Short-Range Responses of the Kissing Bug Triatoma rubida (Hemiptera: Reduviidae) to Carbon Dioxide, Moisture, and Artificial Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
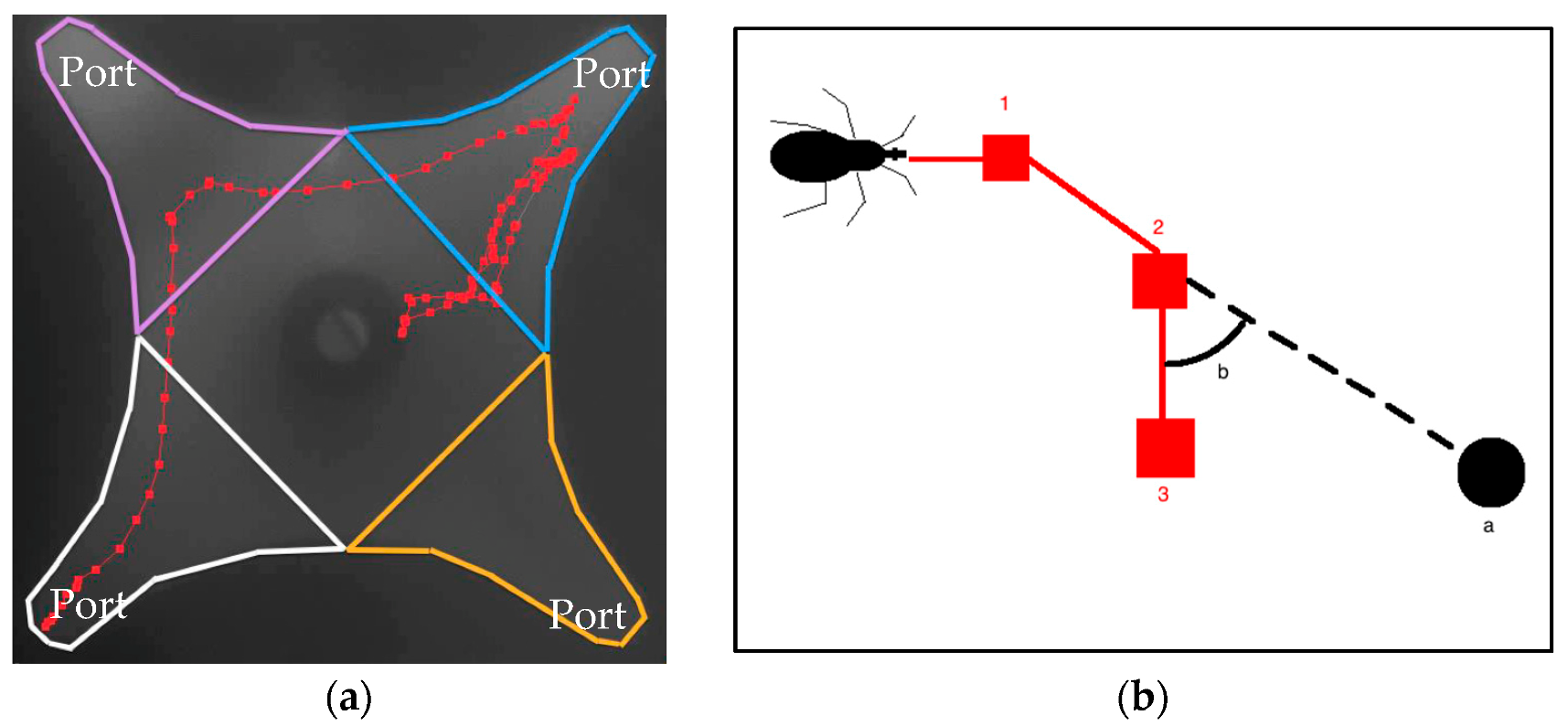
2.2. Olfactometer and Tracking of Activity
2.3. Response to Carbon Dioxide
2.4. Response to Moisture
2.5. Light Experiments
2.6. Data Analysis
3. Results
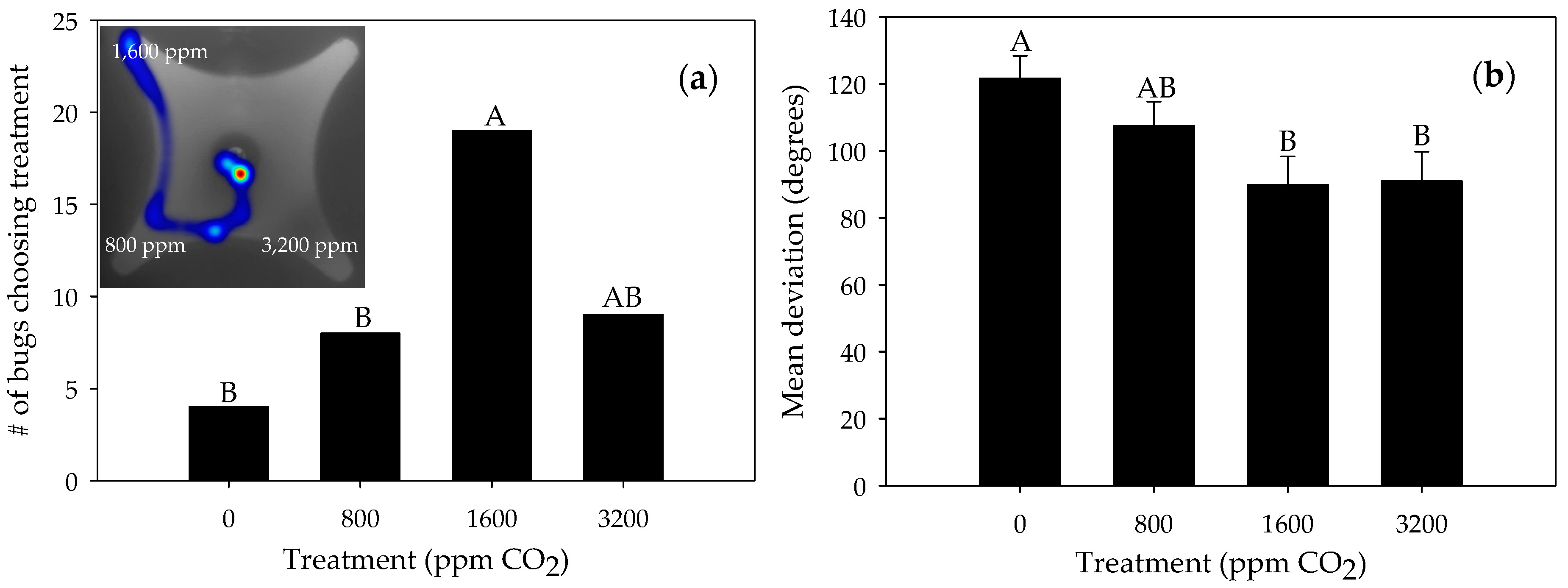
3.1. Responses to Carbon Dioxide
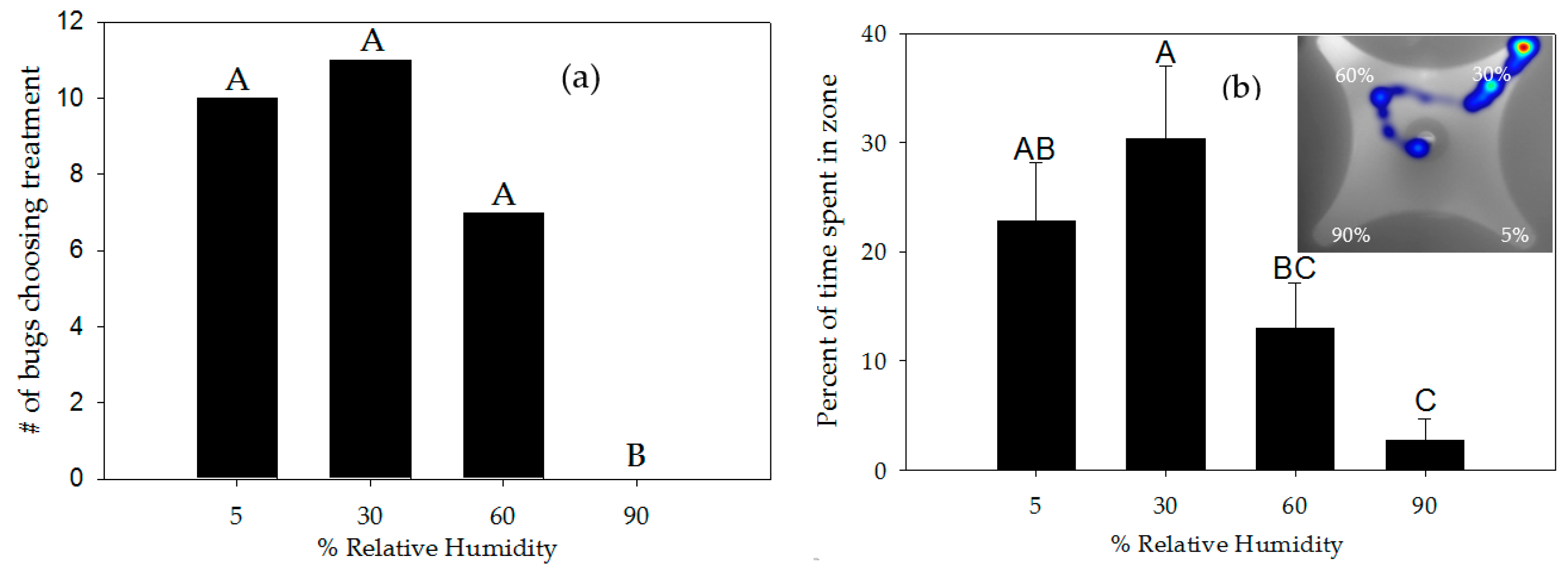
3.2. Response to Moisture
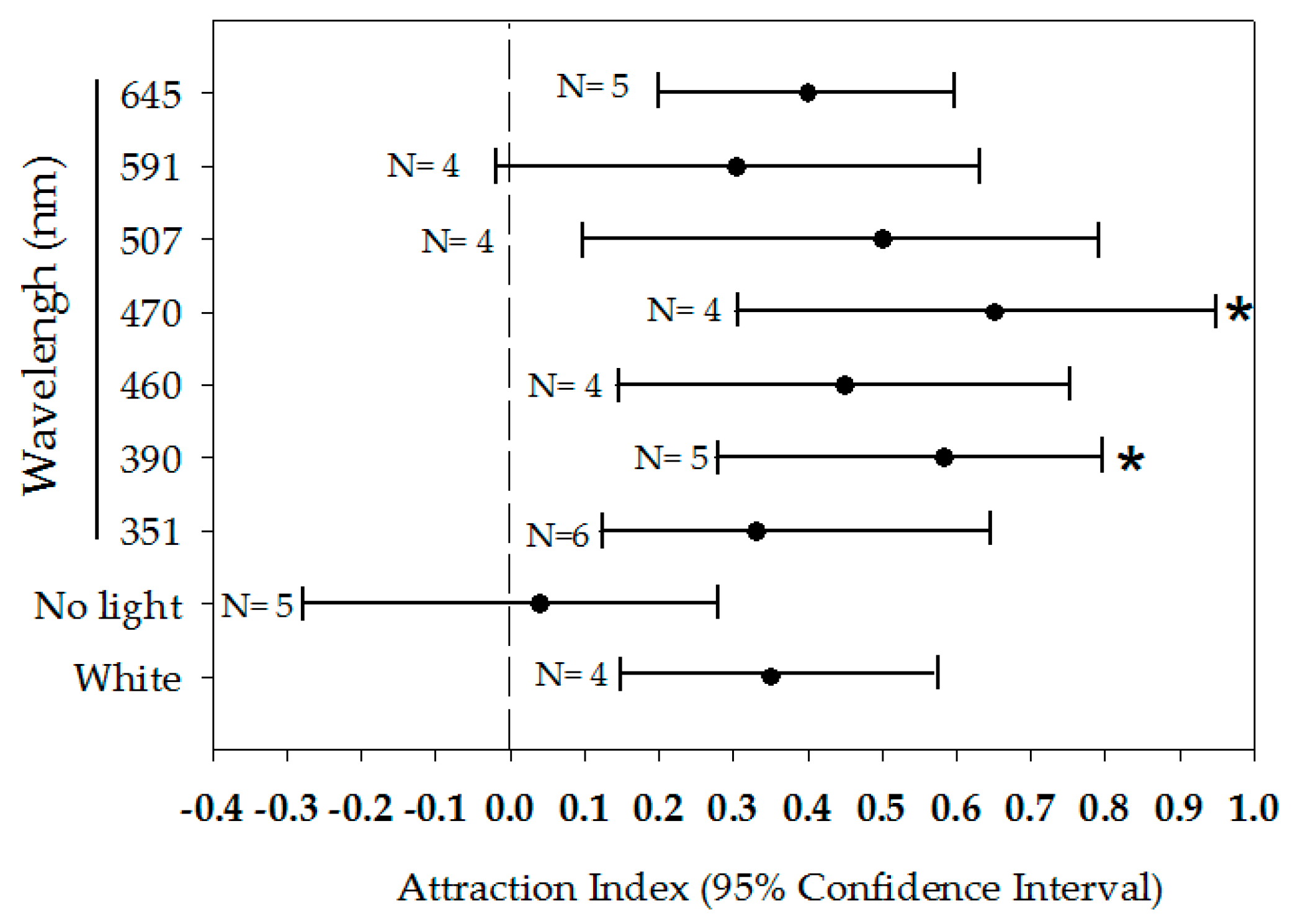
3.3. Responses to Light
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klotz, J.H.; Dorn, P.L.; Logan, J.L.; Stevens, L.; Pinnas, J.L.; Schmidt, J.O.; Klotz, S.A. “Kissing bugs”: Potential disease vectors and cause of anaphylaxis. Clin. Infect. Dis. 2010, 50, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.S.; Janovy, J.; Nadler, S. Foundations in Parasitology; New York McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Lee, B.Y.; Bacon, K.M.; Bottazzi, M.E.; Hotez, P.J. Global economic burden of Chagas disease: A computational simulation model. Lancet Infect. Dis. 2013, 13, 342–348. [Google Scholar] [CrossRef]
- Garza, M.; Feria Arroyo, T.P.; Casillas, E.A.; Sanchez-Cordero, V.; Rivaldi, C.-L.; Sarkar, S. Projected future distributions of vectors of Trypanosoma cruzi in North America under climate change scenarios. PLoS Negl. Trop. Dis. 2014, 8, e2818. [Google Scholar] [CrossRef] [PubMed]
- Reisenman, C.E.; Lawrence, G.; Guerenstein, P.G.; Gregory, T.; Dotson, E.; Hildebrand, J.G. Infection of kissing bugs with Trypanosoma cruzi, Tucson, Arizona, USA. Emerg. Infect. Dis. 2010, 16, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ibarra, J.A.; Paredes-González, E.; Licón-Trillo, A.; Montañez-Valdez, O.D.; Rocha-Chávez, G.; Nogueda-Torres, B. The