The Ternary Heterostructures of BiOBr/Ultrathin g-C3N4/Black Phosphorous Quantum Dot Composites for Photodegradation of Tetracycline
Abstract
:1. Introduction
2. Experimentals
2.1. Materials
2.2. Preparation of UCNs
2.3. Preparation of a BiOBr/UCN (7:3) Photocatalytic System
2.4. Preparation of BiOBr/UCN/BPQDs Ternary Structure
3. Results and Discussion
3.1. Physicochemical Properties of BiOBr/UCN/BPQDs Heterojunctions
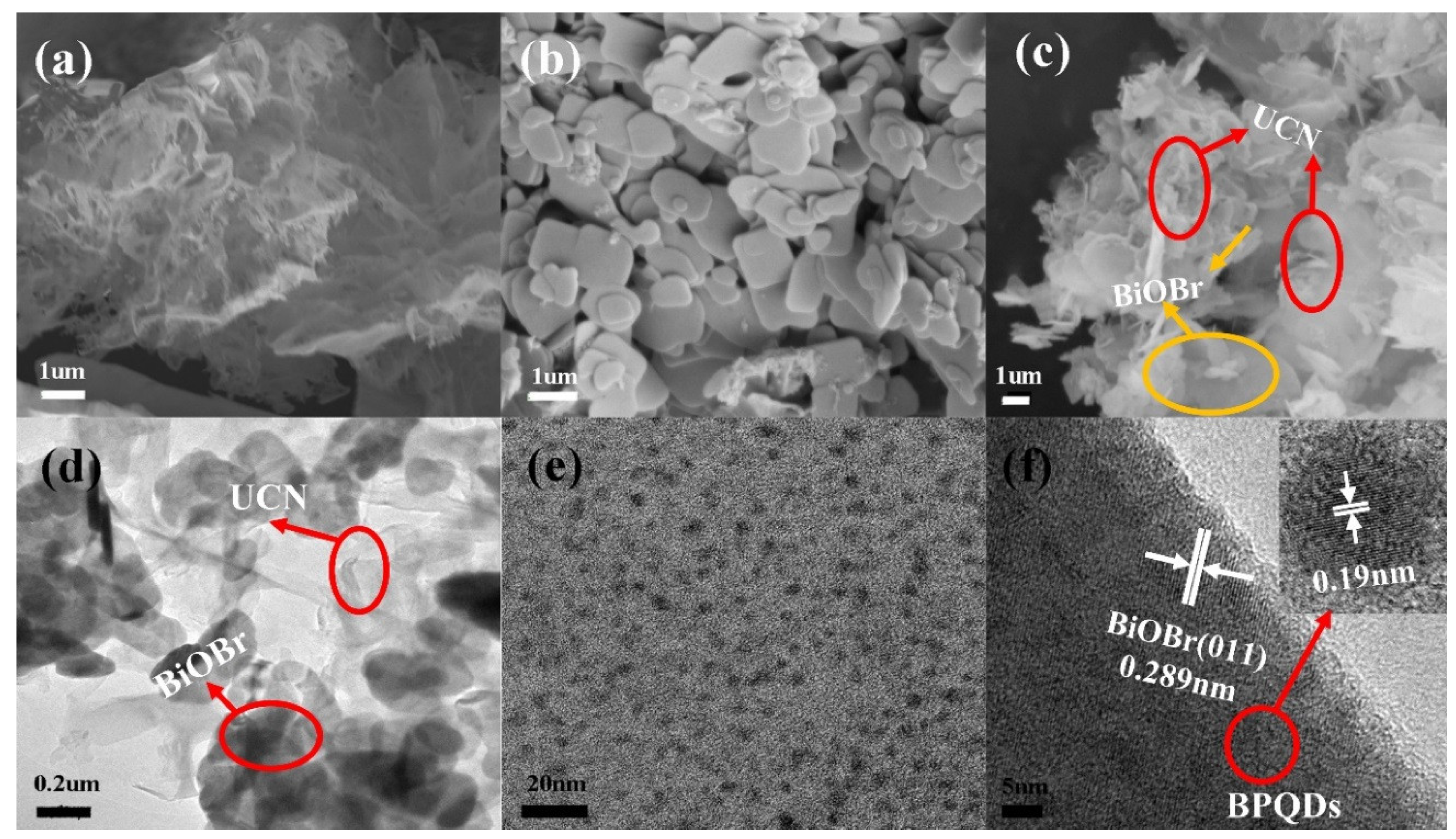
3.1.1. SEM and TEM Analysis
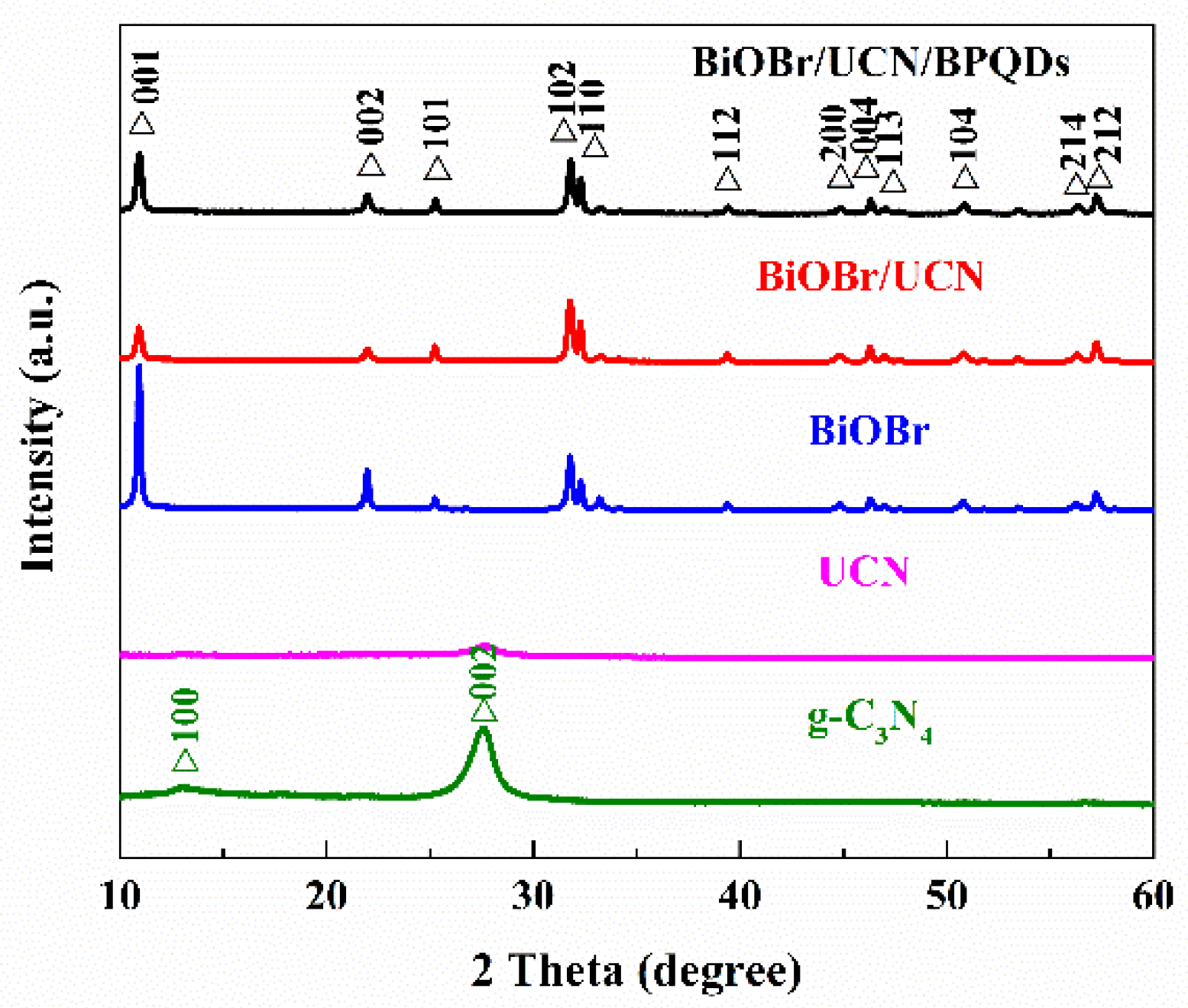
3.1.2. X-ray Diffraction (XRD) Analysis
3.1.3. FT-IR Analysis
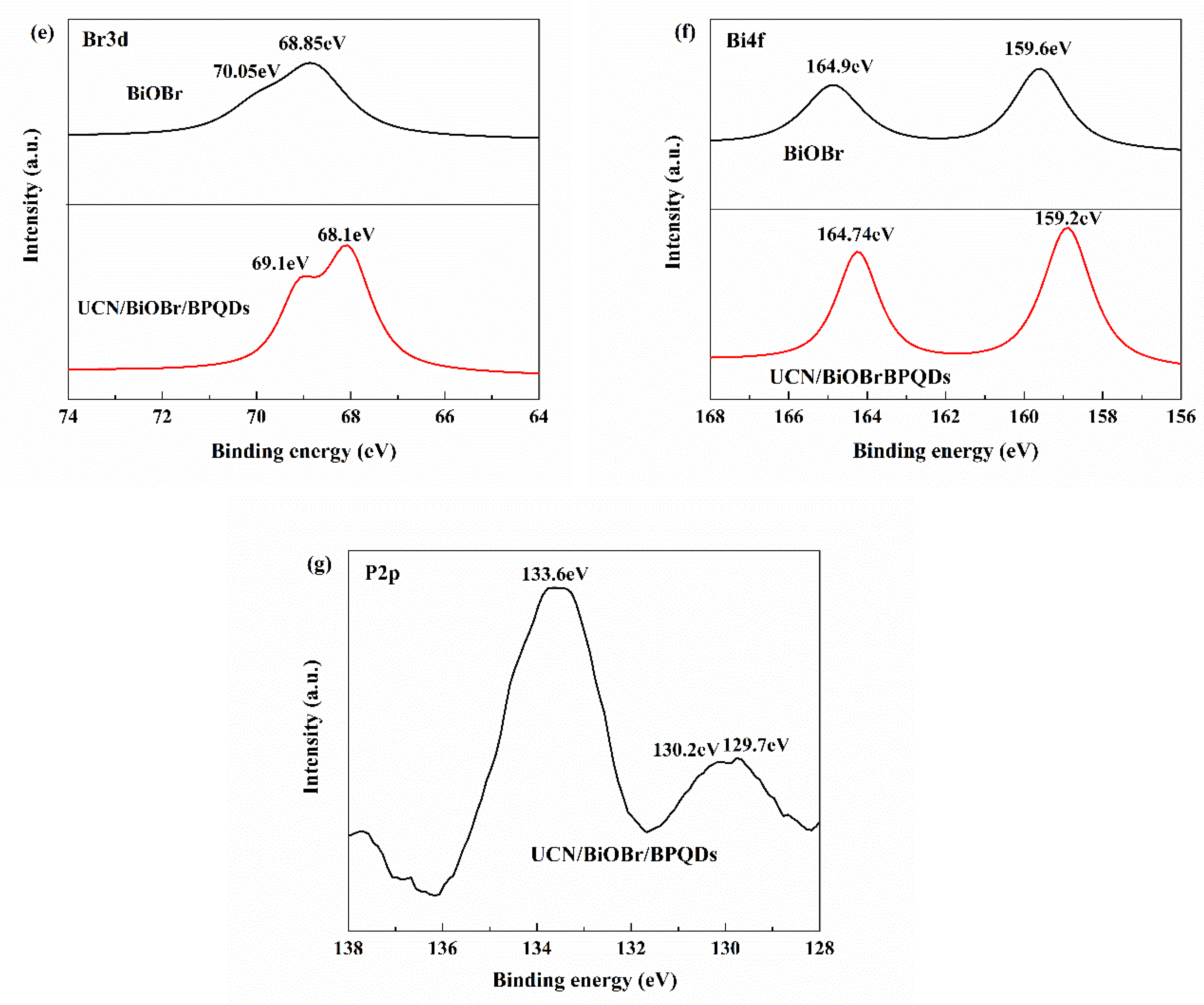
3.1.4. XPS Analysis
3.1.5. Photoluminescence Spectra and Optical Performance Analysis
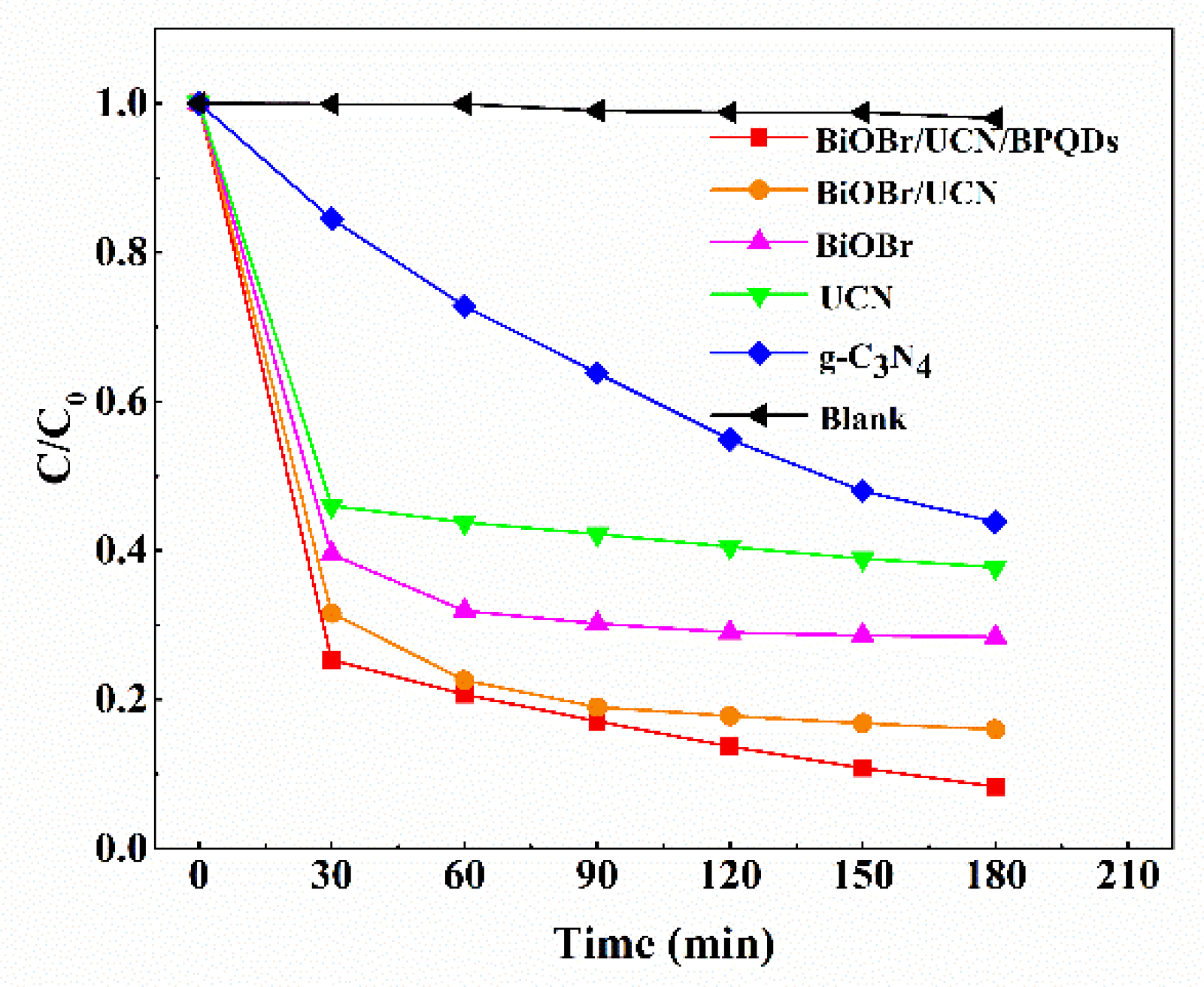
3.2. Photocatalytic Performance of BiOBr/UCN/BPQDs Heterostructures
3.3. Possible Photocatalytic Mechanism of BiOBr/UCN/BPQDs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, M.H.; Bae, H.; Jung, J.Y. Tetracycline degradation by ozonation in the aqueous phase: Proposed degradation intermediates and pathway. J. Hazard. Mater. 2010, 181, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Wang, Y.J.; Sun, R.J.; Zhou, D.M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sorensen, B.; Sengelov, G.; Tjornelund, J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Y.; Zhang, X.; Xu, G.; Wang, D.; Lv, J.; Zheng, Z.; Wu, Y. NiS and MoS2 nanosheet co-modified graphitic C3N4 ternary heterostructure for high efficient visible light photodegradation of antibiotic. J. Hazard. Mater. 2018, 341, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhou, S.; Meng, Q.; Lv, H.; Chen, Z.; Zhang, Y.; Jin, M.; Yuan, M.; Wang, X.; Zhou, G. Synergistic Effects of Ag Nanoparticles/BiV1−xMoxO4 with Enhanced Photocatalytic Activity. Nanoscale Res. Lett. 2017, 12, 588. [Google Scholar] [CrossRef] [PubMed]
