Boron Trifluoride Anionic Side Groups in Polyphosphazene Based Polymer Electrolyte with Enhanced Interfacial Stability in Lithium Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Synthesis of Precursor Polymer
2.3. Characterization of the Final Polymers
2.4. Preparation of Solid Polymer Electrolyte Membranes
2.5. Preparation of Gel Polymer Electrolyte Membranes
2.6. Cell Set Up
3. Results and Discussion
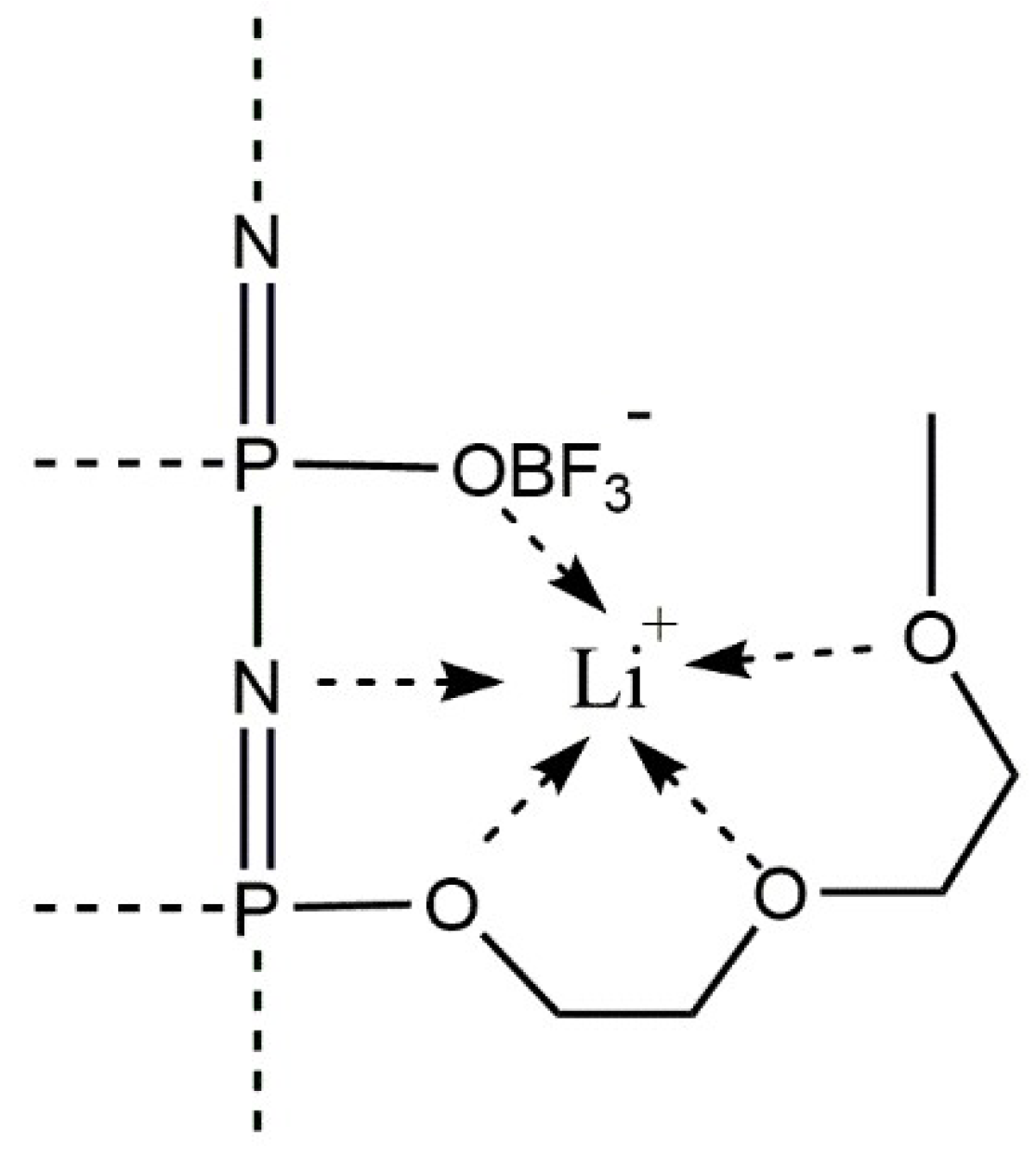
3.1. Polymer Characterization
3.2. Thermal Properties
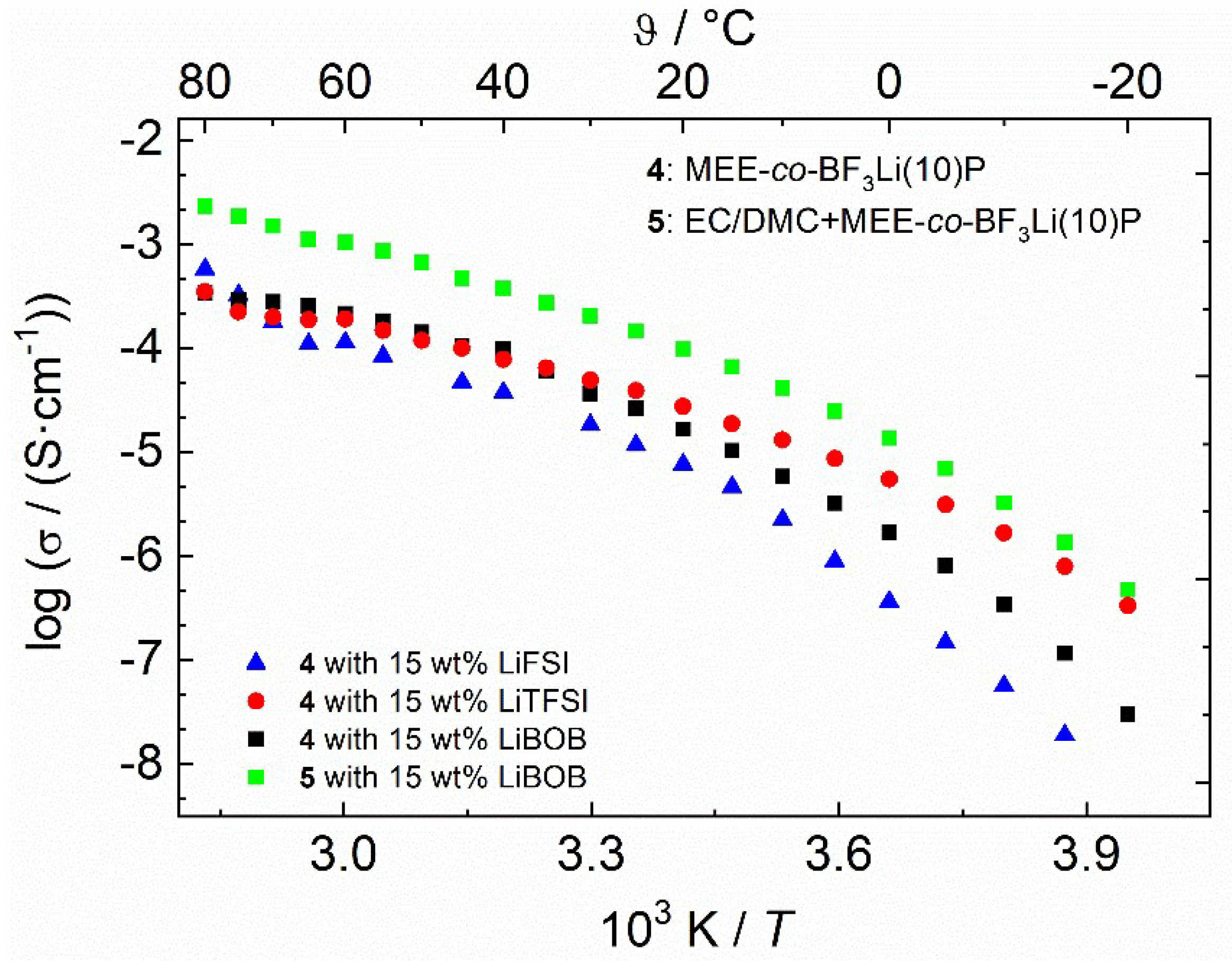
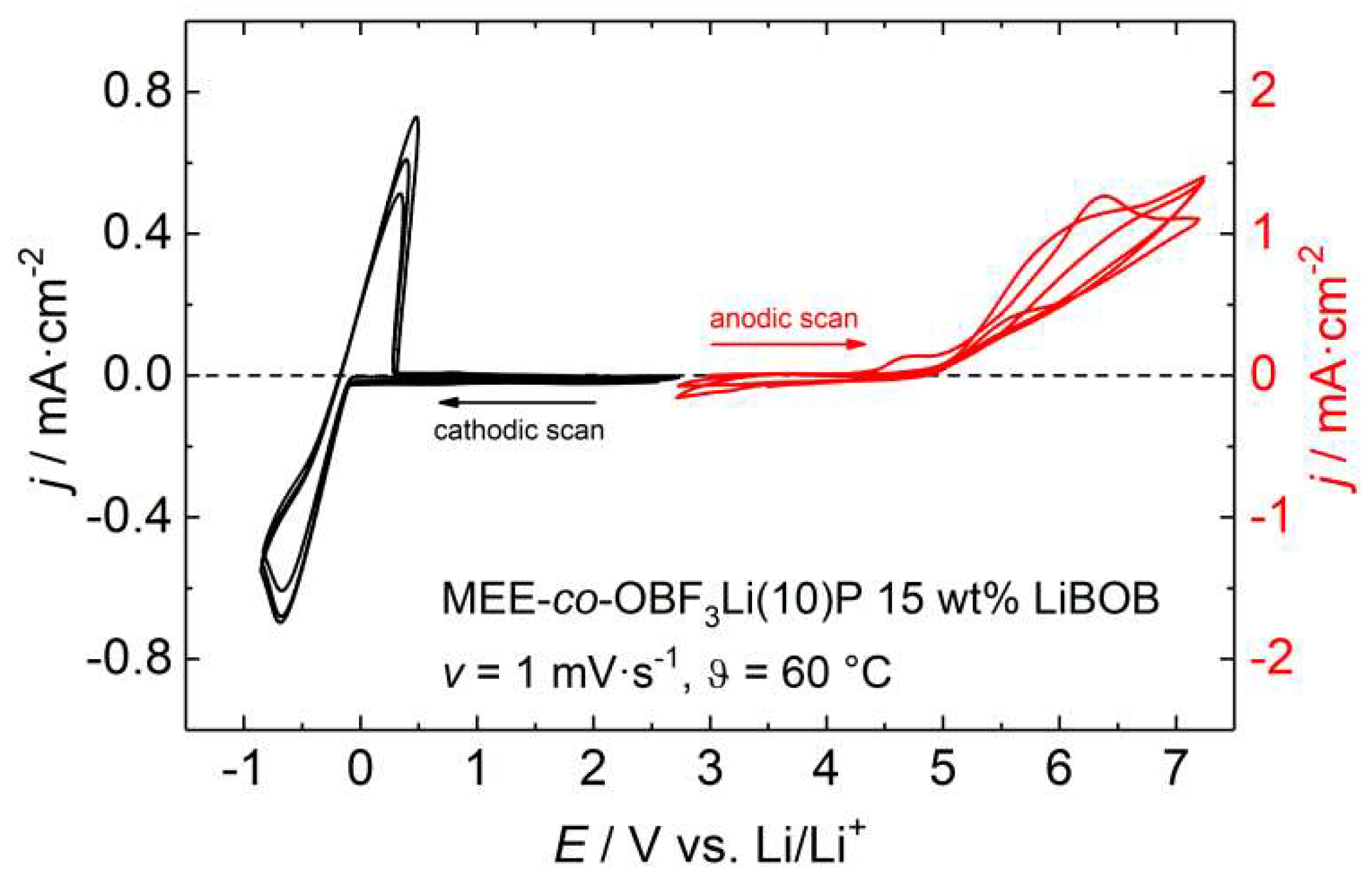
3.3. Ionic Conductivity and Electrochemical Stability
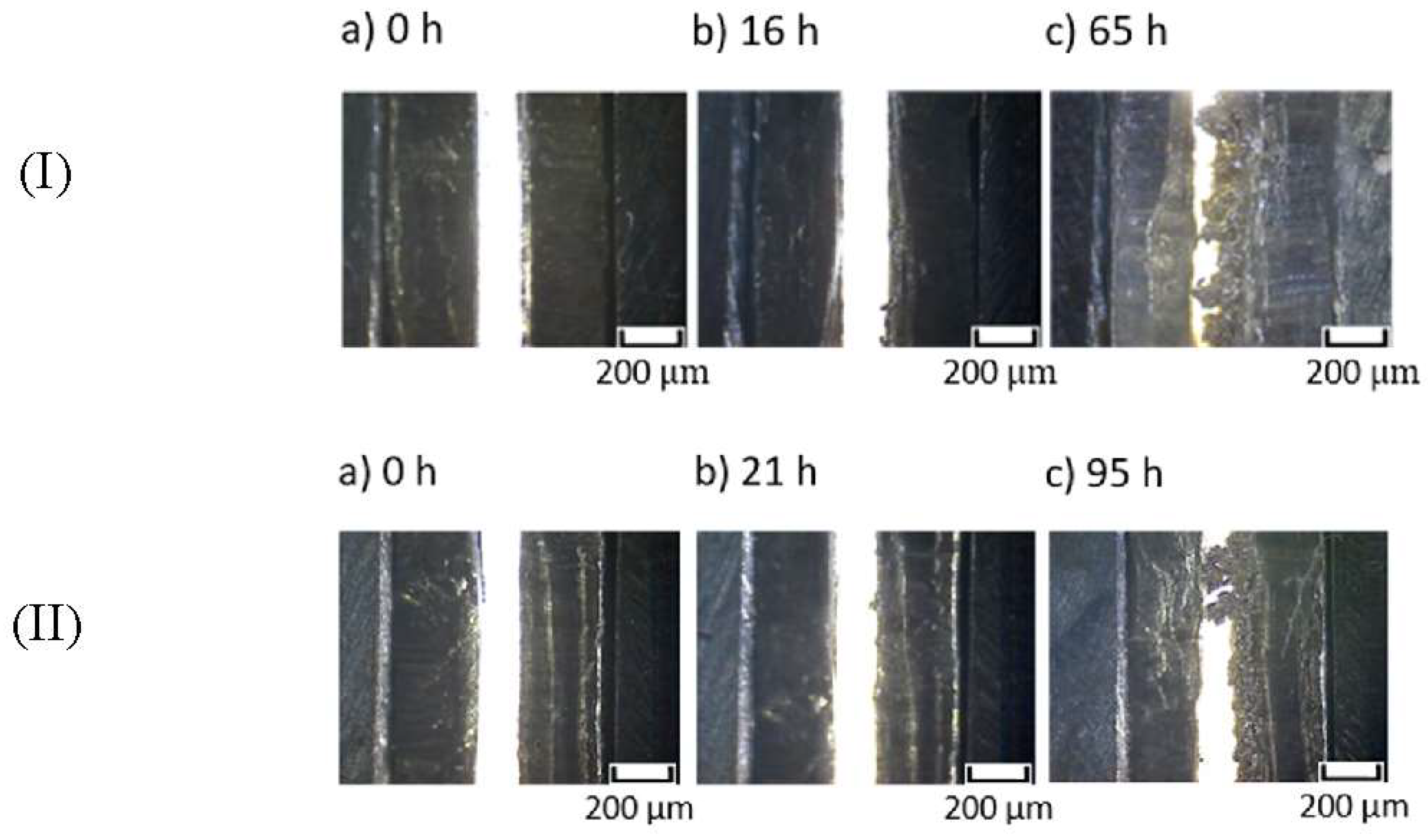
3.4. Analysis of Conditions for Li Dendrite Formation at the Li/MEE-co-OBF3LiP (+LiBOB) Interface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Souquet, J.L.; Duclot, M. Thin film lithium batteries. Solid State Ion. 2002, 148, 375–379. [Google Scholar] [CrossRef]
- Belharouak, I. Lithium Ion Batteries—New Developments; InTech: Londun, UK, 2012; p. 236. [Google Scholar]
- Patil, A.; Patil, V.; Shin, D.W.; Choi, J.-W.; Paik, D.-S.; Yoon, S.-J. Issue and challenges facing rechargeable thin film lithium batteries. Mater. Res. Bull. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Hardy, L.C.; Shriver, D.F. Preparation and electrical response of solid polymer electrolytes with only one mobile species. J. Am. Chem. Soc. 1985, 107, 3823–3828. [Google Scholar] [CrossRef]
- Jiang, Y.Y.X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the peo based solid polymer electrolytes for all-solid state lithium ion batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L.M.; Armand, M.; Zhou, Z. Single lithium-ion conducting solid polymer electrolytes: Advances