biology of three Mexican-American species of Triatominae (Hemiptera: Reduviidae): Triatoma recurva, Triatoma protracta and Triatoma rubida. Mem. Inst. Oswaldo Cruz 2012, 107, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Reisenman, C.E.; Savary, W.; Cowles, J.; Gregory, T.L.; Hildebrand, J.G. The distribution and abundance of Triatomine insects, potential vectors of Chagas disease, in a metropolitan area in Southern Arizona, United States. J. Med. Entomol. 2012, 49, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.A.; Dorn, P.L.; Mosbacher, M.; Schmidt, J.O. Kissing bugs in the United States: Risk for vector-borne disease in humans. Environ. Health Insights 2014, 8, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ryckman, R.E. The vertebrate hosts of the Triatominae of North and Central America and the West Indies (Hemiptera: Reduviidae: Triatominae). Bull. Soc. Vector Ecol. 1986, 11, 221–241. [Google Scholar]
- Wood, S.F. Notes on the distribution and habits of Reduviid vectors of Chagas disease in the Southwest United States. Pan Pac. Entomol. 1941, 27, 115–118. [Google Scholar]
- Wood, S.F. Dispersal flight of Triatoma in southern Arizona. J. Parasitol. 1950, 36, 498–499. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Tucuch, F.S.; Ramirez-Sierra, M.J.; Gourbière, S.; Dumonteil, E. Public street lights increase house infestation by the Chagas disease vector Triatoma dimidiate. PLoS ONE 2012, 7, e36207. [Google Scholar] [CrossRef] [PubMed]
- Zeledon, R.B.C.; Pinto, D.J.; Leiby, D.; Dorn, P.; Coura, J.R. An Appraisal of the Status of Chagas Disease in the United States; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Walter, J.; Fletcher, E.; Moussaoui, R.; Gandhi, K.; Weirauch, C. Do bites of kissing bugs cause unexplained allergies? Results from a survey in triatomine-exposed and unexposed areas in Southern California. PLoS ONE 2012, 7, e44016. