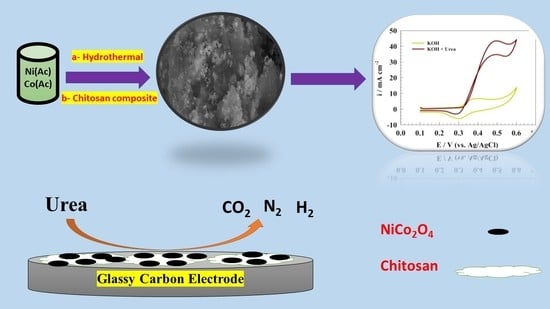
Chitosan Supports Boosting NiCo2O4 for Catalyzed Urea Electrochemical Removal Application
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of NiCo2O4
2.2. Synthesis of NiCo2O4 Supported Chitosan
2.3. Electrode Fabrication
3. Result and Discussion
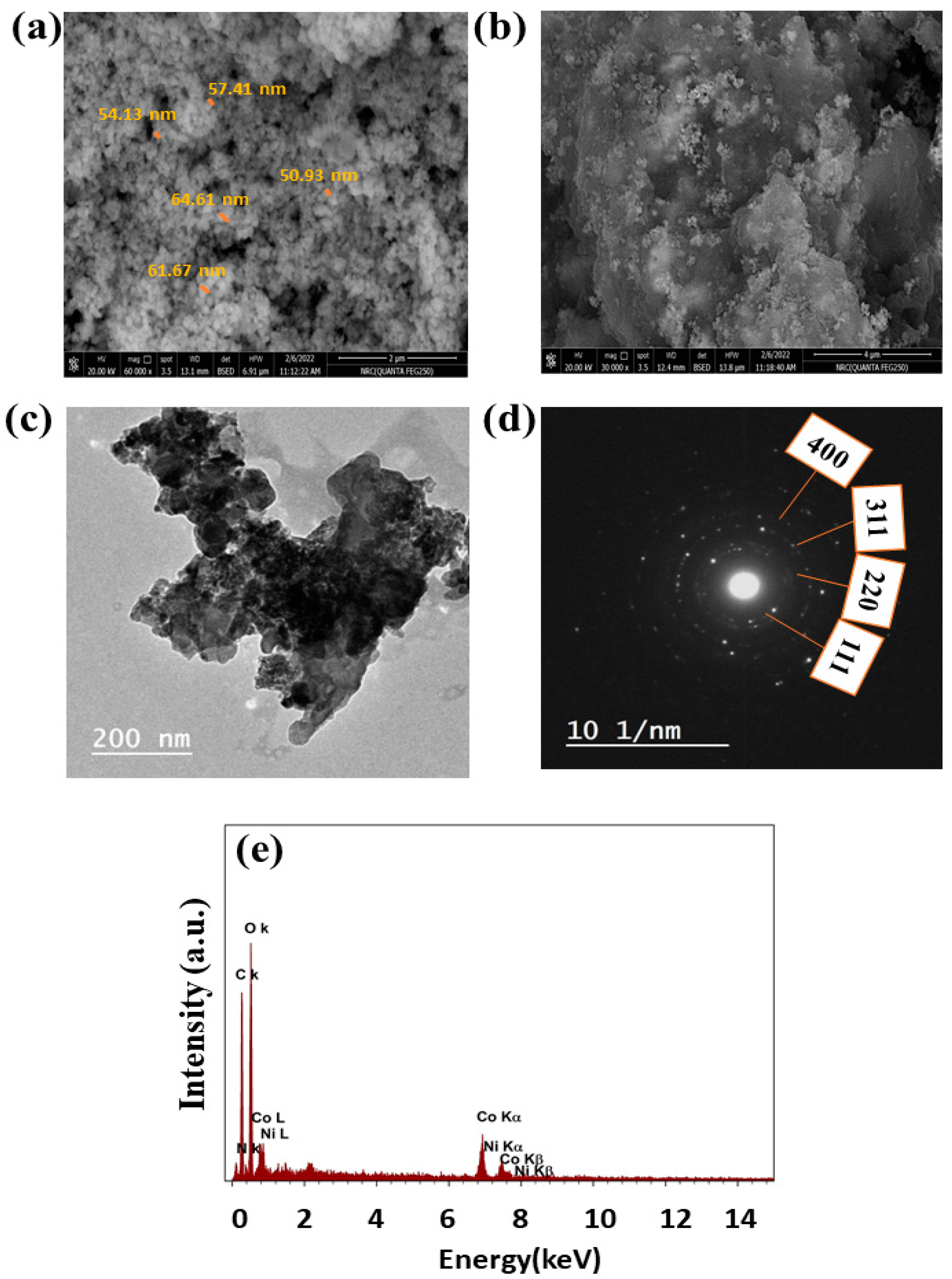
3.1. Characterizations of Morphology, Microstructure, and Composition
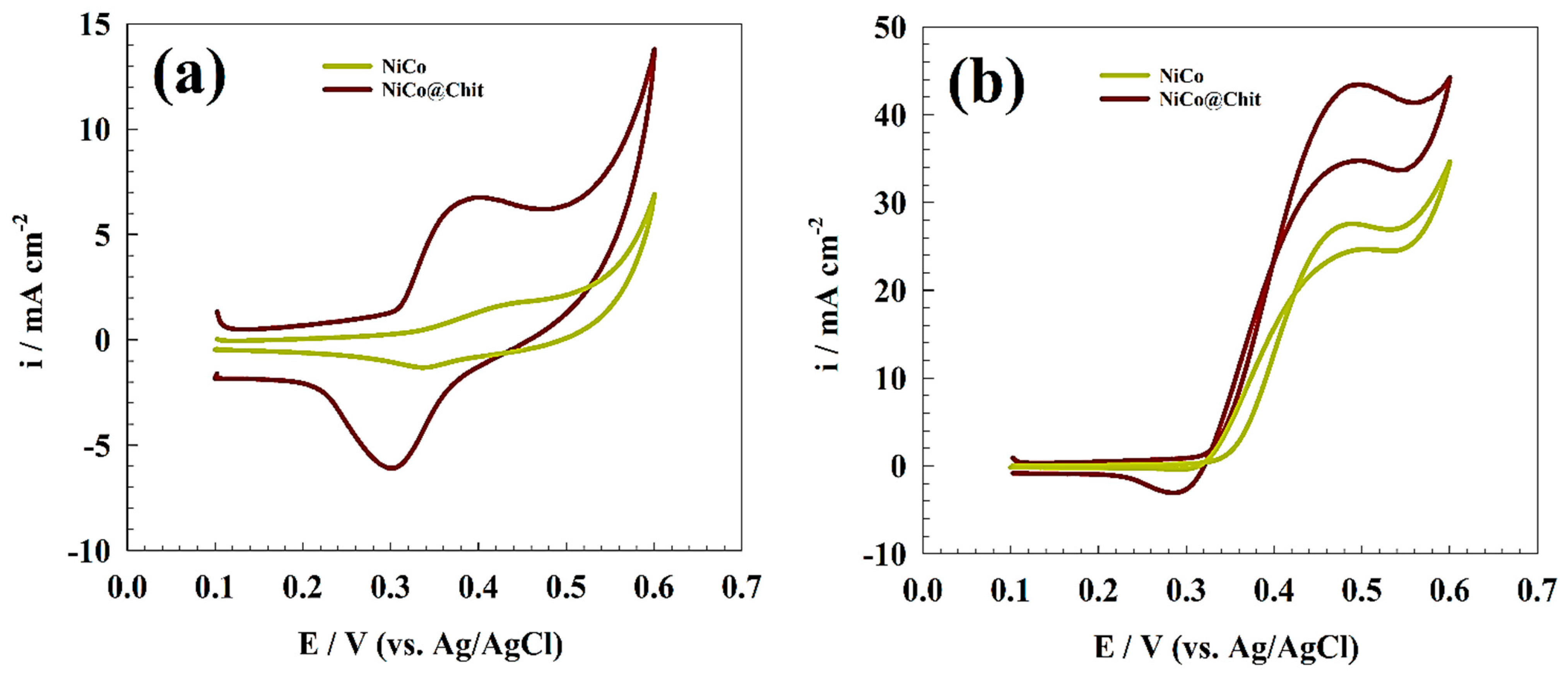
3.2. Urea Electrooxidation
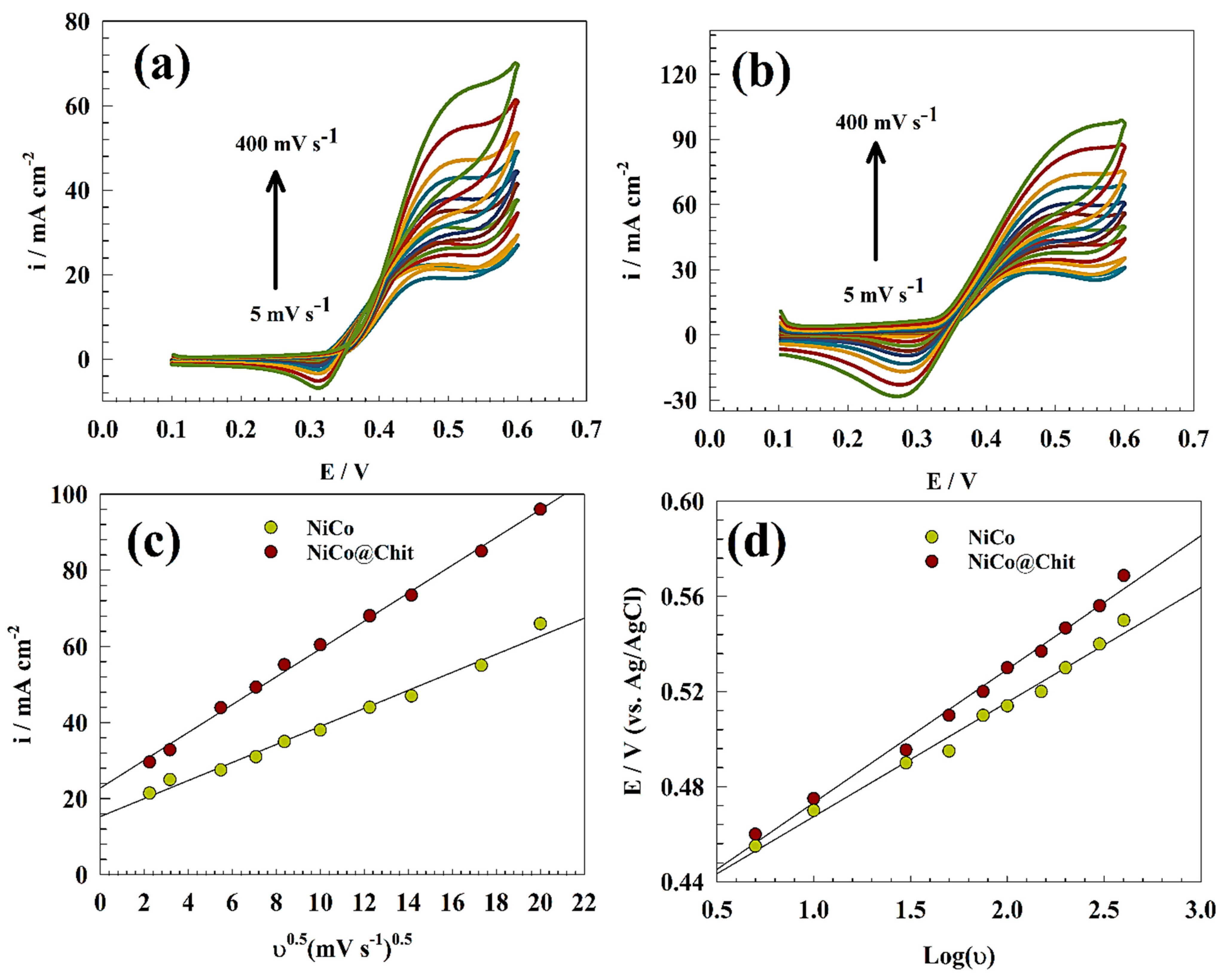
3.3. Urea Oxidation Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, B.; Liang, Z.; Zou, R. Designing advanced catalysts for energy conversion based on urea oxidation reaction. Small 2020, 16, 1906133. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Sowa, M.; Simka, W. Urea removal from aqueous solutions—A review. J. Appl. Electrochem. 2016, 46, 1011–1029. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zheng, X.; Fang, L.; Lu, S. Urea Electrooxidation in Alkaline Environment: Fundamentals and Applications. ChemElectroChem 2023, 10, e202300138. [Google Scholar] [CrossRef]
- Cho, K.; Hoffmann, M.R. Urea degradation by electrochemically generated reactive chlorine species: Products and reaction pathways. Environ. Sci. Technol. 2014, 48, 11504–11511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, J.; Duan, Y.; Li, J.; Wang, H.; Yang, X.; Gong, M. Electrochemical Urea Oxidation in Different Environment: From Mechanism to Devices. ChemCatChem 2022, 14, e202101906. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Z.; Tao, S. Urea-Based Fuel Cells and Electrocatalysts for Urea Oxidation. Energy Technol. 2016, 4, 1329–1337. [Google Scholar] [CrossRef] [Green Version]
- Gurrola, M.P.; Cruz, J.C.; Espinosa-Lagunes, F.I.; Martínez-Lázaro, A.; Ledesma-García, J.; Arriaga, L.G.; Escalona-Villalpando, R.A. Perspective of Use of Pd/rGO in a Direct Urea Microfluidic Fuel Cell. Catalysts 2023, 13, 788. [Google Scholar] [CrossRef]
