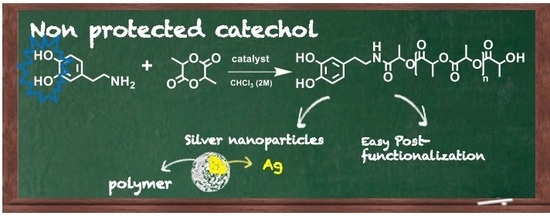
Catechol End-Functionalized Polylactide by Organocatalyzed Ring-Opening Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Synthesis of l-Lactide
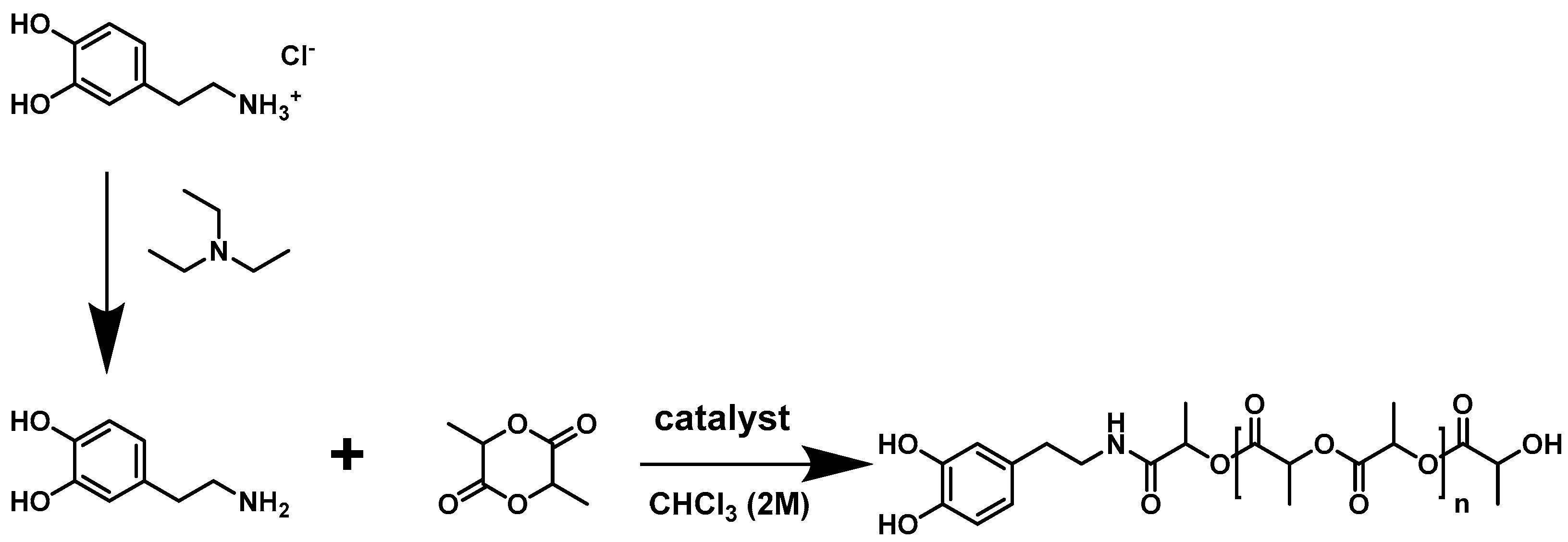
2.3.1. Ring-Opening Polymerization of l-Lactide Using Dopamine as Initiator
2.3.2. Ring-Opening Polymerization of l-Lactide Using Phenethylamine as Initiator
2.3.3. Synthesis of Silver Nanoparticles (AgNPs)
3. Results and Discussion
3.1. Polymerization and Characterization of Catechol-PLLA
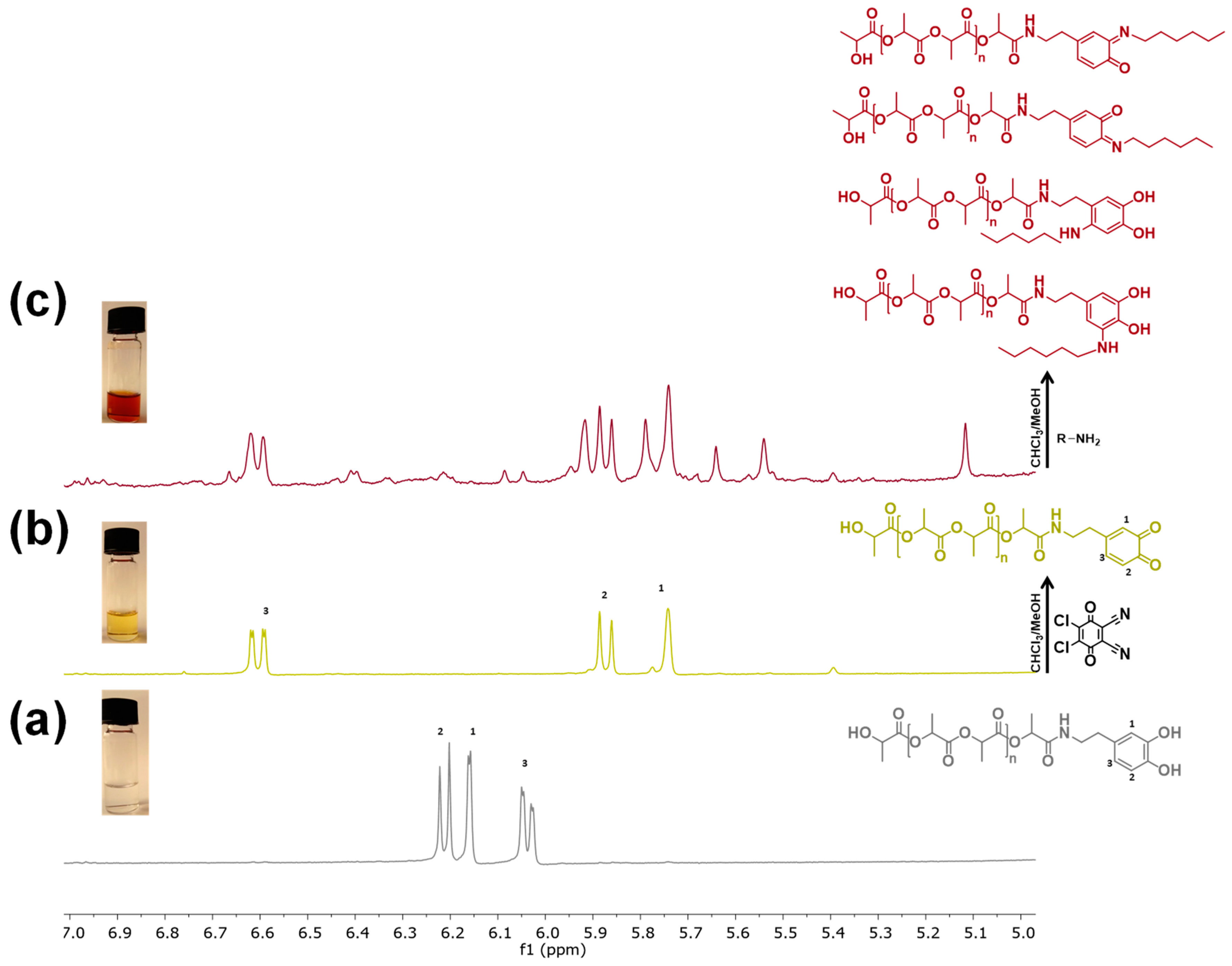
3.2. Characterization of the Redox Behaviour of Catechol-PLLA
3.3. Post-Functionalization of Semitelechelic Catechol-PLLA
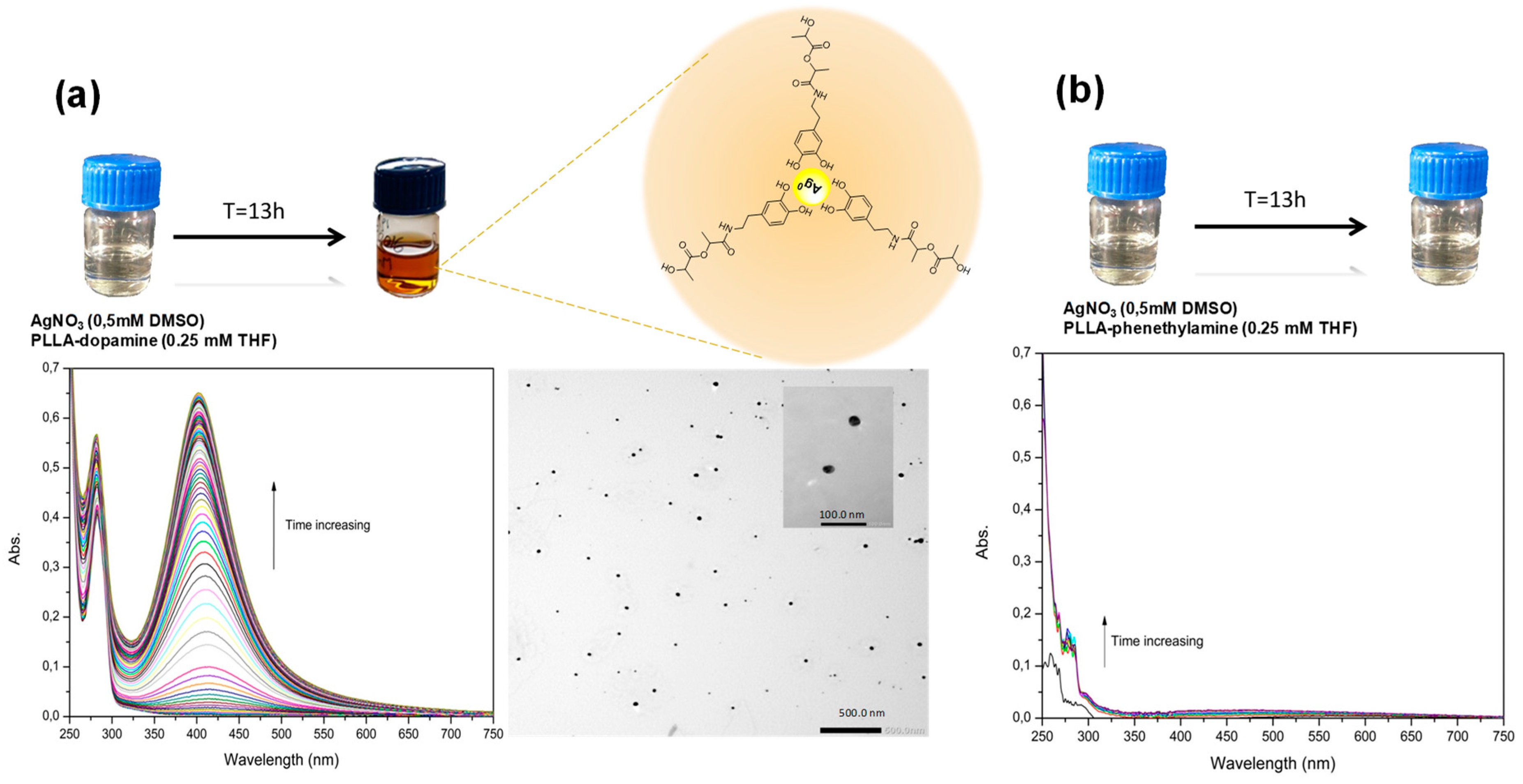
3.4. Synthesis and Characterization of Silver Nanoparticles Using Catechol End-Capped PLLA Polymer
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Kord Forooshani, P.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Moulay, S. Dopa/Catechol-Tethered Polymers: Bioadhesives and Biomimetic Adhesive Materials. Polym. Rev. 2014, 54, 436–513. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Aqil, A.; Ouhib, F.; Admassie, S.; Inganäs, O.; Jérôme, C.; Detrembleur, C. Bioinspired Redox-Active Catechol-Bearing Polymers as Ultrarobust Organic Cathodes for Lithium Storage. