Physicochemical Characteristics of Individual Aerosol Particles during the 2015 China Victory Day Parade in Beijing
Abstract
:1. Introduction
2. Experimental
2.1. Aerosol Sampling
2.2. TEM Analysis
3. Results
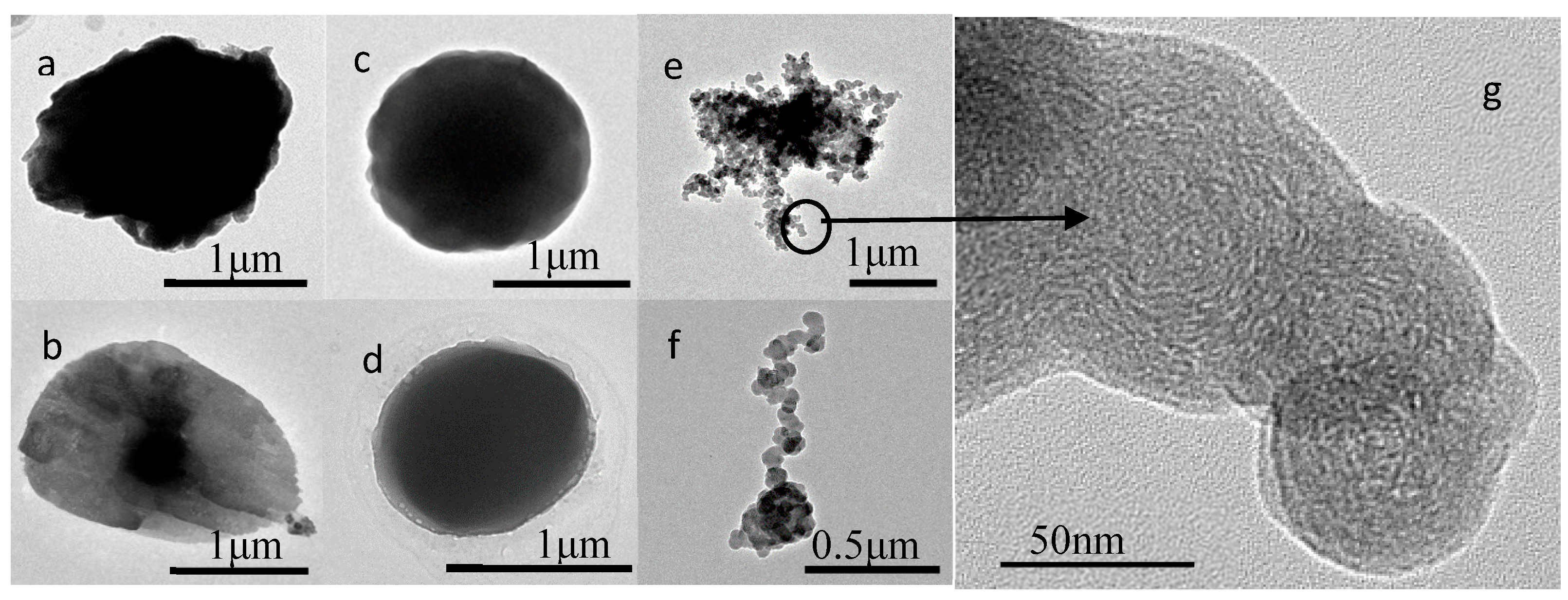
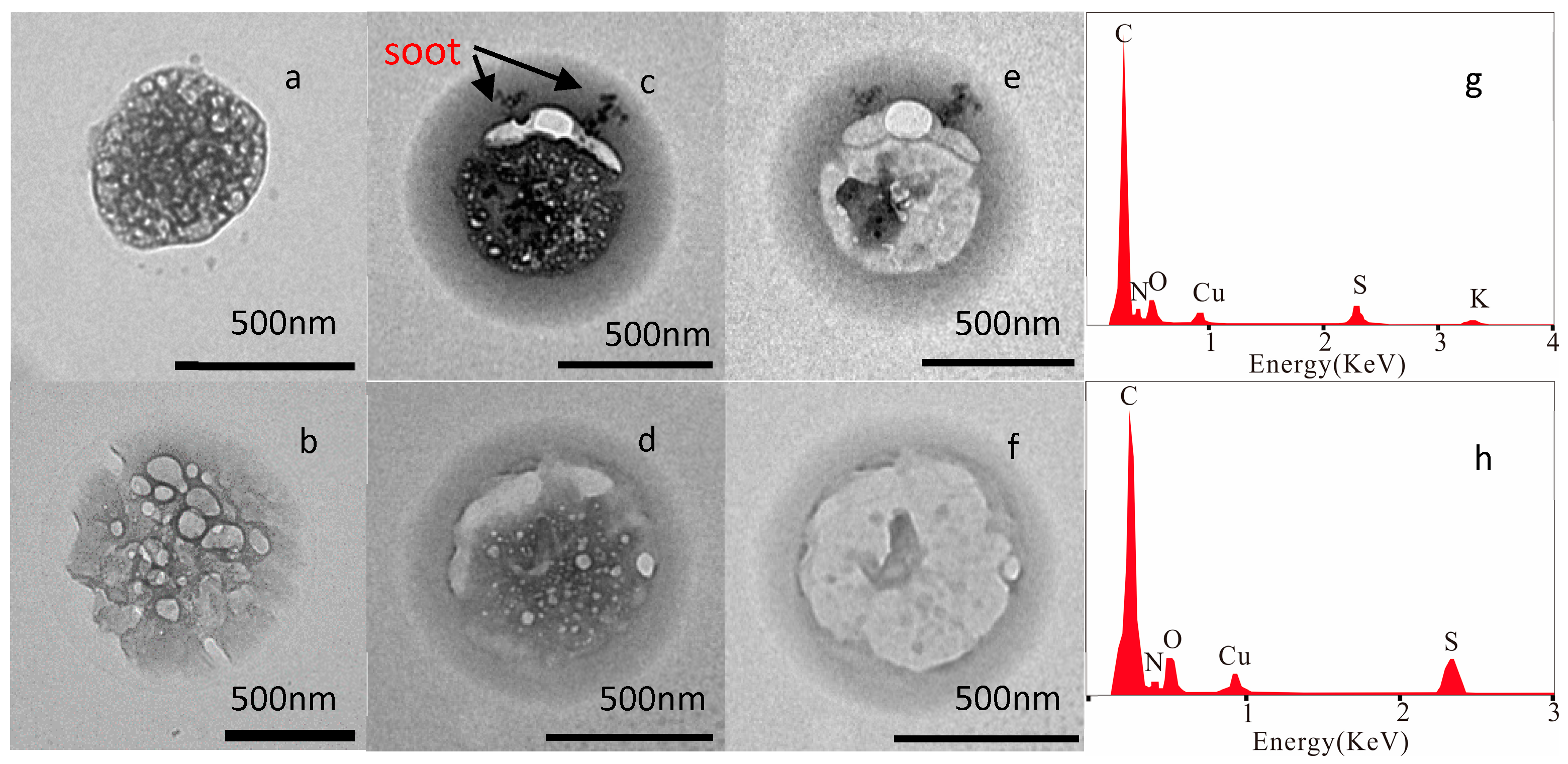
3.1. Nature of Individual Aerosol Particles
3.2. Relative Number Percentage and Size Distribution
4. Discussion and Atmospheric Implication
4.1. Influence of RH on Secondary Particle Formation
4.2. Atmospheric Implication of CS Particles
4.3. Possible Phase Transformation of CS Particles
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, R.J.; Zhang, Y.; Bozzetti, C.; Ho, K.F.; Cao, J.J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, J.J.; Cohen, A.; Dentener, F.; Brunekreef, B.; Zhu, T.; Armstrong, B.; Bell, M.L.; Brauer, M.; Carmichael, G.; Costa, D.L.; et al. What We Breathe Impacts Our Health: Improving Understanding of the Link between Air Pollution and Health. Environ. Sci. Technol. 2016, 50, 4895–4904. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.Y.; Hu, Y.; Shen, R.; Schäfer, K.; Wang, J.; Wang, J.; Schnelle-Kreis, J.; Zimmermann, R.; BéruBé, K.; Suppan, P. Seasonal variation of particle-induced oxidative potential of airborne particulate matter in Beijing. Sci. Total Environ. 2017, 579, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Planche, C.; Mann, G.W.; Carslaw, K.S.; Dalvi, M.; Marsham, J.H.; Field, P.R. Spatial and temporal CCN variations in convection-permitting aerosol microphysics simulations in an idealised marine tropical domain. Atmos. Chem. Phys. 2017, 17, 3371–3384. [Google Scholar] [CrossRef]