- Kumar, S.; Bukkitgar, S.D.; Singh, S.; Pratibha; Singh, V.; Reddy, K.R.; Shetti, N.P.; Venkata Reddy, C.; Sadhu, V.; Naveen, S. Electrochemical sensors and biosensors based on graphene functionalized with metal oxide nanostructures for healthcare applications. ChemistrySelect 2019, 4, 5322–5337. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. Nanostructured titanium oxide hybrids-based electrochemical biosensors for healthcare applications. Colloids Surf. B Biointerfaces 2019, 178, 385–394. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Fadlallah, S.A.; El-Sherif, R.M.; Medany, S.S. Competition between enzymatic and non-enzymatic electrochemical determination of cholesterol. J. Electroanal. Chem. 2023, 930, 117169. [Google Scholar] [CrossRef]
- Eliwa, A.S.; Hefnawy, M.A.; Medany, S.S.; Deghadi, R.G.; Hosny, W.M.; Mohamed, G.G. Ultrasonic-assisted synthesis of nickel metal-organic framework for efficient urea removal and water splitting applications. Synth. Met. 2023, 294, 117309. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Nafady, A.; Mohamed, S.K.; Medany, S.S. Facile green synthesis of Ag/carbon nanotubes composite for efficient water splitting applications. Synth. Met. 2023, 294, 117310. [Google Scholar] [CrossRef]
- Najafinejad, M.S.; Chianese, S.; Fenti, A.; Iovino, P.; Musmarra, D. Application of Electrochemical Oxidation for Water and Wastewater Treatment: An Overview. Molecules 2023, 28, 4208. [Google Scholar] [CrossRef] [PubMed]
- Galindo-de-la-Rosa, J.; Álvarez, A.; Gurrola, M.P.; Rodríguez-Morales, J.A.; Oza, G.; Arriaga, L.G.; Ledesma-García, J. Alcohol Dehydrogenase Immobilized on TiO2 Nanotubes for Ethanol Microfluidic Fuel Cells. ACS Sustain. Chem. Eng. 2020, 8, 10900–10910. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Lang, C.-C.; Gao, M.-R.; Chen, Y.; Fu, Q.-Q.; Duan, Y.; Yu, S.-H. Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis. Energy Environ. Sci. 2018, 11, 1890–1897. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, X.; Zeng, W.; Zhang, Y.; Jiao, Y. Electrodeposition NiMoSe ternary nanoshperes on nickel foam as bifunctional electrocatalyst for urea electrolysis and hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 37792–37801. [Google Scholar] [CrossRef]