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Ferraz, M.P.; Monteiro, F.J.; Fernandes, M.H.; Beppu, M.M.; Mantione, D.; Sardon, H. Antibacterial silk fibroin/nanohydroxyapatite hydrogels with silver and gold nanoparticles for bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Jus, S.; Kokol, V.; Guebitz, G.M. Tyrosinase-catalysed coupling of functional molecules onto protein fibres. Enzym. Microb. Technol. 2008, 42, 535–542. [Google Scholar] [CrossRef]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Charlot, A.; Sciannaméa, V.; Lenoir, S.; Faure, E.; Jérôme, R.; Jérôme, C.; Weerdt, C.V.D.; Martial, J.; Archambeau, C.; Willet, N.; et al. All-in-one strategy for the fabrication of antimicrobial biomimetic films on stainless steel. J. Mater. Chem. 2009, 19, 4117–4125. [Google Scholar] [CrossRef]
- Yang, J.; Keijsers, J.; van Heek, M.; Stuiver, A.; Stuart, M.A.C.; Kamperman, M. The effect of molecular composition and crosslinking on adhesion of a bio-inspired adhesive. Polym. Chem. 2015, 6, 3121–3130. [Google Scholar] [CrossRef]
- Yang, J.; Stuart, M.A.C.; Kamperman, M. Jack of all trades: versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Deming, T.J. Synthetic Polypeptide Mimics of Marine Adhesives. Macromolecules 1998, 31, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