- Phillips, V.T.J.; DeMott, P.J.; Andronache, C. An empirical parameterization of heterogeneous ice nucleation for multiple chemical species of aerosol. J. Atmos. Sci. 2008, 65, 2757–2783. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, Z.; Wang, X.; Andersson, A.; Chen, J.; Zhang, Q.; Gustafsson, Ö. Reconciling modeling with observations of radiative absorption of black carbon aerosols. J. Geophys. Res. Atmos. 2017, 122, 5932–5942. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Ho, K.; Zhang, R.; Lin, Z.; Zhang, Z.; Lin, M.; Cao, J.; Liu, S.; Wang, G. Impact of PM2.5 chemical compositions on aerosol light scattering in Guangzhou—The largest megacity in South China. Atmos. Res. 2014, 135, 48–58. [Google Scholar] [CrossRef]
- Heald, C.L.; Jacob, D.J.; Park, R.J.; Alexander, B.; Fairlie, T.D.; Yantosca, R.M.; Chu, D.A. Transpacific transport of Asian anthropogenic aerosols and its impact on surface air quality in the United States. J. Geophys. Res. Atmos. 2006, 111, D14. [Google Scholar] [CrossRef]
- Ma, Q.X.; Wu, Y.; Zhang, D.; Wang, X.; Xia, Y.; Liu, X.; Tian, P.; Han, Z.; Xia, X.; Wang, Y. Roles of regional transport and heterogeneous reactions in the PM2.5 increase during winter haze episodes in Beijing. Sci. Total Environ. 2017, 599, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wang, Y.Q.; Niu, T.; Zhang, X.C.; Gong, S.L.; Zhang, Y.M.; Sun, J.Y. Atmospheric aerosol compositions in China: Spatial/temporal variability, chemical signature, regional haze distribution and comparisons with global aerosols. Atmos. Chem. Phys. 2012, 12, 779–799. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wang, L.; Yang, J.; Chen, M.; Wei, Z.; Su, J. The characteristics of air pollution episodes in autumn over the southern Hebei, China. World J. Eng. 2015, 12, 221–236. [Google Scholar] [CrossRef]
- Niu, H.; Cheng, W.; Pian, W.; Hu, W. The physicochemical properties of submicron particles from emissions of industrial furnace. World J. Eng. 2016, 13, 218–224. [Google Scholar] [CrossRef]