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.A.; Mazda Shirazi, F.; Boesen, K.; Beatty, N.L.; Dorn, P.L.; Smith, S.; Schmidt, J.O. Kissing bug (Triatoma spp.) intrusion into homes: Troublesome bites and domiciliation. Environ. Health Insights 2016, 10, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Sfara, V.; Zerba, E.N.; Alzogaray, R.A. Toxicity of pyrethroids and repellency of diethyltoluamide in tow deltamethrin-resistant colonies of Triatoma infestans Klug, 1834 (Hemiptera: Reduviidae). Mem. Inst. Oswaldo Cruz 2006, 101, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Rozendaal, J.A. Triatomine bugs. In Vector Control. Methods for Use by Individuals and Communities; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Buhaya, M.H.; Galvan, S.; Maldonado, R.A. Incidence of Trypanosoma cruzi infection in triatomines collected at Indio Mountains Research Station. Acta Trop. 2015, 150, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.; Dorn, P.L.; Hobson, J.; de la Rua, N.M.; Lucero, D.E.; Klotz, J.H.; Schmidt, J.O.; Klotz, S.A. Vector blood meals and Chagas disease transmission potential, United States. Emerg. Infect. Dis. 2012, 18, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.; New Mexico State University, Artesia, NM, USA. Personal communication, 2017.
- Reisenman, C.E.; Gregory, T.; Guerenstein, P.G.; Hildebrand, J.G. Feeding and defecation behavior of Triatoma rubida (Uhler, 1894) (Hemiptera: Reduviidae) under laboratory conditions, and its potential role as a vector of Chagas disease in Arizona, USA. Am. J. Trop. Med. Hyg. 2011, 85, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, C.R.; Lorenzo, M.G. Exploiting triatomine behaviour: Alternative perspectives for their control. Mem. Inst. Oswaldo Cruz 2009, 104, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gibb, T.; Bennett, G.W.; McKnight, S. Bed bug (Heteroptera: Cimicidae) attraction to pitfall traps baited with carbon dioxide, heat, and chemical lure. J. Econ. Entomol. 