and perspectives. Chem. Soc. Rev. 2017, 46, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Scaccia, S.; Passerini, S. Investigation on the stability of the lithium-polymer electrolyte interface. J. Electrochem. Soc. 2000, 147, 4448–4452. [Google Scholar] [CrossRef]
- Ben Youcef, H.; Garcia-Calvo, O.; Lago, N.; Devaraj, S.; Armand, M. Cross-linked solid polymer electrolyte for all-solid-state rechargeable lithium batteries. Electrochim Acta 2016, 220, 587–594. [Google Scholar] [CrossRef]
- Duan, H.; Yin, Y.-X.; Zeng, X.-X.; Li, J.-Y.; Shi, J.-L.; Shi, Y.; Wen, R.; Guo, Y.-G.; Wan, L.-J. In-situ plasticized polymer electrolyte with double-network for flexible solid-state lithium-metal batteries. Energy Storage Mater. 2018, 10, 85–91. [Google Scholar] [CrossRef]
- Lu, Q.; He, Y.B.; Yu, Q.; Li, B.; Kaneti, Y.V.; Yao, Y.; Kang, F.; Yang, Q.H. Dendrite-free, high-rate, long-life lithium metal batteries with a 3d cross-linked network polymer electrolyte. Adv. Mater. 2017, 29, 1604460. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.; Fuller, T.F.; Newman, J. The importance of the lithium ion transference number in lithium/polymer cells. Electrochim. Acta 1994, 39, 2073–2081. [Google Scholar] [CrossRef]
- Jankowsky, S.; Hiller, M.M.; Wiemhöfer, H.D. Preparation and electrochemical performance of polyphosphazene based salt-in-polymer electrolyte membranes for lithium ion batteries. J Power Sources 2014, 253, 256–262. [Google Scholar] [CrossRef]
- Edman, L.; Doeff, M.M.; Ferry, A.; Kerr, J.; De Jonghe, L.C. Transport properties of the solid polymer electrolyte system p(eo)(n)litfsi. J. Phys. Chem. B 2000, 104, 3476–3480. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Girard, G.M.; Yunis, R.; MacFarlane, D.R.; Mecerreyes, D.; Bhattacharyya, A.J.; Howlett, P.C.; Forsyth, M. Preparation and characterization of gel polymer electrolytes using poly (ionic liquids) and high lithium salt concentration ionic liquids. J. Mater. Chem. A 2017, 5, 23844–23852. [Google Scholar] [CrossRef]