- Posfai, M.; Buseck, P.R. Nature and Climate Effects of Individual Tropospheric Aerosol Particles. Rev. Earth Planet. Sci. 2010, 38, 17–43. [Google Scholar] [CrossRef]
- Liu, S.; Aiken, A.C.; Gorkowski, K.; Dubey, M.K.; Cappa, C.D.; Williams, L.R.; Herndon, S.C.; Massoli, P.; Fortner, E.C.; Chhabra, P.S.; et al. Enhanced light absorption by mixed source black and brown carbon particles in UK winter. Nat. Commun. 2015, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Du, W.; Zhang, Y.; Wang, Q.; Chen, C.; Xu, W.; Han, T.; Wang, Y.; Fu, P.; Wang, Z.; et al. Insights into aerosol chemistry during the 2015 China Victory Day parade: Results from simultaneous measurements at ground level and 260 m in Beijing. Atmos. Chem. Phys. 2017, 17, 3215–3232. [Google Scholar] [CrossRef]
- Wang, T.; Nie, W.; Gao, J.; Xue, L.K.; Gao, X.M.; Wang, X.; Qiu, J.; Poon, C.N.; Meinardi, S.; Blake, D.; Wang, S.L. Air quality during the 2008 Beijing Olympics: Secondary pollutants and regional impact. Atmos. Chem. Phys. 2010, 10, 7603–7615. [Google Scholar] [CrossRef]
- Chen, C.; Sun, Y.L.; Xu, W.Q.; Du, W.; Zhou, L.B.; Han, T.T.; Wang, Q.Q.; Fu, P.Q.; Wang, Z.F.; Gao, Z.Q.; et al. Characteristics and sources of submicron aerosols above the urban canopy (260 m) in Beijing, China, during the 2014 APEC summit. Atmos. Chem. Phys. 2015, 15, 12879–12895. [Google Scholar] [CrossRef]
- Li, W.J.; Sun, J.; Xu, L.; Shi, Z.; Riemer, N.; Sun, Y.; Fu, P.; Zhang, J.; Lin, Y.; Wang, X.; et al. A conceptual framework for mixing structures in individual aerosol particles. J. Geophys. Res. Atmos. 2016, 121, 13784–13798. [Google Scholar] [CrossRef]
- Li, W.J.; Shao, L.Y. Characterization of mineral particles in winter fog of Beijing analyzed by TEM and SEM. Environ. Monit. Assess. 2010, 161, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, L.; Zhang, J.; Zhang, Y.; Ren, Y.; Wang, X.; Li, W. Morphology, composition, and mixing state of individual aerosol particles in Northeast China during wintertime. Atmosphere 2017, 8, 10. [Google Scholar] [CrossRef]