- Ashok Kumar, K.V.; Chandana, L.; Ghosal, P.; Subrahmanyam, C. Simultaneous photocatalytic degradation of p-cresol and Cr(VI) by metal oxides supported reduced graphene oxide. Mol. Catal. 2018, 451, 87–95. [Google Scholar] [CrossRef]
- Ribao, P.; Rivero, M.J.; Ortiz, I. TiO2 structures doped with noble metals and/or graphene oxide to improve the photocatalytic degradation of dichloroacetic acid. Environ. Sci. Pollut. Res. Int. 2017, 24, 12628–12637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.; He, Y.; Li, K.; Jiang, Y.; Xu, X.; Yuan, Y.; Lin, K. In situ fabrication of hierarchically porous g-C3N4 and understanding on its enhanced photocatalytic activity based on energy absorption. Appl. Catal. B Environ. 2018, 236, 64–75. [Google Scholar] [CrossRef]
- Li, L.; Meng, Q.; Lv, H.; Shui, L.; Zhang, Y.; Zhang, Z.; Chen, Z.; Yuan, M.; Nötzel, R.; Wang, X.; et al. Synthesis of barbituric acid doped carbon nitride for efficient solar-driven photocatalytic degradation of aniline. Appl. Surf. Sci. 2018, 428, 739–747. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Xia, Y.; Lv, K.; Li, Q.; Sun, J.; Li, M. Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. B Environ. 2016, 185, 225–232. [Google Scholar] [CrossRef]
- Lu, X.; Xu, K.; Chen, P.; Jia, K.; Liu, S.; Wu, C. Facile one step method realizing scalable production of g-C3N4 nanosheets and study of their photocatalytic H2 evolution activity. J. Mater. Chem. A 2014, 2, 18924–18928. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Z.; Yan, L.; Luo, S.; Luo, X.; Huo, M.; Guo, Y. Fabrication of platinum-deposited carbon nitride nanotubes by a one-step solvothermal treatment strategy and their efficient visible-light photocatalytic activity. Appl. Catal. B Environ. 2015, 165, 428–437. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. Nano Energy 2015, 13, 757–770. [Google Scholar] [CrossRef]
- Chang, F.; Xie, Y.; Li, C.; Chen, J.; Luo, J.; Hu, X.; Shen, J. A facile modification of g-C3N4 with enhanced photocatalytic activity for degradation of methylene blue. Appl. Surf. Sci. 2013, 280, 967–974. [Google Scholar] [CrossRef]
- Li, K.; Su, F.-Y.; Zhang, W.D. Modification of g-C3N4 nanosheets by carbon quantum dots for highly efficient photocatalytic generation of hydrogen. Appl. Surf. Sci. 2016, 375, 110–117. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, T.; Guo, Y.; Zhang, Z.; Fang, X. Ultrathin g-C3N4 nanosheets coupled with carbon nanodots as 2D/0D composites for efficient photocatalytic H2 evolution. Appl. Catal. B Environ. 2016, 193, 248–258. [Google Scholar] [CrossRef]
- Lv, J.; Dai, K.; Zhang, J.; Liu, Q.; Liang, C.; Zhu, G. Facile constructing novel 2D porous g-C3N4 /BiOBr hybrid with enhanced visible-light-driven photocatalytic activity. Sep. Purif. Technol. 2017, 178, 6–17. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, X.; Liu, D.; Wang, J.; Zhu, Y. Enhanced catalytic activity of potassium-doped graphitic carbon nitride induced by lower valence position. Appl. Catal. B Environ. 2015, 164, 77–81. [Google Scholar] [CrossRef]
- Bao, Y.; Chen, K. Novel Z-scheme BiOBr/reduced graphene oxide/protonated g-C3N4 photocatalyst: Synthesis, characterization, visible light photocatalytic activity and mechanism. Appl. Surf. Sci. 2018, 437, 51–61. [Google Scholar] [CrossRef]
