mesoporous carbon materials via enhanced hydrogen-bonding interaction. J. Am. Chem. Soc. 2006, 128, 5216–5317. [Google Scholar] [CrossRef]
- Lee, J.S.; Joo, S.H.; Ryoo, R. Synthesis of mesoporous silicas of controlled pore wall thickness and their replication to ordered nanoporous carbons with various pore diameters. J. Am. Chem. Soc. 2002, 124, 1156–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Q.; Meng, Y.; Gu, D.; Yan, Y.; Yu, C.Z.; Tu, B.; Zhao, D.Y. A facile aqueous route to synthesize highly ordered mesoporous polymers and carbon frameworks with Ia(3)over-bard bicontinuous cubic structure. J. Am. Chem. Soc. 2005, 127, 13508–13509. [Google Scholar] [CrossRef]
- Kasahara, N.; Shiraishi, S.; Oya, A. Heterogeneous graphitization of thin carbon fiber derived from phenol-formaldehyde resin. Carbon 2003, 41, 1654–1656. [Google Scholar] [CrossRef]
- Eslava, J.L.; Iglesias-Juez, A.; Agostini, G.; Fernández-García, M.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Time-resolved XAS investigation of the local environment and evolution of oxidation states of a Fischer–Tropsch Ru–Cs/C catalyst. ACS Catal. 2016, 6, 1437–1445. [Google Scholar] [CrossRef]
- Rossetti, I.; Forni, L. Effect of Ru loading and of Ru precursor in Ru/C catalysts for ammonia synthesis. Appl. Catal. A Gen. 2005, 282, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.K.; Torrente-Murciano, L. Low temperature H2 production from ammonia using ruthenium-based catalysts: Synergetic effect of promoter and support. Appl. Catal. B Environ. 2015, 172–173, 129–135. [Google Scholar] [CrossRef]
- Lin, B.; Guo, Y.; Lin, J.; Ni, J.; Lin, J.; Jiang, L.; Wang, Y. Deactivation study of carbon-supported ruthenium catalyst with potassium promoter. Appl. Catal. A Gen. 2017, 541, 1–7. [Google Scholar] [CrossRef]
- Addoun, A.; Dentzer, J.; Ehrburger, P. Porosity of carbons obtained by chemical activation: effect of the nature of the alkaline carbonates. Carbon 2002, 40, 1140–1143. [Google Scholar] [CrossRef]
- Li, C.; Shao, Z.; Pang, M.; Williams, C.T.; Zhang, X.; Liang, C. Carbon nanotubes supported mono- and bimetallic Pt and Ru catalysts for selective hydrogenation of phenylacetylene. Ind. Eng. Chem. Res. 2012, 51, 4934–4941. [Google Scholar] [CrossRef]
- Li, Z.; Liang, C.; Feng, Z.; Ying, P.; Wang, D.; Li, C. Ammonia synthesis on graphitic-nanofilament supported Ru catalysts. J. Mol. Catal. A Chem. 2004, 211, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, C.J.H.; Dahl, S.; Hansen, P.L.; Törnqvist, E.; Jensen, L.; Topsøe, H.; Prip, D.V.; Møenshaug, P.B.; Chorkendorff, I. Structure sensitivity of supported ruthenium catalysts for ammonia synthesis. J. Mol. Catal. A Chem. 2000, 163, 19–26. [Google Scholar] [CrossRef]
- Aika, K. Role of alkali promoter in ammonia synthesis over ruthenium catalysts—Effect on reaction mechanism. Catal. Today 2017, 286, 14–20. [Google Scholar] [CrossRef]
- Liang, C.; Wei, Z.; Xin, Q.; Li, C. Ammonia synthesis over Ru/C catalysts with different carbon supports promoted by barium and potassium compounds. Appl. Catal. A Gen. 2001, 208, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Morishita, T.; Tsumura, T.; Toyoda, M.; Przepiorski, J.; Morawski, A.W.; Konno, H.; Inagaki, M. A review of the control of pore structure in MgO-templated nanoporous carbons. Carbon 2010, 48, 2707. [Google Scholar] [CrossRef]
- Overview of the High Pressure Gas Safety Act. Available online: https://www.khk.or.jp/Portals/0/resources/english/dl/overview_hpg_act.pdf (accessed on 1 October 2016).