- Wang, X.-H.; Hong, Q.-L.; Zhang, Z.-N.; Ge, Z.-X.; Zhai, Q.-G.; Jiang, Y.-C.; Chen, Y.; Li, S.-N. Two-dimensional nickel–cobalt bimetallic hydroxides towards urea electrooxidation. Appl. Surf. Sci. 2022, 604, 154484. [Google Scholar] [CrossRef]
- Khalafallah, D.; Ouyang, C.; Zhi, M.; Hong, Z. Carbon Anchored Epitaxially Grown Nickel Cobalt-Based Carbonate Hydroxide for Urea Electrooxidation Reaction with a High Activity and Durability. ChemCatChem 2020, 12, 2283–2294. [Google Scholar] [CrossRef]
- Mirzaei, P.; Bastide, S.; Dassy, A.; Bensimon, R.; Bourgon, J.; Aghajani, A.; Zlotea, C.; Muller-Bouvet, D.; Cachet-Vivier, C. Electrochemical oxidation of urea on nickel-rhodium nanoparticles/carbon composites. Electrochim. Acta 2019, 297, 715–724. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Fadlallah, S.A.; El-Sherif, R.M.; Medany, S.S. Nickel-manganese double hydroxide mixed with reduced graphene oxide electrocatalyst for efficient ethylene glycol electrooxidation and hydrogen evolution reaction. Synth. Met. 2021, 282, 116959. [Google Scholar] [CrossRef]
- Basumatary, P.; Lee, U.H.; Konwar, D.; Yoon, Y.S. An efficient tri-metallic anodic electrocatalyst for urea electro-oxidation. Int. J. Hydrogen Energy 2020, 45, 32770–32779. [Google Scholar] [CrossRef]
- Wei, D.; Tang, W.; Wang, Y. Hairy sphere-like Ni9S8/CuS/Cu2O composites grown on nickel foam as bifunctional electrocatalysts for hydrogen evolution and urea electrooxidation. Int. J. Hydrogen Energy 2021, 46, 20950–20960. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Fadlallah, S.A.; El-Sherif, R.M.; Medany, S.S. Synergistic effect of Cu-doped NiO for enhancing urea electrooxidation: Comparative electrochemical and DFT studies. J. Alloys Compd. 2022, 896, 162857. [Google Scholar] [CrossRef]
- Wala, M.; Blacha–Grzechnik, A.; Stolarczyk, A.; Bajkacz, S.; Dydo, P.; Simka, W. Unexpected electrochemical oxidation of urea on a new NiCuGO composite catalyst. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Jayaprakash, J. Carbon cloth/nickel cobaltite (NiCo2O4)/polyaniline (PANI) composite electrodes: Preparation, characterization, and application in microbial fuel cells. Fuel 2021, 301, 121016. [Google Scholar] [CrossRef]