- Wang, H.T.; Shi, M.S.; Yang, H.F.; Chang, N.; Zhang, H.; Liu, Y.P.; Lu, M.C.; Ao, D.; Chu, D.Q. Template-free synthesis of nanosliced BiOBr hollow microspheres with high surface area and efficient photocatalytic activity. Mater. Lett. 2018, 222, 164–167. [Google Scholar] [CrossRef]
- Qin, X.; Cheng, H.; Wang, W.; Huang, B.; Zhang, X.; Dai, Y. Three dimensional BiOX (X = Cl, Br and I) hierarchical architectures: Facile ionic liquid-assisted solvothermal synthesis and photocatalysis towards organic dye degradation. Mater. Lett. 2013, 100, 285–288. [Google Scholar] [CrossRef]
- Lu, J.; Meng, Q.; Lv, H.; Shui, L.; Jin, M.; Zhang, Z.; Chen, Z.; Yuan, M.; Wang, X.; Liu, J.-M.; et al. Synthesis of visible-light-driven BiOBrx I1−x solid solution nanoplates by ultrasound-assisted hydrolysis method with tunable bandgap and superior photocatalytic activity. J. Alloy Compd. 2018, 732, 167–177. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, J.; Yin, S.; Li, H.; Xu, H.; He, M.; Huang, L.; Zhang, Q. Improvement of visible light photocatalytic activity over flower-like BiOCl/BiOBr microspheres synthesized by reactable ionic liquids. Colloid Surf. A 2013, 420, 89–95. [Google Scholar] [CrossRef]
- Yan, P.; Jiang, D.; Tian, Y.; Xu, L.; Qian, J.; Li, H.; Xia, J.; Li, H. A sensitive signal-on photoelectrochemical sensor for tetracycline determination using visible-light-driven flower-like CN/BiOBr composites. Biosens. Bioelectron. 2018, 111, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Krivtsov, I.; García, J.R.; Palmisano, L. Selective photocatalytic oxidation of aromatic alcohols in water by using P-doped g-C3N4. Appl. Catal. B Environ. 