- Xing, J.P.; Shao, L.; Zheng, R.; Peng, J.; Wang, W.; Guo, Q.; Wang, Y.; Qin, Y.; Shuai, S.; Hu, M. Individual particles emitted from gasoline engines: Impact of engine types, engine loads and fuel components. J. Clean. Prod. 2017, 149, 461–471. [Google Scholar] [CrossRef]
- Li, W.J.; Shao, L.; Zhang, D.; Ro, C.U.; Hu, M.; Bi, X.; Geng, H.; Matsuki, A.; Niu, H.; Chen, J. A review of single aerosol particle studies in the atmosphere of East Asia: Morphology, mixing state, source, and heterogeneous reactions. J. Clean. Prod. 2016, 112, 1330–1349. [Google Scholar] [CrossRef]
- China, S.; Scarnato, B.; Owen, R.C.; Zhang, B.; Ampadu, M.T.; Kumar, S.; Dzepina, K.; Dziobak, M.P.; Fialho, P.; Perlinger, J.A.; et al. Morphology and mixing state of aged soot particles at a remote marine free troposphere site: Implications for optical properties. Geophys. Res. Lett. 2015, 42, 1243–1250. [Google Scholar] [CrossRef]
- Li, W.J.; Shao, L.Y. Mixing and water-soluble characteristics of particulate organic compounds in individual urban aerosol particles. J. Geophys. Res. Atmos. 2010, 115, 9. [Google Scholar] [CrossRef]
- Ghosal, S.; Weber, P.K.; Laskin, A. Spatially resolved chemical imaging of individual atmospheric particles using nanoscale imaging mass spectrometry: Insight into particle origin and chemistry. Anal. Methods 2014, 6, 2444–2451. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Tian, H.; Gao, J.; Chen, Y.; Zhu, C.; Liu, H.; Wang, K.; Hua, S.; Liu, S.; et al. Effectiveness of temporary control measures for lowering PM2.5 pollution in Beijing and the implications. Atmos. Environ. 2017, 157, 75–83. [Google Scholar] [CrossRef]