- Hua, B.; Zhang, W.; Wu, J.; Pu, J.; Chi, B.; Jian, L. A promising NiCo2O4 protective coating for metallic interconnects of solid oxide fuel cells. J. Power Sources 2010, 195, 7375–7379. [Google Scholar] [CrossRef]
- Chen, R.; Wang, H.-Y.; Miao, J.; Yang, H.; Liu, B. A flexible high-performance oxygen evolution electrode with three-dimensional NiCo2O4 core-shell nanowires. Nano Energy 2015, 11, 333–340. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Li, X.; Xu, J. Fabrication of NiCo2O4 nanobelt by a chemical co-precipitation method for non-enzymatic glucose electrochemical sensor application. J. Alloys Compd. 2020, 831, 154796. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Liu, Y.; Ju, Z.; Qian, Y. High electrochemical performance of monodisperse NiCo2O4 mesoporous microspheres as an anode material for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 981–988. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L.; Qi, T.; Xia, D.; Wan, H. Facilely synthesized porous NiCo2O4 flowerlike nanostructure for high-rate supercapacitors. J. Power Sources 2014, 248, 28–36. [Google Scholar] [CrossRef]
- Annu; Raja, A.N. Recent development in chitosan-based electrochemical sensors and its sensing application. Int. J. Biol. Macromol. 2020, 164, 4231–4244. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kas, H.S. Chitosan: Properties, preparations and application to microparticulate systems. J. Microencapsul. 1997, 14, 689–711. [Google Scholar] [CrossRef] [PubMed]
- de Alvarenga, E.S. Characterization and properties of chitosan. Biotechnol. Biopolym. 2011, 91, 48–53. [Google Scholar]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [Green Version]
- Hao, C.; Zhou, S.; Wang, J.; Wang, X.; Gao, H.; Ge, C. Preparation of hierarchical spinel NiCo2O4 nanowires for high-performance supercapacitors. Ind. Eng. Chem. Res. 2018, 57, 2517–2525. [Google Scholar] [CrossRef]
- Marco, J.F.; Gancedo, J.R.; Gracia, M.; Gautier, J.L.; Ríos, E.; Berry, F.J. Characterization of the nickel cobaltite, NiCo2O4, prepared by several methods: An XRD, XANES, EXAFS, and XPS study. J. Solid State Chem. 2000, 153, 74–81. [Google Scholar] [CrossRef]