2018, 220, 222–233. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, T.; Wang, P.; Wang, L.; Ye, L.; Shi, X.; Bai, W. Size-dependent role of gold in g-C3N4/BiOBr/Au system for photocatalytic CO2 reduction and dye degradation. Sol. Energy Mater. Sol. C 2016, 157, 406–414. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Cheng, F.; Chen, Z.; Dong, X. BiOBr/protonated graphitic C3N4 heterojunctions: Intimate interfaces by electrostatic interaction and enhanced photocatalytic activity. J. Alloys Compd. 2015, 634, 215–222. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Facets coupling of BiOBr-g-C3N4 composite photocatalyst for enhanced visible-light-driven photocatalytic activity. Appl. Catal. B Environ. 2013, 142, 1–7. [Google Scholar] [CrossRef]
- Yasaei, P.; Kumar, B.; Foroozan, T.; Wang, C.; Asadi, M.; Tuschel, D.; Indacochea, J.E.; Klie, R.F.; Salehi-Khojin, A. High-quality black phosphorus atomic layers by liquid-phase exfoliation. Adv. Mater. 2015, 27, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.F.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, W.; Ge, Y.; Guo, H.; Zhang, X.; Chen, S.; Deng, Y.; Lu, Z.; Zhang, H. Stabilization of Black Phosphorous Quantum Dots in PMMA Nanofiber Film and Broadband Nonlinear Optics and Ultrafast Photonics Application. Adv. Funct. Mater. 2017, 27, 1702437. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, T.; Sun, Z.; Chen, H.; Guan, J.; Chen, X.; Ji, H.; Du, P.; Yang, S. Black Phosphorus Revisited: A Missing Metal-Free Elemental Photocatalyst for Visible Light Hydrogen Evolution. Adv. Mater. 2017, 29, 1605776. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Ji, Y.; Dang, Z.; Yan, J.; Li, P.; Li, Y.; Liu, S. g-C3N4 Loading Black Phosphorus Quantum Dot for Efficient and Stable Photocatalytic H2 Generation under Visible Light. Adv. Funct. Mater. 2018, 28, 1800668. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, B.; Xiang, D.; Zhu, Y. Effect of solvents on morphology and photocatalytic activity of BiOBr synthesized by solvothermal method. Mater. Res. Bull. 2012, 47, 3753–3757. [Google Scholar] [CrossRef]
- Xiong, X.; Ding, L.; Wang, Q.; Li, Y.; Jiang, Q.; Hu, J. Synthesis and photocatalytic activity of BiOBr nanosheets with tunable exposed {0 1 0} facets. Appl. Catal. B Environ. 2016, 188, 283–291. [Google Scholar] [CrossRef]
- Lee, H.U.; Park, S.Y.; Lee, S.C.; Choi, S.; Seo, S.; Kim, H.; Won, J.; Choi, K.; Kang, K.S.; Park, H.G.; et al. Black Phosphorus (BP) Nanodots for Potential Biomedical Applications. Small 2016, 12, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Li, R. Photocatalytic nitrogen fixation: An attractive approach for artificial photocatalysis. Chin. J. Catal. 2018, 39, 1180–1188. [Google Scholar] [CrossRef]
- Chang, C.; Fu, Y.; Hu, M.; Wang, C.; Shan, G.; Zhu, L. Photodegradation of bisphenol A by highly stable palladium-doped mesoporous graphite carbon nitride (Pd/mpg-C3N4) under simulated solar light irradiation. Appl. Catal. B Environ. 2013, 142, 553–560. [Google Scholar] [CrossRef]
- Ma, G.; Chen, Z.; Chen, Z.; Jin, M.; Meng, Q.; Yuan, M.; Wang, X.; Liu, J.M.; Zhou, G. Constructing novel WO3/Fe(III) nanofibers photocatalysts with enhanced visible-light-driven photocatalytic activity via interfacial charge transfer effect. Mater. Today Energy 2017, 3, 45–52. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, X.; Xiao, W.; Zhuang, Y. Photocatalytic degradation of sulfamethazine by graphitic carbon nitride-modified zinc molybdate: Effects of synthesis method on performance, degradation kinetics, and mechanism. Chin. J. Catal. 2017, 38, 2009–2020. [Google Scholar] [CrossRef]