- Wang, Z.S.; Li, Y.T.; Zhang, D.W.; Chen, T.; Wei, Q.; Sun, T.H.; Wang, B.Y.; Pan, J.X.; Cui, J.X.; Pi, S. Analysis on air quality in Beijing during the military parade period in 2015. China Environ. Sci. 2017, 37, 1628–1636. (In Chinese) [Google Scholar]
- Wang, W.; Shao, L.; Guo, M.; Hou, C.; Xing, J.; Wu, F. Physicochemical properties of individual airborne particles in Beijing during pollution periods. Aerosol Air Qual. Res. 2017, 17, 3209–3219. [Google Scholar] [CrossRef]
- Bian, Y.X.; Zhao, C.S.; Ma, N.; Chen, J.; Xu, W.Y. A study of aerosol liquid water content based on hygroscopicity measurements at high relative humidity in the North China Plain. Atmos. Chem. Phys. 2014, 14, 6417–6426. [Google Scholar] [CrossRef] [Green Version]
- Villani, P.; Sellegri, K.; Monier, M.; Laj, P. Influence of semi-volatile species on particle hygroscopic growth. Atmos. Environ. 2013, 79, 129–137. [Google Scholar] [CrossRef]
- Fajardo, O.A.; Jiang, J.K.; Hao, J.M. Continuous Measurement of Ambient Aerosol Liquid Water Content in Beijing. Aerosol Air Qual. Res. 2016, 16, 1152–1164. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wu, Z.; Tan, T.; Wang, Y.; Qin, Y.; Zheng, J.; Li, M.; Hu, M. Estimation of the PM2.5 effective hygroscopic parameter and water content based on particle chemical composition: Methodology and case study. Sci. China-Earth Sci. 2016, 59, 1683–1691. [Google Scholar] [CrossRef]
- Wang, G.H.; Zhang, R.; Gomez, M.E.; Yang, L.; Zamora, M.L.; Hu, M.; Lin, Y.; Peng, J.; Guo, S.; Meng, J.; et al. Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. USA 2016, 113, 13630–13635. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, P.F.; Hanna, S.J.; Li, Y.J.; Martin, S.T.; Bertram, A.K. Relative humidity-dependent viscosities of isoprene-derived secondary organic material and atmospheric implications for isoprene-dominant forests. Atmos. Chem. Phys. 2015, 15, 5145–5159. [Google Scholar] [CrossRef]