- Zuo, X.; Ma, X.; Wu, J.; Deng, X.; Xiao, X.; Liu, J.; Nan, J. Self-supporting ethyl cellulose/poly (vinylidene fluoride) blended gel polymer electrolyte for 5 v high-voltage lithium-ion batteries. Electrochim. Acta 2018, 271, 582–590. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Shao, H. Co-polymerization and blending based peo/pmma/p (vdf-hfp) gel polymer electrolyte for rechargeable lithium metal batteries. J. Membr. Sci. 2018, 547, 1–10. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, X.; Xia, X.; Xu, R.; Zhang, S.; Wu, J.; Liang, Y.; Gu, C.; Tu, J. A newly designed composite gel polymer electrolyte based on poly (vinylidene fluoride-hexafluoropropylene)(pvdf-hfp) for enhanced solid-state lithium-sulfur batteries. Chem. A Euro. J. 2017, 23, 15203–15209. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Shimazaki, Y.; Mehta, M.A.; Fujinami, T. Synthesis and characterization of aluminate polymer electrolytes and their blends with poly(ether)s. Solid State Ion. 2000, 133, 295–301. [Google Scholar] [CrossRef]
- Fujinami, T.; Tokimune, A.; Mehta, M.A.; Shriver, D.F.; Rawsky, G.C. Siloxyaluminate polymers with high li+ ion conductivity. Chem. Mater. 1997, 9, 2236–2239. [Google Scholar] [CrossRef]
- Tada, Y.; Sato, M.; Takeno, N.; Nakacho, Y.; Shigehara, K. Attempts at lithium single-ionic conduction by anchoring sulfonate anions as terminating groups of oligo(oxyethylene) side-chains in comb-type polyphosphazenes. Chem. Mater. 1994, 6, 27–30. [Google Scholar] [CrossRef]