- Shim, J.-J. Three-dimensional nickel foam/graphene/NiCo2O4 as high-performance electrodes for supercapacitors. J. Power Sources 2015, 273, 110–117. [Google Scholar]
- Adhikari, S.; Kwon, Y.; Kim, D.-H. Three-dimensional core–shell structured NiCo2O4@CoS/Ni-Foam electrocatalyst for oxygen evolution reaction and electrocatalytic oxidation of urea. Chem. Eng. J. 2020, 402, 126192. [Google Scholar] [CrossRef]
- Kim, J.-G.; Pugmire, D.L.; Battaglia, D.; Langell, M.A. Analysis of the NiCo2O4 spinel surface with Auger and X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2000, 165, 70–84. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-C.; Lo, W.-C.; Li, J.-X.; Wang, Y.-K.; Tang, J.-F.; Fong, Z.-Y. In situ XPS investigation of the X-ray-triggered decomposition of perovskites in ultrahigh vacuum condition. npj Mater. Degrad. 2021, 5, 13. [Google Scholar] [CrossRef]
- Dementjev, A.P.; de Graaf, A.; van de Sanden, M.C.M.; Maslakov, K.I.; Naumkin, A.V.; Serov, A.A. X-Ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Medany, S.S.; Hefnawy, M.A. Nickel–cobalt oxides decorated Chitosan electrocatalyst for ethylene glycol oxidation. Surf. Interfaces 2023, 40, 103077. [Google Scholar] [CrossRef]
- Tiwari, S.; Rathore, G.; Patra, N.; Yadav, A.K.; Bhattacharya, D.; Jha, S.N.; Tseng, C.M.; Liu, S.W.; Biring, S.; Sen, S. Oxygen and cerium defects mediated changes in structural, optical and photoluminescence properties of Ni substituted CeO2. J. Alloys Compd. 2019, 782, 689–698. [Google Scholar] [CrossRef]
- Ikram, M.; Shahzadi, A.; Hayat, S.; Nabgan, W.; Ul-Hamid, A.; Haider, A.; Noor, M.; Goumri-Said, S.; Kanoun, M.B.; Ali, S. Novel Ta/chitosan-doped CuO nanorods for catalytic purification of industrial wastewater and antimicrobial applications. RSC Adv. 2022, 12, 16991–17004. [Google Scholar] [CrossRef] [PubMed]