- Liu, Z.R.; Hu, B.; Zhang, J.; Yu, Y.; Wang, Y. Characteristics of aerosol size distributions and chemical compositions during wintertime pollution episodes in Beijing. Atmos. Res. 2016, 168, 1–12. [Google Scholar] [CrossRef]
- Semeniuk, T.A.; Wise, M.E.; Martin, S.T.; Russell, L.M.; Buseck, P.R. Water uptake characteristics of individual atmospheric particles having coatings. Atmos. Environ. 2007, 41, 6225–6235. [Google Scholar] [CrossRef]
- Davies, J.F.; Miles, R.E.; Haddrell, A.E.; Reid, J.P. Influence of organic films on the evaporation and condensation of water in aerosol. Proc. Natl. Acad. Sci. USA 2013, 110, 8807–8812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.J.; Marcolli, C.; Krieger, U.K.; Lienhard, D.M.; Peter, T. Morphologies of mixed organic/inorganic/aqueous aerosol droplets. Faraday Discuss. 2013, 165, 289–316. [Google Scholar] [CrossRef] [PubMed]
- Zuend, A.; Marcolli, C.; Peter, T.; Seinfeld, J.H. Computation of liquid-liquid equilibria and phase stabilities: Implications for RH-dependent gas/particle partitioning of organic-inorganic aerosols. Atmos. Chem. Phys. 2010, 10, 7795–7820. [Google Scholar] [CrossRef]
- Schill, G.P.; Tolbert, M.A. Heterogeneous ice nucleation on phase-separated organic-sulfate particles: Effect of liquid vs. glassy coatings. Atmos. Chem. Phys. 2013, 13, 4681–4695. [Google Scholar] [CrossRef]
- Stewart, D.J.; Cai, C.; Nayler, J.; Preston, T.C.; Reid, J.P.; Krieger, U.K.; Marcolli, C.; Zhang, Y.H. Liquid-Liquid Phase Separation in Mixed Organic/Inorganic Single Aqueous Aerosol Droplets. J. Phys. Chem. A 2015, 119, 4177–4190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Marcolli, C.; Krieger, U.K.; Zuend, A.; Peter, T. Liquid-liquid phase separation and morphology of internally mixed dicarboxylic acids/ammonium sulfate/water particles. Atmos. Chem. Phys. 2012, 12, 2691–2712. [Google Scholar] [CrossRef]