- Tsuchida, E.; Kobayashi, N.; Ohno, H. Single-ion conduction in poly[(oligo(oxyethylene)methacrylate)-co-(alkali-metal methacrylates)]. Macromolecules 1988, 21, 96–100. [Google Scholar] [CrossRef]
- Zhao, C.-Z.; Zhang, X.-Q.; Cheng, X.-B.; Zhang, R.; Xu, R.; Chen, P.-Y.; Peng, H.-J.; Huang, J.-Q.; Zhang, Q. An anion-immobilized composite electrolyte for dendrite-free lithium metal anodes. Proc. Natl. Acad. Sci. USA 2017, 114, 11069–11074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diederichsen, K.M.; McShane, E.J.; McCloskey, B.D. Promising routes to a high li+ transference number electrolyte for lithium ion batteries. ACS Energy Letters 2017, 2, 2563–2575. [Google Scholar] [CrossRef]
- Allcock, H.R.; Welna, D.T.; Maher, A.E. Single ion conductors–polyphosphazenes with sulfonimide functional groups. Solid State Ion. 2006, 177, 741–747. [Google Scholar] [CrossRef]
- Fiedler, C.; Luerssen, B.; Lucht, B.; Janek, J. Synthesis and characterization of polyphosphazene electrolytes including cyclic ether side groups. J. Power Sources 2018, 384, 165–171. [Google Scholar] [CrossRef]
- Grünebaum, M.; Buchheit, A.; Krause, D.; Hiller, M.M.; Schmidt, C.; Winter, M.; Wiemhöfer, H.-D. Development of new pyrazole-based lithium salts for battery applications – do established basic design concepts really work? Electrochim. Acta 2018, 286, 313–323. [Google Scholar] [CrossRef]
- Kaskhedikar, N.; Paulsdorf, J.; Burjanadze, M.; Karatas, Y.; Wilmer, D.; Roling, B.; Wiemhofer, H.D. Ionic conductivity of polymer electrolyte membranes based on polyphosphazene with oligo(propylene oxide) side chains. Solid State Ion. 2006, 177, 703–707. [Google Scholar] [CrossRef]