- Alba, A.; du Boullay, O.T.; Martin-Vaca, B.; Bourissou, D. Direct ring-opening of lactide with amines: application to the organo-catalyzed preparation of amide end-capped PLA and to the removal of residual lactide from PLA samples. Polym. Chem. 2015, 6, 989–997. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, B.-H.; Messersmith, P.B. Acetonide Protection of Dopamine for the Synthesis of Highly Pure N-docosahexaenoyldopamine. Tetrahedron Lett. 2010, 51, 2403–2405. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Cordella, D.; Aqil, A.; Debuigne, A.; Admassie, S.; Jérôme, C.; Detrembleur, C. Surface- and Redox-Active Multifunctional Polyphenol-Derived Poly(ionic liquid)s: Controlled Synthesis and Characterization. Macromolecules 2016, 49, 7676–7691. [Google Scholar] [CrossRef]
- Zhang, X.; Jones, G.O.; Hedrick, J.L.; Waymouth, R.M. Fast and selective ring-opening polymerizations by alkoxides and thioureas. Nat. Chem. 2016, 8, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nurumbetov, G.; Simula, A.; Zhu, C.; Li, M.; Wilson, P.; Kempe, K.; Yang, B.; Tao, L.; Haddleton, D.M. Synthesis of well-defined catechol polymers for surface functionalization of magnetic nanoparticles. Polym. Chem. 2016, 7, 7002–7010. [Google Scholar] [CrossRef]