- You, Y.; Renbaum-Wolff, L.; Carreras-Sospedra, M.; Hanna, S.J.; Hiranuma, N.; Kamal, S.; Smith, M.L.; Zhang, X.; Weber, R.J.; Shilling, J.E.; et al. Images reveal that atmospheric particles can undergo liquid-liquid phase separations. Proc. Natl. Acad. Sci. USA 2012, 109, 13188–13193. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Smith, M.L.; Song, M.; Martin, S.T.; Bertram, A.K. Liquid-liquid phase separation in atmospherically relevant particles consisting of organic species and inorganic salts. Int. Rev. Phys. Chem. 2014, 33, 43–77. [Google Scholar] [CrossRef]
- Hou, C.; Shao, L.; Hu, W.; Zhang, D.; Zhao, C.; Xing, J.; Huang, X.; Hu, M. Characteristics and aging of traffic-derived particles in a highway tunnel at a coastal city in southern China. Sci. Total Environ. 2018, 619, 1385–1393. [Google Scholar] [CrossRef]
- Marcolli, C.; Krieger, U.K. Phase changes during hygroscopic cycles of mixed organic/inorganic model systems of tropospheric aerosols. J. Phys. Chem. A 2006, 110, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Hodas, N.; Zuend, A.; Mui, W.; Flagan, R.C.; Seinfeld, J.H. Influence of particle-phase state on the hygroscopic behavior of mixed organic-inorganic aerosols. Atmos. Chem. Phys. 2015, 15, 5027–5045. [Google Scholar] [CrossRef]
- Bondy, A.L.; Kirpes, R.M.; Merzel, R.L.; Pratt, K.A.; Banaszak Holl, M.M.; Ault, A.P. Atomic Force Microscopy-Infrared Spectroscopy of Individual Atmospheric Aerosol Particles: Subdiffraction Limit Vibrational Spectroscopy and Morphological Analysis. Anal. Chem. 2017, 89, 8594–8598. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, R.E.; Wang, B.; Kelly, S.T.; Lundt, N.; You, Y.; Bertram, A.K.; Leone, S.R.; Laskin, A.; Gilles, M.K. Liquid-Liquid Phase Separation in Aerosol Particles: Imaging at the Nanometer Scale. Environ. Sci. Technol. 2015, 49, 4995–5002. [Google Scholar] [CrossRef] [PubMed]