- Jankowsky, S.; Hiller, M.M.; Stolina, R.; Wiemhöfer, H.D. Performance of polyphosphazene based gel polymer electrolytes in combination with lithium metal anodes. J. Power Sources 2015, 273, 574–579. [Google Scholar] [CrossRef]
- Jankowsky, S.; Hiller, M.M.; Fromm, O.; Winter, M.; Wiemhöfer, H.D. Enhanced lithium-ion transport in polyphosphazene based gel polymer electrolytes. Electrochim. Acta 2015, 155, 364–371. [Google Scholar] [CrossRef]
- Wang, B. Development of a one-pot in situ synthesis of poly(dichlorophosphazene) from pcl3. Macromolecules 2005, 38, 643–645. [Google Scholar] [CrossRef]
- Wang, B.; Rivard, E.; Manners, I. A new high-yield synthesis of cl3pnsime3, a monomeric precursor for the controlled preparation of high molecular weight polyphosphazenes. Inorg. Chem. 2002, 41, 1690. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R.; Crane, C.A.; Morrissey, C.T.; Nelson, J.M.; Reeves, S.D.; Honeyman, C.H.; Manners, I. “Living” cationic polymerization of phosphoranimines as an ambient temperature route to polyphosphazenes with controlled molecular weights. Macromolecules 1996, 29, 7740–7747. [Google Scholar] [CrossRef]
- Paulsdorf, J.; Kaskhedikar, N.; Burjanadze, M.; Obeidi, S.; Stolwijk, N.A.; Wilmer, D.; Wiemhofer, H.D. Synthesis and ionic conductivity of polymer electrolytes based on a polyphosphazene with short side groups. Chem. Mater. 2006, 18, 1281–1288. [Google Scholar] [CrossRef]
- Hiller, M.M.; Joost, M.; Gores, H.J.; Passerini, S.; Wiemhöfer, H.D. The influence of interface polarization on the determination of lithium transference numbers of salt in polyethylene oxide electrolytes. Electrochim. Acta 2013, 114, 21–29. [Google Scholar] [CrossRef]
- He, X.; Schmohl, S.; Wiemhöfer, H.D. Direct observation and suppression effect of lithium dendrite growth for polyphosphazene based polymer electrolytes in lithium metal cells. ChemElectroChem. 2018. [Google Scholar] [CrossRef]