2009, 102, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Milne, M.A.; Ross, E.J.; Sonenshine, D.E.; Kirsch, P. Attraction of Triatoma dimidiata and Rhodnius prolixus (Hemiptera: Reduviidae) to combinations of host cues tested at two distances. J. Med. Entomol. 2009, 46, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, R.B.; Manrique, G.; Lazzari, C.R. The role of water vapour in the orientation behaviour of the blood-sucking bug Triatoma infestans (Hemiptera, Reduviidae). J. Insect Physiol. 2003, 49, 315–321. [Google Scholar] [CrossRef]
- Lazzari, C.R. Orientation towards hosts in haematophagous insects: An integrative perspective. Adv. Insect Physiol. 2009, 37, 1–58. [Google Scholar]
- Gillies, M.T. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): A review. Bull. Entomol. Res. 1980, 70, 525–532. [Google Scholar] [CrossRef]
- Guerenstein, P.G.; Lorenzo, M.G.; Núñez, J.A.; Lazzari, C.R. Baker's yeast, an attractant for baiting traps for Chagas’ disease vectors. Experientia 1995, 51, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Honda, K.-I. Insect reactions to light and its applications to pest management. App. Entomol. Zool. 2013, 48, 413–421. [Google Scholar] [CrossRef]
- Anderson, J.F.; Ferrandino, F.J.; McKnight, S.; Nolen, J.; Miller, J. A carbon dioxide, heat and chemical lure trap for the bedbug, Cimex lectularius. Med. Vet. Entomol. 2009, 23, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Montes, C.; Cuadrillero, C.; Vilella, D. Maintenance of a laboratory colony of Cimex lectularius (Hemiptera: Cimicidae) using an artificial feeding technique. J. Med. Entomol. 