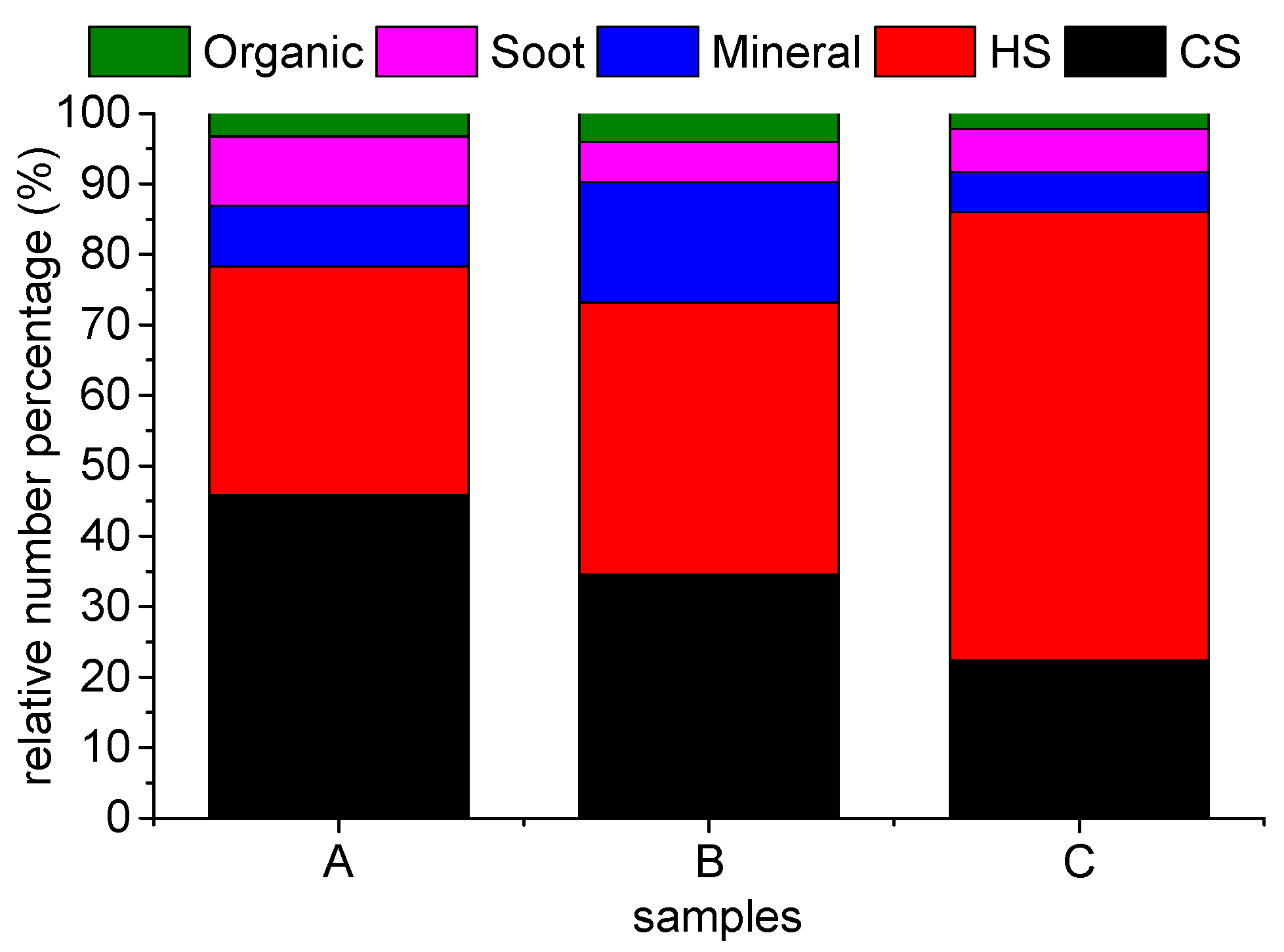
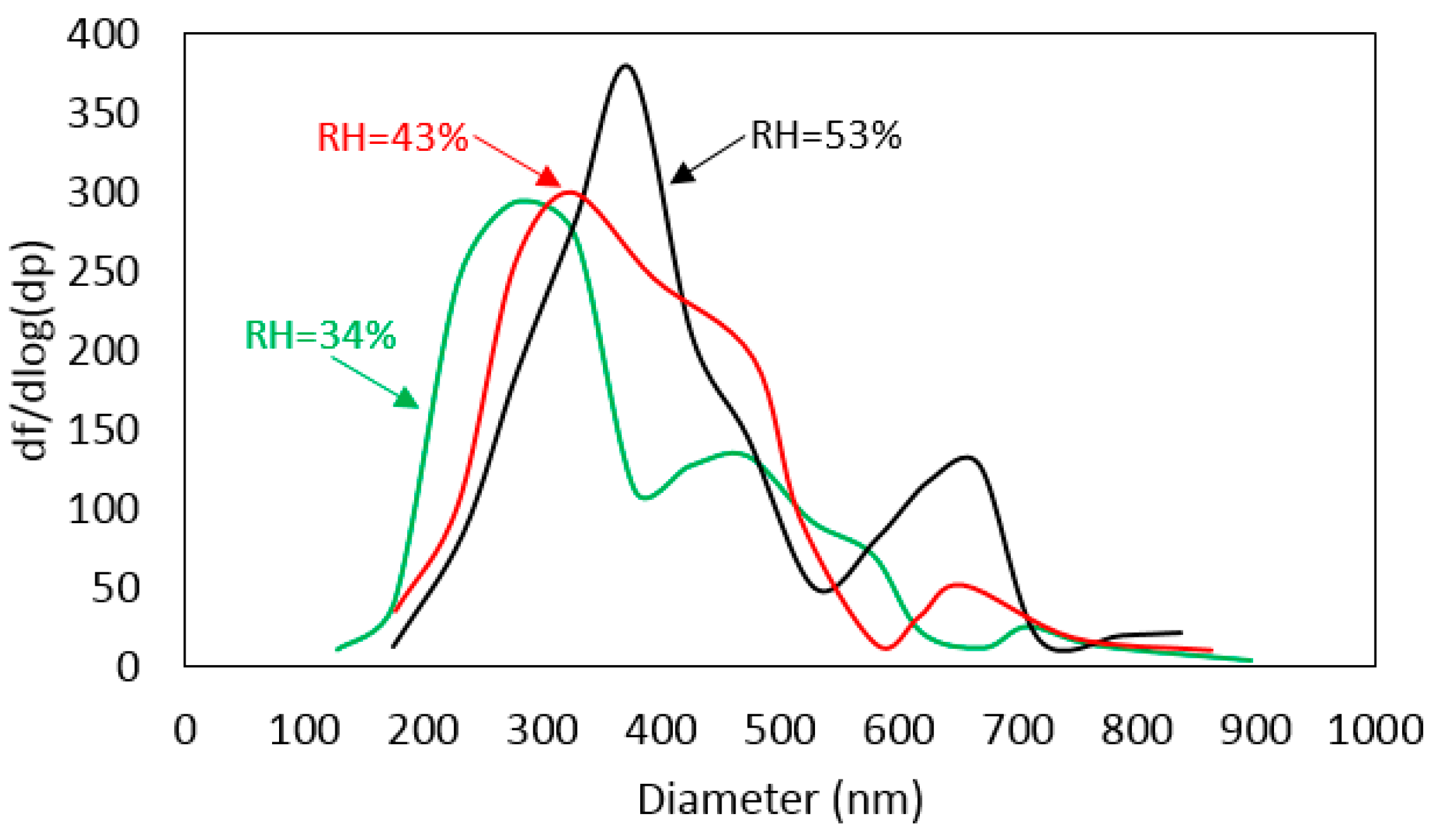
| ID | Collection Date | T (°C) | RH (%) | P (hPa) |
|---|---|---|---|---|
| A | 2015/8/28 | 34.8 | 34.3 | 1000.1 |
| B | 2015/9/3 | 29.3 | 43.5 | 1005.1 |
| C | 2015/9/2 | 27.2 | 53.3 | 1006.5 |
| Types | Sub-Types | Physical and Chemical Characteristics |
|---|---|---|
| Primary particles | Mineral | Irregular shaped; mainly composed O, Si, Al, Fe, Ca, Na, K, Mg; soil, road dust and construction dust sources |
| Soot | Aggregate shaped spheres; composed of C and minor O; sourced from incomplete combustion. | |
| Organic | Spherical or near spherical shaped; composed of C and O; combustion sources | |
| Secondary particles | Homogeneous mixed S-rich particles (HS) | Spherical or near spherical shaped; composed of C, S, O, N and sometimes with minor K; beam-sensitive; secondary formation in atmosphere |
| Organic coated S-rich particles (CS) | Core-shell structure; composed of C, S, O, N and sometimes with minor K; beam-sensitive; secondary formation in atmosphere |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Shao, L.; Xing, J.; Li, J.; Chang, L.; Li, W. Physicochemical Characteristics of Individual Aerosol Particles during the 2015 China Victory Day Parade in Beijing. Atmosphere 2018, 9, 40. https://doi.org/10.3390/atmos9020040
Wang W, Shao L, Xing J, Li J, Chang L, Li W. Physicochemical Characteristics of Individual Aerosol Particles during the 2015 China Victory Day Parade in Beijing. Atmosphere. 2018; 9(2):40. https://doi.org/10.3390/atmos9020040
Chicago/Turabian StyleWang, Wenhua, Longyi Shao, Jiaoping Xing, Jie Li, Lingli Chang, and Wenjun Li. 2018. "Physicochemical Characteristics of Individual Aerosol Particles during the 2015 China Victory Day Parade in Beijing" Atmosphere 9, no. 2: 40. https://doi.org/10.3390/atmos9020040