- Myers, E.L.; Butts, C.P.; Aggarwal, V.K. Bf3 center dot oet2 and tmsotf: A synergistic combination of lewis acids. Chem. Commun. 2006, 4434–4436. [Google Scholar] [CrossRef]
- Feng, S.W.; Shi, D.Y.; Liu, F.; Zheng, L.P.; Nie, J.; Feng, W.F.; Huang, X.J.; Armand, M.; Zhou, Z.B. Single lithium-ion conducting polymer electrolytes based on poly (4-styrenesulfonyl)(trifluoromethanesulfonyl)imide anions. Electrochim. Acta 2013, 93, 254–263. [Google Scholar] [CrossRef]
- Van Wullen, L.; Koster, T.K.J.; Wiemhofer, H.D.; Kaskhedikar, N. Local cation coordination motifs in polyphosphazene based composite electrolytes. Chem. Mater. 2008, 20, 7399–7407. [Google Scholar] [CrossRef]
- Lee, D.K.; Allcock, H.R. The effects of cations and anions on the ionic conductivity of poly[bis(2-(2-methoxyethoxy)ethoxy)phosphazene] doped with lithium and magnesium salts of trifluoromethanesulfonate and bis(trifluoromethanesulfonyl)imidate. Solid State Ion. 2010, 181, 1721–1726. [Google Scholar] [CrossRef]
- Wilken, S.; Johansson, P.; Jacobsson, P. Infrared spectroscopy of instantaneous decomposition products of lipf6-based lithium battery electrolytes. Solid State Ion. 2012, 225, 608–610. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, J.; Lee, J.-i.; Lee, C. Ionic conductivity and electrochemical properties of cross-linked solid polymer electrolyte using star-shaped siloxane acrylate. J. Power Sources 2007, 165, 92–96. [Google Scholar] [CrossRef]