- Vijayaprasath, G.; Murugan, R.; Palanisamy, S.; Prabhu, N.M.; Mahalingam, T.; Hayakawa, Y.; Ravi, G. Role of nickel doping on structural, optical, magnetic properties and antibacterial activity of ZnO nanoparticles. Mater. Res. Bull. 2016, 76, 48–61. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium. Electrochim. Acta 2012, 81, 292–300. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Medany, S.S.; El-Sherif, R.M.; Fadlallah, S.A. Green synthesis of NiO/Fe3O4@chitosan composite catalyst based on graphite for urea electro-oxidation. Mater. Chem. Phys. 2022, 290, 126603. [Google Scholar] [CrossRef]
- Galal, A.; Atta, N.F.; Hefnawy, M.A. Lanthanum nickel oxide nano-perovskite decorated carbon nanotubes/poly (aniline) composite for effective electrochemical oxidation of urea. J. Electroanal. Chem. 2020, 862, 114009. [Google Scholar] [CrossRef]
- Atta, N.F.; El-Sherif, R.M.A.; Hassan, H.K.; Hefnawy, M.A.; Galal, A. Conducting Polymer-Mixed Oxide Composite Electrocatalyst for Enhanced Urea Oxidation. J. Electrochem. Soc. 2018, 165, J3310–J3317. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Direct evidence of the mechanism for the electro-oxidation of urea on Ni (OH) 2 catalyst in alkaline medium. Electrochim. Acta 2013, 108, 660–665. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Liang, Z.Y.; Meng, S.X. Adsorption of urea nitrogen onto chitosan coated dialdehyde cellulose under biocatalysis of immobilized urease: Equilibrium and kinetic. Biochem. Eng. J. 2005, 24, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puspita, A.; Prawati, G.; Fatimah, I. Chitosan-modified smectite clay and study on adsorption-desorption of urea. Chem. Eng. Trans. 2017, 56, 1645–1650. [Google Scholar]
- Zhou, Y.; Yang, Y.; Guo, X.; Chen, G. Effect of molecular weight and degree of deacetylation of chitosan on urea adsorption properties of copper chitosan. J. Appl. Polym. Sci. 2003, 89, 1520–1523. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, Y.; Jia, M.; Jia, J.; Wen, Z. Monodisperse Ni0·85Se nanocrystals on rGO for high-performance urea electrooxidation. J. Alloys Compd. 2021, 852, 156751. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Medany, S.S.; El-Sherif, R.M.; El-Bagoury, N.; Fadlallah, S.A. High-performance IN738 superalloy derived from turbine blade waste for efficient ethanol, ethylene glycol, and urea electrooxidation. J. Appl. Electrochem. 