| Samples | Cs/Ru Molar Ratio | Ru Loading (wt %) | SBET (m2 g−1) | VTotal (cm3 g−1) | VMicro. (cm3 g−1) 3 | VMeso. (cm3 g−1) 4 | Pore Size (nm) | ||
|---|---|---|---|---|---|---|---|---|---|
| (Expected) 1 | (Solid) 2 | (Expected) 1 | (Solid) 2 | ||||||
| AC | - | - | - | - | 1260 | 0.62 | 0.51 | 0.11 | 1.6 |
| MPC-15 | - | - | - | - | 1180 | 2.94 | 0.55 | 2.39 | 5.8 |
| MPC-18 | - | - | - | - | 930 | 2.29 | 0.37 | 1.92 | 5.1 |
| MPC-21 | - | - | - | - | 270 | 1.28 | 0.11 | 1.17 | 6.8 |
| 2.5Cs-10Ru/AC | 2.5 | 1.3 | 10 | 13.4 | 580 | 0.30 | 0.24 | 0.06 | 1.6 |
| 2.5Cs-10Ru/MPC/15 | 2.5 | 1.1 | 10 | 13.7 | 680 | 1.81 | 0.27 | 1.54 | 5.8 |
| 2.5Cs-10Ru/MPC-18 | 2.5 | 1.1 | 10 | 13.8 | 430 | 1.33 | 0.17 | 1.16 | 5.8 |
| 5Cs-5Ru/MPC-18 | 5.0 | 2.1 | 5.0 | 8.1 | 440 | 1.40 | 0.18 | 1.22 | 5.8 |
| 10Cs-2.5Ru/MPC-18 | 10 | 3.7 | 2.5 | 4.8 | 500 | 1.52 | 0.20 | 1.32 | 5.8 |
| 2.5Cs/MPC-18 | - | - 5 | - | - | 580 | 1.71 | 0.23 | 1.48 | 5.8 |
| 10Ru/MPC-18 | - | - | 10 | 11.3 | 800 | 1.84 | 0.32 | 1.52 | 5.1 |
| 2.5Cs-10Ru/MPC-21 | 2.5 | 1.1 | 10 | 13.4 | 110 | 0.58 | 0.04 | 0.54 | 7.3 |
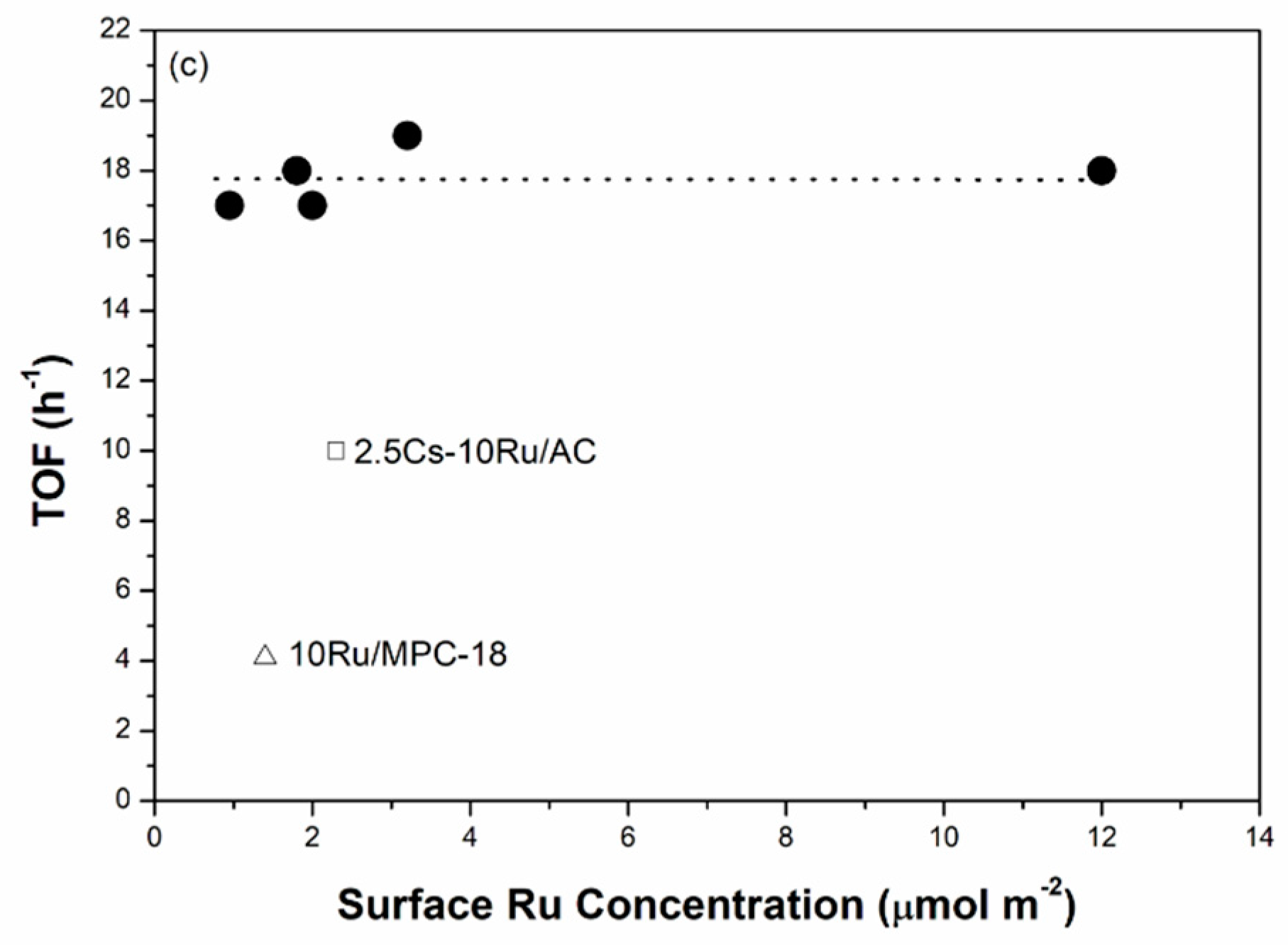
| Samples | Ru Conc. (μmol m−2) | Ru Size (nm) | Cs Conc. (μmol m−2) | CO2 Uptake (mmol g−1) 3 | Ammonia Synthesis Activity | ||
|---|---|---|---|---|---|---|---|
| HRTEM 1 | CO Chem. 2 | Maximum Rate (mmol g−1 h−1) 4 | TOF (h−1) | ||||