- Oh, B.; Vissers, D.; Zhang, Z.; West, R.; Tsukamoto, H.; Amine, K. New interpenetrating network type poly(siloxane-g-ethylene oxide) polymer electrolyte for lithium battery. J. Power Sources 2003, 119–121, 442–447. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Yang, J. Lithium dendrite formation in li/poly(ethylene oxide)–lithium bis(trifluoromethanesulfonyl)imide and n-methyl-n-propylpiperidinium bis(trifluoromethanesulfonyl)imide/li cells. J. Electrochem. Soc. 2010, 157, A1092. [Google Scholar] [CrossRef]
- Liu, S.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Yang, J. Effect of nano-silica filler in polymer electrolyte on li dendrite formation in li/poly(ethylene oxide)–li(cf3so2)2n/li. J. Power Sources 2010, 195, 6847–6853. [Google Scholar] [CrossRef]
- Brissot, C.; Rosso, M.; Chazalviel, J.N.; Lascaud, S. Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 1999, 81–82, 925–929. [Google Scholar] [CrossRef]
- Brissot, C.; Rosso, M.; Chazalviel, J.N.; Baudry, P.; Lascaud, S. In situ study of dendritic growth inlithium/peo-salt/lithium cells. Electrochim. Acta 1998, 43, 1569–1574. [Google Scholar] [CrossRef]
- Kushima, A.; So, K.P.; Su, C.; Bai, P.; Kuriyama, N.; Maebashi, T.; Fujiwara, Y.; Bazant, M.Z.; Li, J. Liquid cell transmission electron microscopy observation of lithium metal growth and dissolution: Root growth, dead lithium and lithium flotsams. Nano Energy 2017, 32, 271–279. [Google Scholar] [CrossRef]
- Lu, Y.; Tu, Z.; Archer, L.A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 2014, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Ozhabes, Y.; Gunceler, D.; Arias, T. Stability and surface diffusion at lithium-electrolyte interphases with connections to dendrite suppression. arXiv preprint, 2015; arXiv:1504.05799. [Google Scholar]





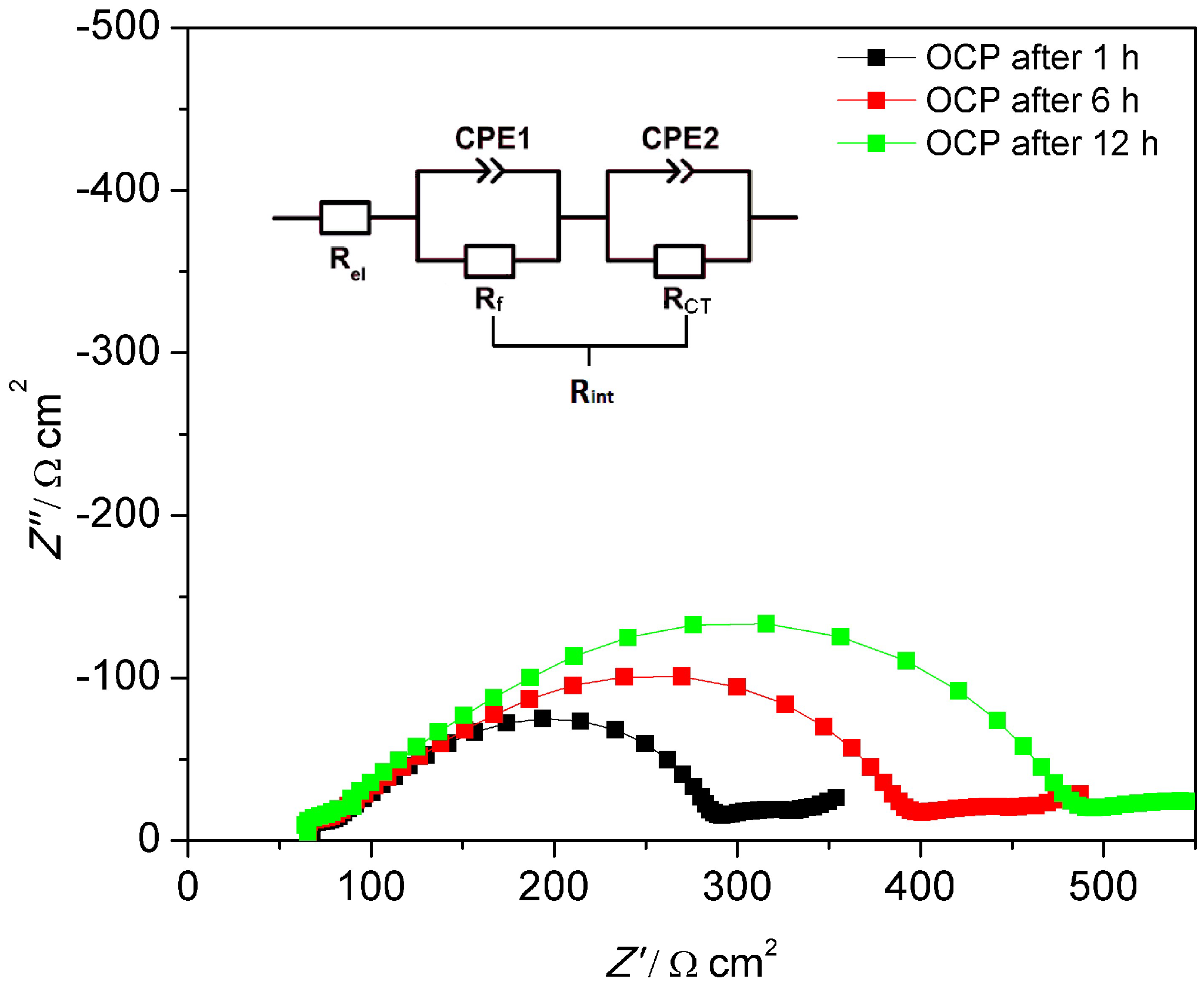
| MEEP/LiBOB (Ω cm2) | |||
| Rel | Rf | Rct | |
| OCP, 1 h | 54 | 163 | 104 |
| OCP, 6 h | 53 | 238 | 109 |