2002, 39, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Noldus, L.P.J.J.; Spink, A.J.; Tegelenbosch, R.A.J. Computerised video tracking, movement analysis and behaviour recognition in insects. Comput. Electron. Agric. 2002, 35, 201–227. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Campbell, J.F.; Phillips, T.W.; Cohnstaedt, L.W.; Throne, J.E. Evaluation of light attraction for the stored-product psocid, Liposcelis bostrychophila. J. Pest Sci. 2015, 1–8. [Google Scholar] [CrossRef]
- Sharpe, D. Your Chi-Square test is statistically significant: Now what? Pract. Assess. Res. Eval. 2015, 20, 1–10. [Google Scholar]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall/CRC: Boca Raton, FL, USA, 1998. [Google Scholar]
- SAS Institute Inc. Base SAS® 9.4 Procedures Guide: Statistical Procedures, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Barrozo, R.B.; Reisenman, C.E.; Guerenstein, P.; Lazzari, C.R.; Lorenzo, M.G. An inside look at the sensory biology of triatomines. J. Insect Physiol. 2017, 97, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, C.R.; Núñez, J.A. Blood temperature and feeding behavior in Triatoma infestans (Heteroptera: Reduviidae). Entomol. Gen. 1989, 14, 183–188. [Google Scholar] [CrossRef]
- Ferreira, R.A.; Lazzari, C.R.; Lorenzo, M.G.; Pereira, M.H. Do haematophagous bugs assess skin surface temperature to detect blood vessels? PLoS ONE 2007, 2, e932. [Google Scholar] [CrossRef] [PubMed]
- Indacochea, A.; New Mexico State University, Las Cruces, NM, USA. Personal communication, 2017.
- Lehane, M.J. The Biology of Blood-Sucking in Insects; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Taneja, J.; Guerin, P.M. Oriented responses of the triatomine bugs Rhodnius prolixus and Triatoma infestans to vertebrate odours on a servosphere. J. Comp. Physiol. A 1995, 176, 455–464. [Google Scholar] [CrossRef]