2023, 53, 1337–1348. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Medany, S.S.; El-Sherif, R.M.; Fadlallah, S.A. NiO-MnOx/Polyaniline/Graphite Electrodes for Urea Electrocatalysis: Synergetic Effect between Polymorphs of MnOx and NiO. ChemistrySelect 2022, 7, e202103735. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Xue, Y.; Miao, B.; Li, S.; Jiang, Y.; Liu, X.; Chen, Y. Atomically thick Ni (OH) 2 nanomeshes for urea electrooxidation. Nanoscale 2019, 11, 1058–1064. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Z.; Yan, G.; Wang, Z.; Li, X.; Guo, H.; Wang, J. New insight into the electrodeposition of NiCo layered double hydroxide and its capacitive evaluation. Electrochim. Acta 2020, 336, 135734. [Google Scholar] [CrossRef]
- Galal, A.; Atta, N.F.; Hefnawy, M.A. Voltammetry study of electrocatalytic activity of lanthanum nickel perovskite nanoclusters-based composite catalyst for effective oxidation of urea in alkaline medium. Synth. Met. 2020, 266, 116372. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Hefnawy, M.A.; SNafee, S.; Alamro, F.S.; Pashameah, R.A.; Ahmed, H.A.; Medany, S.S. Zinc Nanocomposite Supported Chitosan for Nitrite Sensing and Hydrogen Evolution Applications. Polymers 2023, 15, 2357. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Hefnawy, M.A.; Alamro, F.S.; Pashameah, R.A.; Ahmed, H.A.; Medany, S.S. Polyaniline-Supported Nickel Oxide Flower for Efficient Nitrite Electrochemical Detection in Water. Polymers 2023, 15, 1804. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Fadlallah, S.A.; El-Sherif, R.M.; Medany, S.S. Systematic DFT studies of CO-Tolerance and CO oxidation on Cu-doped Ni surfaces. J. Mol. Graph. Model. 2023, 118, 108343. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Shao, Z.; Zhou, P. Preparation and characterization of chitosan/Cu (II) affinity membrane for urea adsorption. J. Appl. Polym. Sci. 2003, 90, 1108–1112. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Bao, W.-W.; Li, M.-Y.; Yang, C.-M.; Zhang, N.-N. Ultrafast formation of an FeOOH electrocatalyst on Ni for efficient alkaline water and urea oxidation. Chem. Commun. 2020, 56, 14713–14716. [Google Scholar] [CrossRef]