| 2.5Cs-10Ru/AC | 2.3 | 1.6 ± 0.4 | 5.8 (16%) | 3.0 | 1.6 | 2.2 (400) | 10 |
| 2.5Cs-10Ru/MPC-15 | 2.0 | 1.9 ± 0.6 | 2.5 (36%) | 2.2 | 2.4 | 8.1 (370) | 17 |
| 2.5Cs-10Ru/MPC-18 | 3.2 | 2.1 ± 0.4 | 2.4 (40%) | 3.5 | 2.4 | 10 (360) | 19 |
| 5Cs-5Ru/MPC-18 | 1.8 | 1.6 ± 0.3 | 1.6 (59%) | 3.8 | 2.6 | 8.5 (380) | 18 |
| 10Cs-2.5Ru/MPC-18 | 0.95 | 1.2 ± 0.3 | 1.3 (79%) | 3.5 | 2.5 | 6.2 (420) | 17 |
| 2.5Cs/MPC-18 | - | - | - | 4.2 | 3.5 | 0 | - |
| 10Ru/MPC-18 | 1.4 | 2.1 ± 0.6 | 2.1 (44%) | - | 0 | 2.0 (510) | 4.0 |
| 2.5Cs-10Ru/MPC-21 | 12 | 3.2 ± 0.8 | 3.7 (31%) | 13 | 2.7 | 7.3 (360) | 18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishi, M.; Chen, S.-Y.; Takagi, H. Energy Efficient and Intermittently Variable Ammonia Synthesis over Mesoporous Carbon-Supported Cs-Ru Nanocatalysts. Catalysts 2019, 9, 406. https://doi.org/10.3390/catal9050406
Nishi M, Chen S-Y, Takagi H. Energy Efficient and Intermittently Variable Ammonia Synthesis over Mesoporous Carbon-Supported Cs-Ru Nanocatalysts. Catalysts. 2019; 9(5):406. https://doi.org/10.3390/catal9050406
Chicago/Turabian StyleNishi, Masayasu, Shih-Yuan Chen, and Hideyuki Takagi. 2019. "Energy Efficient and Intermittently Variable Ammonia Synthesis over Mesoporous Carbon-Supported Cs-Ru Nanocatalysts" Catalysts 9, no. 5: 406. https://doi.org/10.3390/catal9050406