- Thavasi, V.; Bettens, R.P.A.; Leong, L.P. Temperature and Solvent Effects on Radical Scavenging Ability of Phenols. J. Phys. Chem. A 2009, 113, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- Guin, P.S.; Das, S.; Mandal, P.C. Electrochemical Reduction of Quinones in Different Media: A Review. Available online: https://www.hindawi.com/journals/ijelc/2011/816202/ref/ (accessed on 16 October 2017).
- Yang, J.; Saggiomo, V.; Velders, A.H.; Stuart, M.A.C.; Kamperman, M. Reaction Pathways in Catechol/Primary Amine Mixtures: A Window on Crosslinking Chemistry. PLoS ONE 2016, 11, e0166490. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.; Lecomte, P.; Lenoir, S.; Vreuls, C.; Weerdt, C.V.D.; Archambeau, C.; Martial, J.; Jérôme, C.; Duwez, A.-S.; Detrembleur, C. Sustainable and bio-inspired chemistry for robust antibacterial activity of stainless steel. J. Mater. Chem. 2011, 21, 7901–7904. [Google Scholar] [CrossRef]

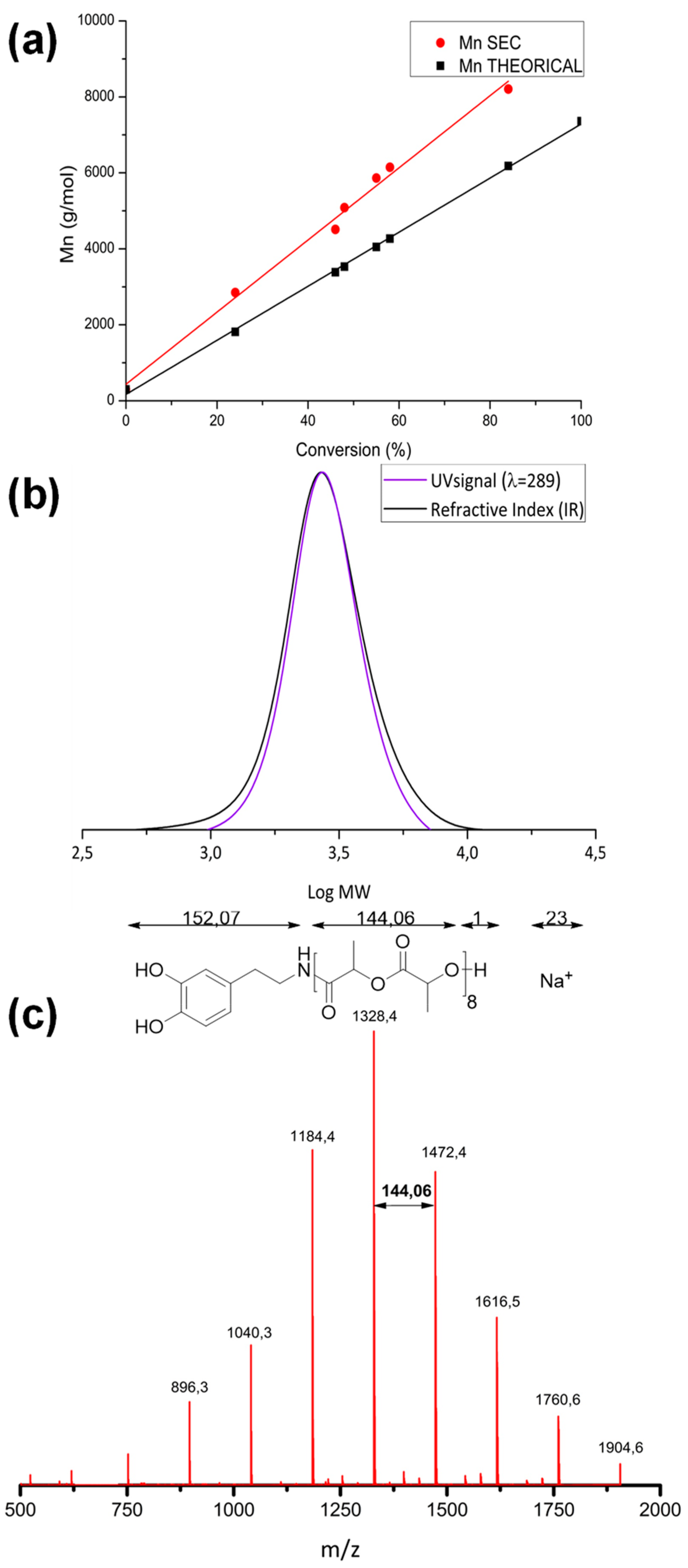

| Entry | Initiator | Time (h) | Conv. 1 (%) | ([M]0/[I]0/[Cat]) | Mn (Theo) 2 (kg/mol) | Mn (Exp) 3 (kg/mol) | Ð 3 |
|---|---|---|---|---|---|---|---|
| 1 | Dopamine | 96 | 1 | 10/1/0 | 0 | - | - |
| 2 | Dopamine | 24 | 93 | 10/1/0.25 | 1.5 | 3.8 | 1.2 |
| 3 | Dopamine | 24 | 93 | 10/1/0.5 | 1.5 | 3.5 | 1.1 |