- Ding, D.; Xu, X.; Tian, P.; Liu, X.; Xu, J.; Han, Y.F. Promotional effects of Sb on Pd-based catalysts for the direct synthesis of hydrogen peroxide at ambient pressure. Chin. J. Catal. 2018, 39, 673–681. [Google Scholar] [CrossRef]
- Zou, C.; Meng, Z.; Ji, W.; Liu, S.; Shen, Z.; Zhang, Y.; Jiang, N. Preparation of a fullerene[60]-iron oxide complex for the photo-fenton degradation of organic contaminants under visible-light irradiation. Chin. J. Catal. 2018, 39, 1051–1059. [Google Scholar] [CrossRef]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Shang, C.; Meng, Q.; Jin, M.; Liao, H.; Li, M.; Chen, Z.; Yuan, M.; Wang, X.; Zhou, G. The Ternary Heterostructures of BiOBr/Ultrathin g-C3N4/Black Phosphorous Quantum Dot Composites for Photodegradation of Tetracycline. Polymers 2018, 10, 1118. https://doi.org/10.3390/polym10101118
Jiang T, Shang C, Meng Q, Jin M, Liao H, Li M, Chen Z, Yuan M, Wang X, Zhou G. The Ternary Heterostructures of BiOBr/Ultrathin g-C3N4/Black Phosphorous Quantum Dot Composites for Photodegradation of Tetracycline. Polymers. 2018; 10(10):1118. https://doi.org/10.3390/polym10101118
Chicago/Turabian StyleJiang, Tianhao, Chaoqun Shang, Qingguo Meng, Mingliang Jin, Hua Liao, Ming Li, Zhihong Chen, Mingzhe Yuan, Xin Wang, and Guofu Zhou. 2018. "The Ternary Heterostructures of BiOBr/Ultrathin g-C3N4/Black Phosphorous Quantum Dot Composites for Photodegradation of Tetracycline" Polymers 10, no. 10: 1118. https://doi.org/10.3390/polym10101118




