Untargeted Metabolomics Insights into Newborns with Congenital Zika Infection
Abstract
:1. Introduction
2. Results
2.1. Clinical Data and ZIKV Diagnosis
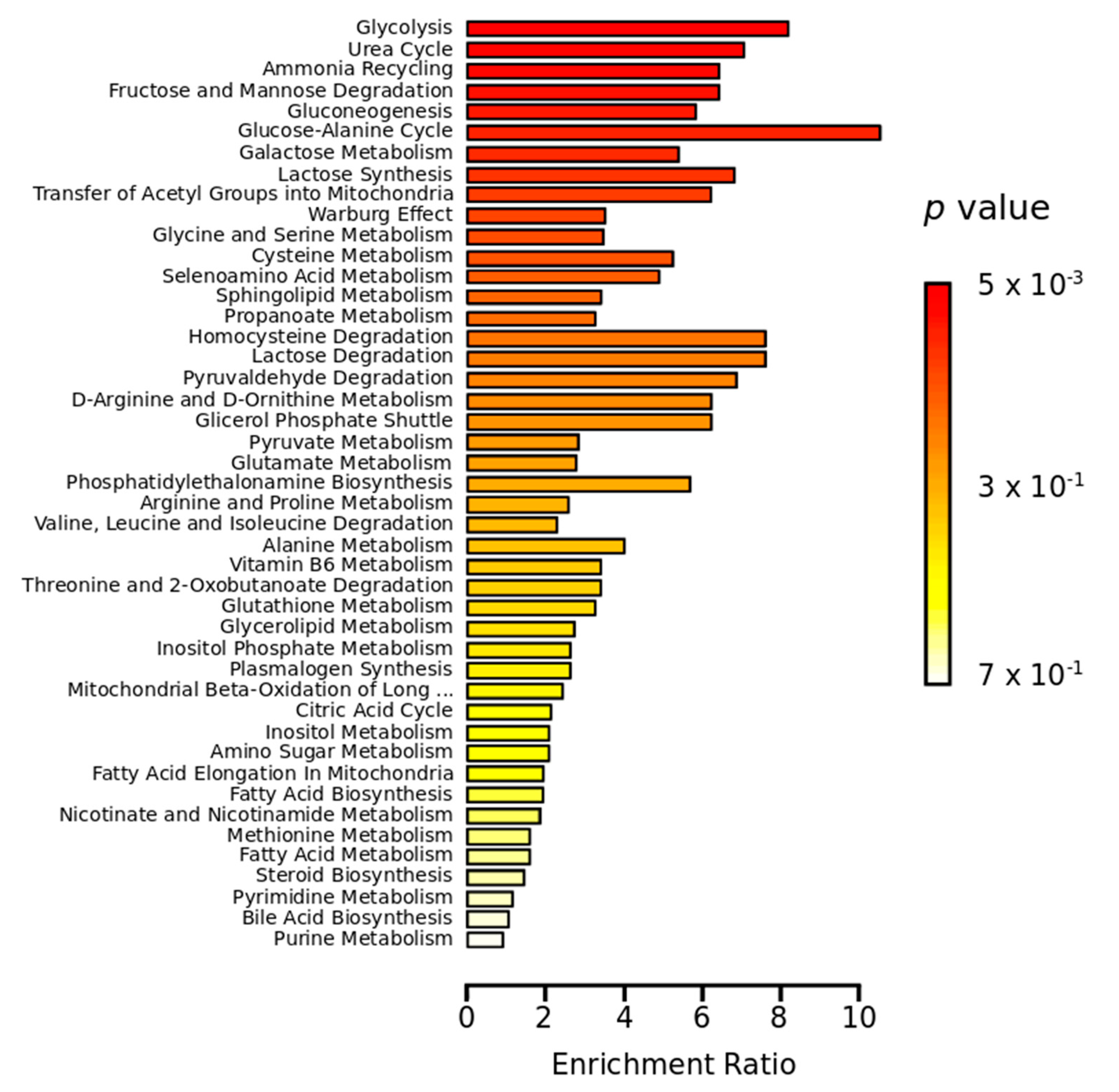
2.2. Metabolomics Study
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Studied Groups and Sample Collection
4.3. ZIKV Diagnosis
4.4. Metabolomics Sample Preparation
4.5. GC-MS Metabolomics Analysis
4.6. Metabolomics Data Processing and Statistical Analysis
4.7. Metabolite Identification
4.8. Machine Learning Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvet, G.A.; Santos, F.B.; Sequeira, P.C. Zika virus infection: Epidemiology, clinical manifestations and diagnosis. Curr. Opin. Infect. Dis. 2016, 29, 459–466. [Google Scholar] [CrossRef]
- Weaver, S.C.; Costa, F.; Garcia-Blanco, M.A.; Ko, A.I.; Ribeiro, G.S.; Saade, G.; Shi, P.Y.; Vasilakis, N. Zika virus: History, emergence, biology, and prospects for control. Antivir. Res. 2016, 130, 69–80. [Google Scholar] [CrossRef]
- Teixeira, M.G.; Costa, M.C.; de Oliveira, W.K.; Nunes, M.L.; Rodrigues, L.C. The Epidemic of Zika Virus-Related Microcephaly in Brazil: Detection, Control, Etiology, and Future Scenarios. Am. J. Public Health 2016, 106, 601–605. [Google Scholar] [CrossRef]
- Tang, H.; Hammack, C.; Ogden, S.C.; Wen, Z.; Qian, X.; Li, Y.; Yao, B.; Shin, J.; Zhang, F.; Lee, E.M.; et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 2016, 18, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Leal, M.C.; Muniz, L.F.; Neto, S.D.S.C.; van der Linden, V.; Ramos, R.C.F. Sensorineural hearing loss in a case of congenital Zika virus. Braz. J. Otorhinolaryngol. 2020, 86, 513–515. [Google Scholar] [CrossRef] [Green Version]
- Miranda, H.A.; Costa, M.C.; Frazão, M.A.M.; Simão, N.; Franchischini, S.; Moshfeghi, D.M. Expanded Spectrum of Congenital Ocular Findings in Microcephaly with Presumed Zika Infection. Ophthalmology 2016, 123, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- van der Linden, V.; Pessoa, A.; Dobyns, W.; Barkovich, A.J.; Júnior, H.V.; Filho, E.L.; Ribeiro, E.M.; Leal, M.C.; Coimbra, P.P.; Aragão, M.F.; et al. Description of 13 Infants Born during October 2015-January 2016 with Congenital Zika Virus Infection without Microcephaly at Birth-Brazil. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1343–1348. [Google Scholar] [CrossRef]
- Subissi, L.; Dub, T.; Besnard, M.; Mariteragi-Helle, T.; Nhan, T.; Lutringer-Magnin, D.; Barboza, P.; Gurry, C.; Brindel, P.; Nilles, E.J.; et al. Zika Virus Infection during Pregnancy and Effects on Early Childhood Development, French Polynesia, 2013–2016. Emerg. Infect. Dis. 2018, 24, 1850–1858. [Google Scholar] [CrossRef] [Green Version]