- Taneja, J.; Guerin, P.M. Ammonia attracts the haematophagous bug Triatoma infestans: Behavioural and neurophysiological data on nymphs. J. Comp. Physiol. A 1997, 181, 21–34. [Google Scholar] [CrossRef]
- Barrozo, R.B.; Lazzari, C.R. The response of the blood-sucking bug Triatoma infestans to carbon dioxide and other host odours. Chem. Senses 2004, 29, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.A. Food source orientation and activity in Rhodnius prolixus. Bull. Entomol. Res. 1982, 72, 253–262. [Google Scholar] [CrossRef]
- Guerenstein, P.G.; Hildebrand, J.G. Roles and effects of environmental carbon dioxide in insect life. Annu. Rev. Entomol. 2007, 53, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Stange, G.; Stowe, S. Carbon-dioxide sensing structures in terrestrial arthropods. Microsc. Res. Tech. 1999, 47, 416–427. [Google Scholar] [CrossRef]
- Omondi, B.A.; Majeed, S.; Ignell, R. Functional development of carbon dioxide detection in the maxillary palp of Anopheles gambiae. J. Exp. Biol. 2015, 218, 2482–2488. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F. How terrestrial organisms sense, signal, and respond to carbon dioxide. Integr. Comp. Biol. 2002, 42, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Warnes, M.L.; Finlayson, L.H. Electroantennogram responses of the stable fly, Stomoxys calcitrans, to carbon dioxide and other odours. Physiol. Entomol. 1986, 11, 469–473. [Google Scholar] [CrossRef]
- Guerenstein, P.G.; Guerin, P.M. Olfactory and behavioural responses of the blood-sucking bug Triatoma infestans to odours of vertebrate hosts. J. Exp. Biol. 2001, 204, 585–597. [Google Scholar] [PubMed]
- Romero, A.; New Mexico State University, Las Cruces, NM, USA. Unpublished data. 2017.