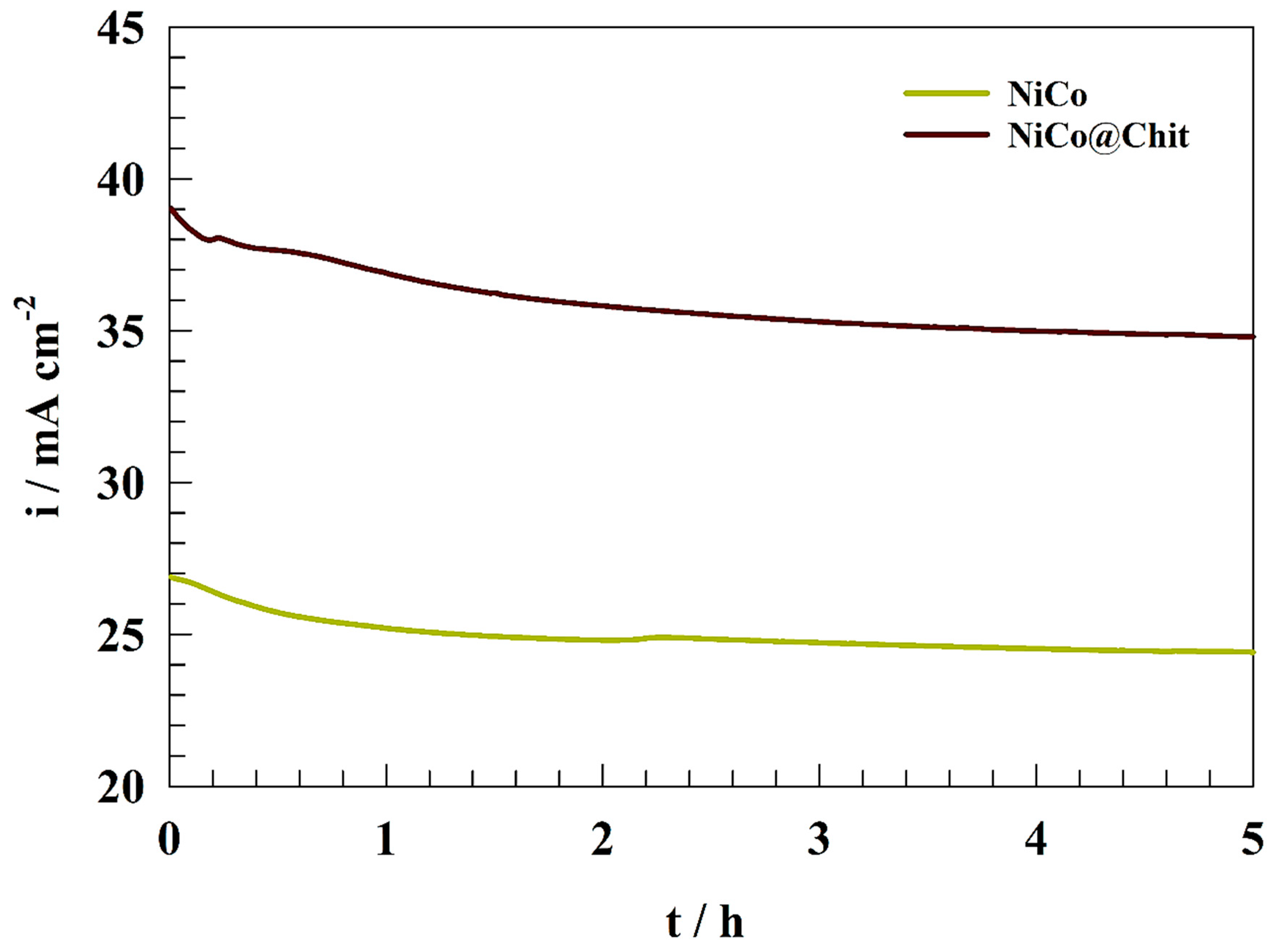
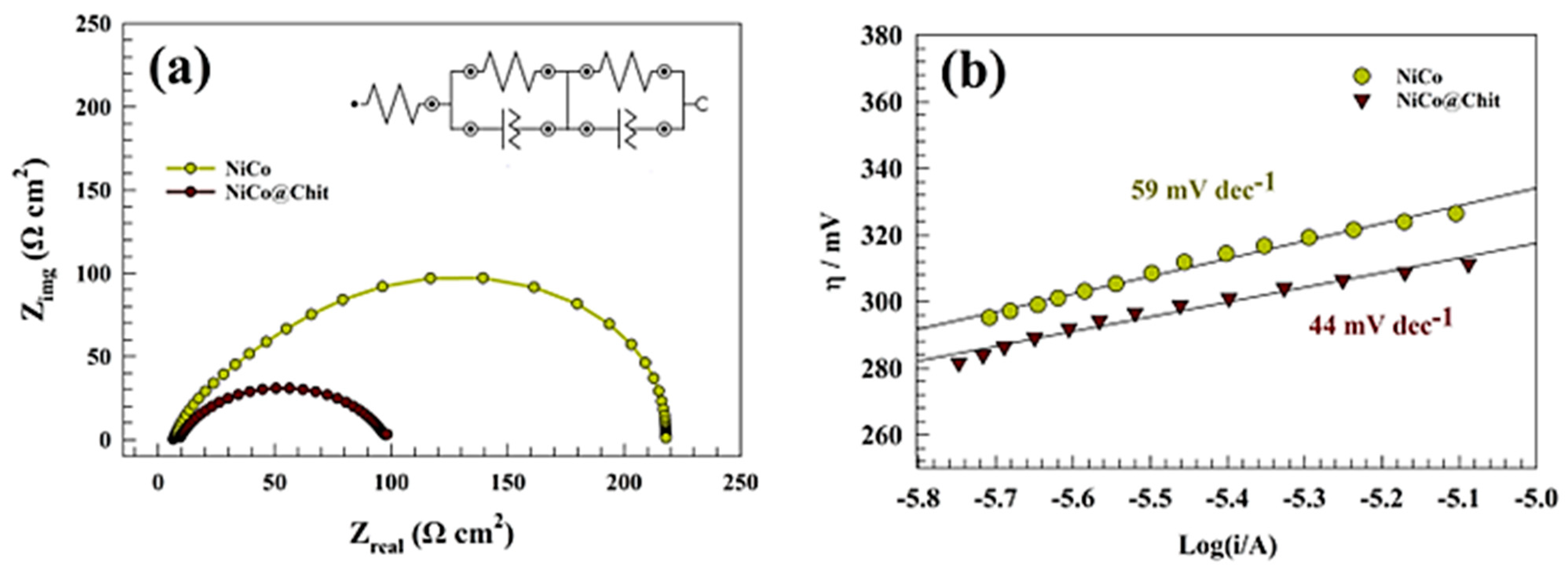
| Electrode | Anodic Current (mA cm−2) | Onset Potential (V) | Epa (V) | Tafel Slope mV dec−1 | Diffusion Coefficient (cm2 s−1) | Surface Coverage (Γ)/(mol cm−2) |
|---|---|---|---|---|---|---|
| NiCo | 27 | 0.35 | 0.5 | 44 | 5.98 × 10−6 | 9.34 × 10−10 |
| NiCo@Chit | 43 | 0.32 | 0.49 | 29 | 1.87 × 10−5 | 4.29 × 10−9 |
| Electrode | Fuel Concentration (M) | Electrolyte Concentration (M) | Scan Rate (mV s−1) | Oxidation Current (mA cm−2) | References |
|---|---|---|---|---|---|
| NiCo2O4@Chitosan | 1.0 | 1.0 | 20 | 43 | This work |
| Ni0.85Se/rGO | 0.5 | 1.0 | 50 | 10 | [56] |
| Ni0.9Cu0.1 | 0.3 | 0.5 | 20 | 32 | [23] |
| IN738 supper alloy | 1.0 | 1.0 | 20 | 12 | [57] |
| NiO-MnOx/Polyaniline | 0.3 | 0.5 | 20 | 16 | [58] |
| Ni(OH)2 meshes | 0.3 | 1.0 | 50 | 20 | [59] |
| Electrode | Rs | R1 | Q1 | R2 | Q2 | ||
|---|---|---|---|---|---|---|---|
| Ω cm2 | Ω cm2 | Y0 | N | Ω cm2 | Y0 | m | |
| NiCo | 3.2 | 6.76 | 0.0005621 | 0.5154 | 230 | 0.002180 | 0.8322 |
| NiCo@Chit | 2.5 | 7.56 | 0.0013547 | 0.6523 | 103 | 0.003715 | 0.7354 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamro, F.S.; Hefnawy, M.A.; Nafee, S.S.; Al-Kadhi, N.S.; Pashameah, R.A.; Ahmed, H.A.; Medany, S.S. Chitosan Supports Boosting NiCo2O4 for Catalyzed Urea Electrochemical Removal Application. Polymers 2023, 15, 3058. https://doi.org/10.3390/polym15143058
Alamro FS, Hefnawy MA, Nafee SS, Al-Kadhi NS, Pashameah RA, Ahmed HA, Medany SS. Chitosan Supports Boosting NiCo2O4 for Catalyzed Urea Electrochemical Removal Application. Polymers. 2023; 15(14):3058. https://doi.org/10.3390/polym15143058
Chicago/Turabian StyleAlamro, Fowzia S., Mahmoud A. Hefnawy, Sherif S. Nafee, Nada S. Al-Kadhi, Rami Adel Pashameah, Hoda A. Ahmed, and Shymaa S. Medany. 2023. "Chitosan Supports Boosting NiCo2O4 for Catalyzed Urea Electrochemical Removal Application" Polymers 15, no. 14: 3058. https://doi.org/10.3390/polym15143058