- Waldorf, K.M.A.; Nelson, B.R.; Stencel-Baerenwald, J.E.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med. 2018, 24, 368–374. [Google Scholar] [CrossRef]
- Klassen, A.; Faccio, A.T.; Canuto, G.A.B.; da Cruz, P.L.R.; Ribeiro, H.C.; Tavares, M.F.M.; Sussulini, A. Metabolomics: Definitions and Significance in Systems Biology. In Metabolomics: From Fundamentals to Clinical Applications, 1st ed.; Sussulini, A., Ed.; Springer: Cham, Switzerland, 2017; Volume 1, pp. 3–17. [Google Scholar]
- Mastrangelo, A.; Ferrarini, A.; Rey-Stolle, F.; García, A.; Barbas, C. From sample treatment to biomarker discovery: A tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta 2015, 900, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Thaker, S.K.; Chapa, T.; Garcia, G.; Gong, D.; Schmid, E.W.; Arumugaswami, V.; Sun, R.; Christofk, H.R. Differential Metabolic Reprogramming by Zika Virus Promotes Cell Death in Human versus Mosquito Cells. Cell Metab. 2019, 29, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.F.O.R.; Delafiori, J.; de Oliveira, D.N.; Guerreiro, T.M.; Esteves, C.Z.; Lima, E.O.; Pando-Robles, V.; Catharino, R.R. Serum Metabolic Alterations upon Zika Infection. Front. Microbiol. 2017, 8, 1954. [Google Scholar] [CrossRef]
- Melo, C.F.O.R.; Navarro, L.C.; de Oliveira, D.N.; Guerreiro, T.M.; Lima, E.O.; Delafiori, J.; Dabaja, M.Z.; Ribeiro, M.D.S.; de Menezes, M.; Rodrigues, R.G.M.; et al. A Machine Learning Application Based in Random Forest for Integrating Mass Spectrometry-Based Metabolomic Data: A Simple Screening Method for Patients with Zika Virus. Front. Bioeng. Biotechnol. 2018, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Diop, F.; Vial, T.; Ferraris, P.; Wichit, S.; Bengue, M.; Hamel, R.; Talignani, L.; Liegeois, F.; Pompon, J.; Yssel, H.; et al. Zika virus infection modulates the metabolomic profile of microglial cells. PLoS ONE 2018, 13, e0206093. [Google Scholar] [CrossRef]
- Nunes, E.C.; Canuto, G.A.B.C. Metabolomics applied in the study of emerging arboviruses caused by Aedes aegypti mosquitoes: A review. Electrophoresis 2020, 41, 2102–2113. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, J.; Rabeneck, D.B.; Martines, R.B.; Reagan-Steiner, S.; Ermias, Y.; Estetter, L.B.C.; Suzuki, T.; Ritter, J.; Keating, M.K.; Hale, G.; et al. Zika Virus RNA Replication and Persistence in Brain and Placental Tissue. Emerg. Infect. Dis. 2017, 23, 405–414. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Nogueira, R.M.R.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Ajaz, S.; McPhail, M.J.; Singh, K.K.; Mujib, S.; Trovato, F.M.; Napoli, S.; Agarwal, K. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am. J. Physiol. Cell Physiol. 2021, 320, C57–C65. [Google Scholar] [CrossRef] [PubMed]