| 4 | Dopamine | 12 | 94 | 10/1/0.75 | 1.5 | 3.4 | 1.2 |
| 5 | Dopamine | 12 | 94 | 10/1/1 | 1.5 | 3.6 | 1.1 |
| 6 | Dopamine | 24 | 93 | 20/1/0.25 | 2.8 | 4.0 | 1.2 |
| 7 | Dopamine | 48 | 92 | 50/1/0.25 | 6.9 | 9.5 | 1.2 |
| 8 | Dopamine | 48 | 91 | 100/1/0.25 | 13.4 | 21.0 | 1.4 |
| 9 | Phenethylamine | 24 | 90 | 10/1/0.25 | 1.5 | 1.6 | 1.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadaba, N.; Salsamendi, M.; Casado, N.; Zuza, E.; Muñoz, J.; Sarasua, J.-R.; Mecerreyes, D.; Mantione, D.; Detrembleur, C.; Sardon, H. Catechol End-Functionalized Polylactide by Organocatalyzed Ring-Opening Polymerization. Polymers 2018, 10, 155. https://doi.org/10.3390/polym10020155
Sadaba N, Salsamendi M, Casado N, Zuza E, Muñoz J, Sarasua J-R, Mecerreyes D, Mantione D, Detrembleur C, Sardon H. Catechol End-Functionalized Polylactide by Organocatalyzed Ring-Opening Polymerization. Polymers. 2018; 10(2):155. https://doi.org/10.3390/polym10020155
Chicago/Turabian StyleSadaba, Naroa, Maitane Salsamendi, Nerea Casado, Ester Zuza, Jone Muñoz, Jose-Ramon Sarasua, David Mecerreyes, Daniele Mantione, Christophe Detrembleur, and Haritz Sardon. 2018. "Catechol End-Functionalized Polylactide by Organocatalyzed Ring-Opening Polymerization" Polymers 10, no. 2: 155. https://doi.org/10.3390/polym10020155