- Bogner, F. Response properties of sensitive receptors in tsetse flies (Diptera: Glossina palpalis). Physiol. Entomol. 1992, 17, 19–24. [Google Scholar] [CrossRef]
- Kellogg, F.E. Water vapor and carbon dioxide receptors in Aedes aegypti. J. Insect Physiol. 1970, 16, 99–108. [Google Scholar] [CrossRef]
- Acree, F.J.; Turner, R.B.; Gouck, H.K.; Beroza, M.; Smith, N. l-lactic acid: A mosquito attractant isolated from humans. Science 1968, 161, 1346–1347. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, R.B.; Lazzari, C.R. Orientation behaviour of the blood-sucking bug Triatoma infestans to short-chain fatty acids: Synergistic effect of l-Lactic acid and carbon dioxide. Chem. Senses 2004, 29, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Otalora-Luna, F.; Perret, J.L.; Guerin, P.M. Appetence behaviours of the triatomine bug Rhodnius prolixus on a servosphere in response to the host metabolites carbon dioxide and ammonia. J. Comp. Physiol. A 2004, 190, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Yokohari, F.; Tateda, H. Moist and dry hygroreceptors for relative humidity of the cockroach, Periplaneta americana L. J. Comp. Physiol. 1976, 106, 137–152. [Google Scholar] [CrossRef]
- Tichy, H.; Kallina, W. Insect hygroreceptor responses to continuous changes in humidity and air pressure. J. Neurophysiol. 2010, 103, 3274–3286. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.G. Humidity-invoked upwind orientation of shore insects (Bambidion obtusidens, Coleoptera: Carabidae). J. Insect Behav. 1997, 10, 355–363. [Google Scholar] [CrossRef]
- Roca, M.J.; Lazzari, C.R. Effects of relative humidity on the hematophagous bug Triatoma infestans: Hygropreference and eclosion success. J. Insect Physiol. 1994, 40, 901–907. [Google Scholar] [CrossRef]
- Guarneri, A.A.; Lazzari, C.; Diotaiuti, L.; Lorenzo, M.G. The effect of relative humidity on the behaviour and development of Triatoma brasiliensis. Physiol. Entomol. 2002, 27, 142–147. [Google Scholar] [CrossRef]
- Reisenman, C.E.; Lorenzo Figueiras, A.N.; Giurfa, M.; Lazzari, C.R. Interaction of visual and olfactory cues in the aggregation behaviour of the haematophagous bug Triatoma infestans. J. Comp. Physiol. A 2000, 186, 961–968. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indacochea, A.; Gard, C.C.; Hansen, I.A.; Pierce, J.; Romero, A. Short-Range Responses of the Kissing Bug Triatoma rubida (Hemiptera: Reduviidae) to Carbon Dioxide, Moisture, and Artificial Light. Insects 2017, 8, 90. https://doi.org/10.3390/insects8030090
Indacochea A, Gard CC, Hansen IA, Pierce J, Romero A. Short-Range Responses of the Kissing Bug Triatoma rubida (Hemiptera: Reduviidae) to Carbon Dioxide, Moisture, and Artificial Light. Insects. 2017; 8(3):90. https://doi.org/10.3390/insects8030090
Chicago/Turabian StyleIndacochea, Andres, Charlotte C. Gard, Immo A. Hansen, Jane Pierce, and Alvaro Romero. 2017. "Short-Range Responses of the Kissing Bug Triatoma rubida (Hemiptera: Reduviidae) to Carbon Dioxide, Moisture, and Artificial Light" Insects 8, no. 3: 90. https://doi.org/10.3390/insects8030090