- Dunn, D.M.; Rodriguez-Sanchez, I.; Schafer, X.; Munger, J.J. Human Cytomegalovirus Induces the Expression of the AMPKa2 Subunit to Drive Glycolytic Activation and Support Productive Viral Infection. J. Virol. 2020, JVI.01321-20. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, K.A.; Sanchez, E.L.; Camarda, R.; Lagunoff, M.J. Dengue virus induces and requires glycolysis for optimal replication. J. Virol. 2015, 89, 2358–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripoli, M.; D’Aprile, A.; Quarato, G.; Sarasin-Filipowicz, M.; Gouttenoire, J.; Scrima, R.; Cela, O.; Boffoli, D.; Heim, M.H.; Moradpour, D.; et al. Hepatitis C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1 alpha-mediated glycolytic adaptation. J. Virol. 2010, 84, 647–660. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, P.K.; Suhail, H.; Arumugaswami, V.; Pellett, P.E.; Gir, S.; Kumar, A.J. AMP-Activated Protein Kinase Restricts Zika Virus Replication in Endothelial Cells by Potentiating Innate Antiviral Responses and Inhibiting Glycolysis. J. Immunol. 2020, 204, 1810–1824. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Dang, J.; Qin, Y.; Lichinchi, G.; Bansal, V.; Rana, T.M. Zika virus infection reprograms global transcription of host cells to allow sustained infection. Emerg. Microbes. Infect. 2017, 6, e24. [Google Scholar] [CrossRef] [Green Version]
- Hay, W.W. Placental-Fetal Glucose Exchange and Fetal Glucose Metabolism. Trans. Am. Clin. Climatol. Assoc. 2006, 117, 321–340. [Google Scholar]
- Gilbert-Jaramillo, J.; Garcez, P.; James, W.; Molnár, Z.; Clarke, K.J. The potential contribution of impaired brain glucose metabolism to congenital Zika syndrome. J. Anat. 2019, 235, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Henderson, A.D.; Ventura, C.V.; Huisman, T.A.G.M.; Meoded, A.; Hazin, A.N.; van der Linden, V.; Petribu, N.C.L.; May, W.N. Characterization of Visual Pathway Abnormalities in Infants With Congenital Zika Syndrome Using Computed Tomography and Magnetic Resonance Imaging. J. Neuroophthalmol. 2020. [Google Scholar] [CrossRef]
- Pacheco, O.; Newton, S.M.; Daza, M.; Cates, J.E.; Reales, J.A.M.; Burkel, V.K.; Mercado, M.; Godfred-Cato, S.; Gonzalez, M.; Anderson, K.N.; et al. Neurodevelopmental findings in children 20-30 months of age with postnatal Zika infection at 1-12 months of age, Colombia, September-November 2017. Paediatr. Perinat. Epidemiol. 2021, 35, 92–97. [Google Scholar] [CrossRef]