| OCP, 12 h | 52 | 316 | 120 |

| MEE-co-OBF3LiP/LiBOB (Ω cm2) | |||
| Rel | Rf | Rct | |
| OCP, 1 h | 72 | 101 | 97 |
| OCP, 6 h | 72 | 155 | 109 |
| OCP, 12 h | 71 | 203 | 114 |
| σtot / [mS·cm−1] 25 °C | σtot / [mS·cm−1] 60 °C | σLi+ / 10−3 [mS·cm−1] 60 °C | T+ 60°C | Tg | n(LiSalt):n(OEO) | n(Litot):n(OEO) | [P=N]:O | |
|---|---|---|---|---|---|---|---|---|
| (3) MEE-co-OBF3(5)LiP | −72.6 | |||||||
| +LiBOB | 0.038 | 0.138 | 5.52 | 0.04 | −49.7 | 1:26 | 1:21 | 1:5.7 |
| +LiFSI | 0.017 | 0.113 | 5.65 | 0.05 | −49.9 | 1:25 | 1:21 | 1:5.7 |
| +LiTFSI | 0.037 | 0.171 | 5.13 | 0.03 | −57.4 | 1:39 | 1:29 | 1:5.7 |
| (4) MEE-co-OBF3(10)LiP | −65.0 | |||||||
| + LiBOB | 0.052 | 0.356 | 17.80 | 0.05 | −53.7 | 1:26 | 1:18 | 1:5.7 |
| (5) EC/DMC+ MEE-co-OBF3(10)LiP | ||||||||
| + LiBOB | 0.15 | 1.05 | 220 | 0.18 | - | 1:26 | 1:18 | 1:5.7 |
| MEEP | −81.2 | |||||||
| +LiBOB | 0.023 | 0.553 | 22.10 | 0.04 | −45.9 | 1:27 | 1:27 | 1:6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmohl, S.; He, X.; Wiemhöfer, H.-D. Boron Trifluoride Anionic Side Groups in Polyphosphazene Based Polymer Electrolyte with Enhanced Interfacial Stability in Lithium Batteries. Polymers 2018, 10, 1350. https://doi.org/10.3390/polym10121350
Schmohl S, He X, Wiemhöfer H-D. Boron Trifluoride Anionic Side Groups in Polyphosphazene Based Polymer Electrolyte with Enhanced Interfacial Stability in Lithium Batteries. Polymers. 2018; 10(12):1350. https://doi.org/10.3390/polym10121350
Chicago/Turabian StyleSchmohl, Sebastian, Xuan He, and Hans-Dieter Wiemhöfer. 2018. "Boron Trifluoride Anionic Side Groups in Polyphosphazene Based Polymer Electrolyte with Enhanced Interfacial Stability in Lithium Batteries" Polymers 10, no. 12: 1350. https://doi.org/10.3390/polym10121350