- Macedo-da-Silva, J.; Rosa-Fernandes, L.; Barbosa, R.H.; Angeli, C.B.; Carvalho, F.R.; Vianna, R.A.O.; Carvalho, P.C.; Larsen, M.R.; Cardoso, C.A.; Palmisano, G. Serum Proteomics Reveals Alterations in Protease Activity, Axon Guidance, and Visual Phototransduction Pathways in Infants With In Utero Exposure to Zika Virus Without Congenital Zika Syndrome. Front. Cell Infect. Microbiol. 2020, 10, 577819. [Google Scholar] [CrossRef]
- Freitas, B.P.; Dias, J.R.O.; Prazeres, J.; Sacramento, G.A.; Ko, A.I.; Maia, M.; Belfort, R., Jr. Ocular Findings in Infants With Microcephaly Associated With Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. 2016, 134, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Atchaneeyasakul, L.O.; Linck, L.; Weleber, R.G. Microcephaly with chorioretinal degeneration. Ophthalmic. Genet. 1998, 19, 39–48. [Google Scholar] [CrossRef]
- Vasconcelos-Santos, D.V.; Andrade, G.M.Q.; Caiaffa, W.T. Zika Virus, Microcephaly, and Ocular Findings-Reply. JAMA Ophthalmol. 2016, 134, 946. [Google Scholar] [CrossRef]
- Kiziltoprak, H.; Tekin, K.; Inanc, M.; Goker, Y.S. Cataract in diabetes mellitus. World J. Diabetes 2019, 10, 140–153. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yaku, K.; Nakagawa, T. Simultaneous Measurement of Amino Acid Enantiomers in Aged Mouse Brain Samples by LC/MS/MS Combined with Derivatization Using Nα-(5-Fluoro-2,4-dinitrophenyl)-l-leucinamide (l-FDLA). Metabolites 2021, 11, 57. [Google Scholar] [CrossRef]
- Ploux, E.; Freret, T.; Billard, J.M. d-serine in physiological and pathological brain aging. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140542. [Google Scholar] [CrossRef]
- Oliveira, J.V.; Carvalho, T.C.X.; Giovanetti, M.; de Jesus, J.G.; Santos, C.S.; Pessoa, L.B.; Magalhães-Filho, C.F.Q.; Lima, J.G.S.; Carvalho, D.A.X.; Figueiredo, E.M.; et al. Neonatal surveillance for congenital Zika infection during the 2016 microcephaly outbreak in Salvador, Brazil: Zika virus detection in asymptomatic newborns. Int. J. Gynaecol. Obstet. 2020, 148 (Suppl. S2), 9–14. [Google Scholar] [CrossRef]
- Villar, J.; Ismail, L.C.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Canuto, G.A.B.; Dörr, F.; Lago, J.H.G.; Tempone, A.G.; Pinto, E.; Pimenta, D.C.; Farah, J.P.S.; Alves, M.J.M.; Tavares, M.F.M. New insights into the mechanistic action of methyldehydrodieugenol B towards Leishmania (L.) infantum via a multiplatform based untargeted metabolomics approach. Metabolomics 2017, 13, 56. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the KDD ’16 Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13 August 2016. [Google Scholar]
- Friedman, J.H. Greedy Function Approximation: A Gradient Boosting Machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Schapire, R.E. The Boosting Approach to Machine Learning: An Overview. In Nonlinear Estimation and Classification. Lecture Notes in Statistics; Denison, D.D., Hansen, M.H., Holmes, C.C., Mallick, B., Yu, B., Eds.; Springer: New York, NY, USA, 2003; Volume 171, pp. 149–171. [Google Scholar]
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. In Advances in Neural Information Processing Systems 30; von Luxburg, U., Ed.; Curran Associates, Inc.: Red Hook, NY, USA, 2017; pp. 4765–4774. [Google Scholar]

| Chemical Class | Metabolite | ZPMP vs. ZNMN | ZPMN vs. ZNMN | ZPMP vs. ZPMN |
|---|---|---|---|---|
| Amino acids and derivatives | DL-isoleucine | 0.97 | ||
| L-serine | 0.71 (b) | 1.21 | ||
| L-threonine/L-allothreonine § | 0.75 (b) | 1.37 | ||
| L-valine | 0.88 | |||
| N-methylglutamic acid | 0.72 (a) | |||
| Carbohydrates and conjugates | Aldohexose (D-glucose/D-mannose) * | 0.60 | 0.28 | |
| Sugar alcohol (D-mannitol/D-sorbitol) * | 0.43 | |||
| Aldohexose (D-mannose/D-altrose) * | 0.61 | 2.30 | 0.26 | |
| Glucoheptonic acid/ribonic acid-gamma-lactone/gluconic acid lactone | 0.88 (a) | 1.67 (b) | ||
| Methyl-beta-D-galactopyranoside | 0.52 (a) | 0.84 (a) | 0.62 | |
| Hexose (tagatose/L-sorbose) */D-lyxosylamine (hexosamine) | 0.88 (a) | |||
| Fatty acids and conjugates | Palmitic acid | 0.62 | ||
| Stearic acid | 0.64 | 0.84 (a) | ||
| Inorganic acids | Phosphoric acid | 0.75 (a) | 0.69 (a) | |
| Organic acids and derivatives | Pyruvic acid | 1.31 (a) | ||
| Urea | 0.50 | 0.41 (b) | 1.21 (a) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, E.d.C.; Filippis, A.M.B.d.; Pereira, T.d.E.S.; Faria, N.R.d.C.; Salgado, Á.; Santos, C.S.; Carvalho, T.C.P.X.; Calcagno, J.I.; Chalhoub, F.L.L.; Brown, D.; et al. Untargeted Metabolomics Insights into Newborns with Congenital Zika Infection. Pathogens 2021, 10, 468. https://doi.org/10.3390/pathogens10040468
Nunes EdC, Filippis AMBd, Pereira TdES, Faria NRdC, Salgado Á, Santos CS, Carvalho TCPX, Calcagno JI, Chalhoub FLL, Brown D, et al. Untargeted Metabolomics Insights into Newborns with Congenital Zika Infection. Pathogens. 2021; 10(4):468. https://doi.org/10.3390/pathogens10040468
Chicago/Turabian StyleNunes, Estéfane da C., Ana M. B. de Filippis, Taiane do E. S. Pereira, Nieli R. da C. Faria, Álvaro Salgado, Cleiton S. Santos, Teresa C. P. X. Carvalho, Juan I. Calcagno, Flávia L. L. Chalhoub, David Brown, and et al. 2021. "Untargeted Metabolomics Insights into Newborns with Congenital Zika Infection" Pathogens 10, no. 4: 468. https://doi.org/10.3390/pathogens10040468







