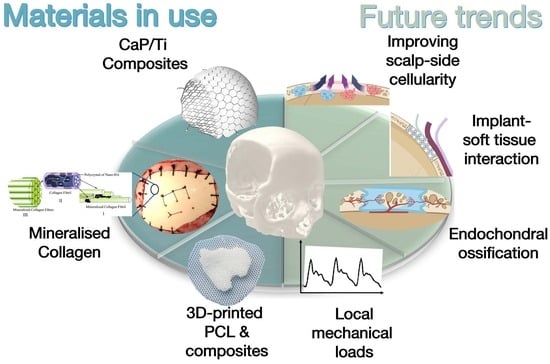
Biomaterials for Regenerative Cranioplasty: Current State of Clinical Application and Future Challenges
Abstract
:1. Introduction
2. Regeneration Capacity of Cranium
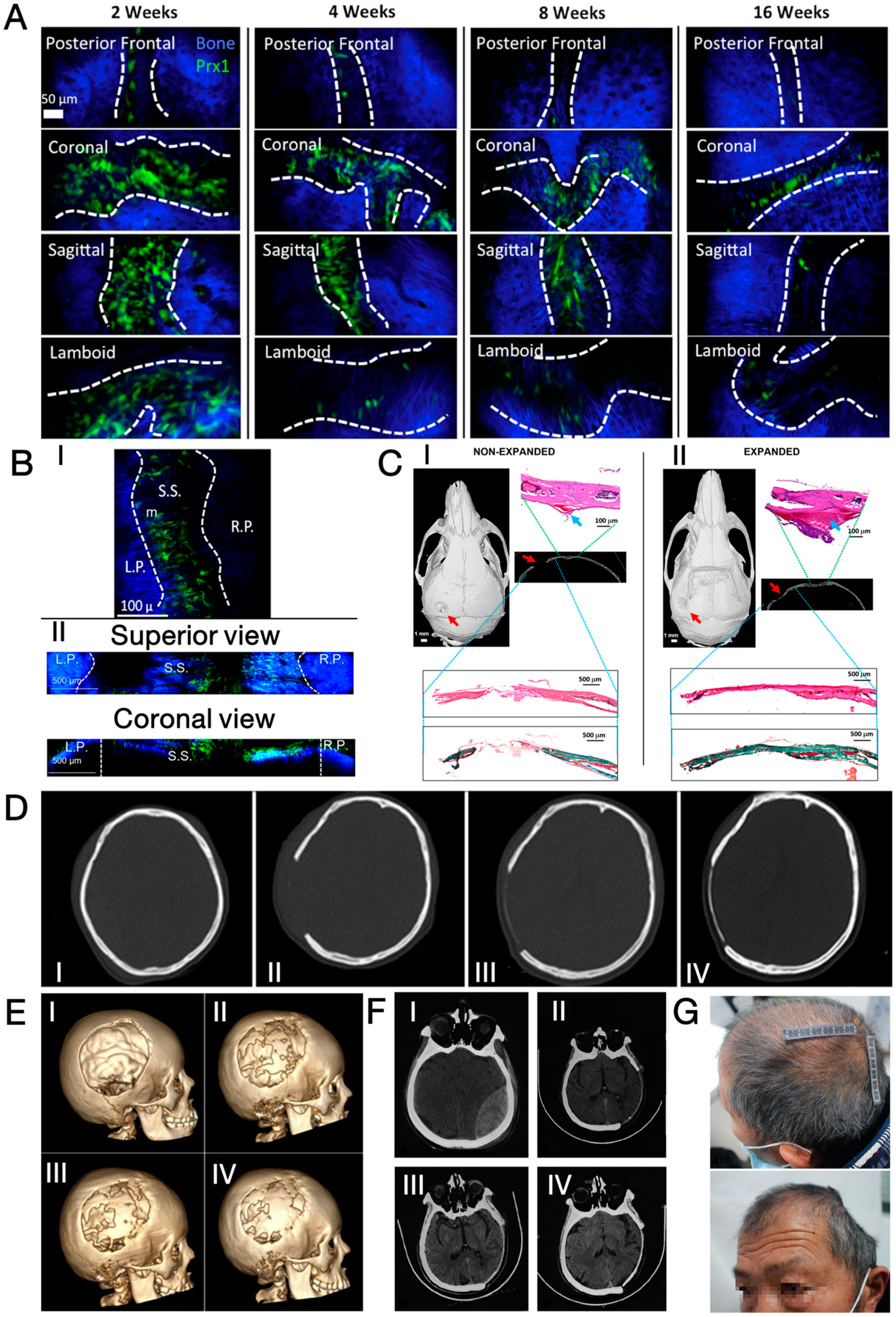
2.1. Biological Basis of Cranium Regeneration
2.2. Clinical Observation of Cranium Regeneration
| Age (Years) | Gender | Indications of Craniectomy | Highlights of the Surgical Process | Post-Operative Outcome | Ref. |
|---|---|---|---|---|---|
| 6 | Male | TBI |
|
| [50] |
| 7 | Female | TBI |
|
| [48] |
| 8 | Female | Brain abscess |
|
| [51] |
| 12 | Female | Tumour |
|
| [52] |
| 15 | Male | TBI |
|
| |
| 18 | Male | TBI |
|
| [53] |
| 20 | Female | TBI |
|
| [47] |
| 29 | Female | TBI |
|
| [54] |
| 64 | Male | TBI |
|
| [49] |
3. Three types of Regenerative Cranioplasty Implants and Progress of Clinical Translation
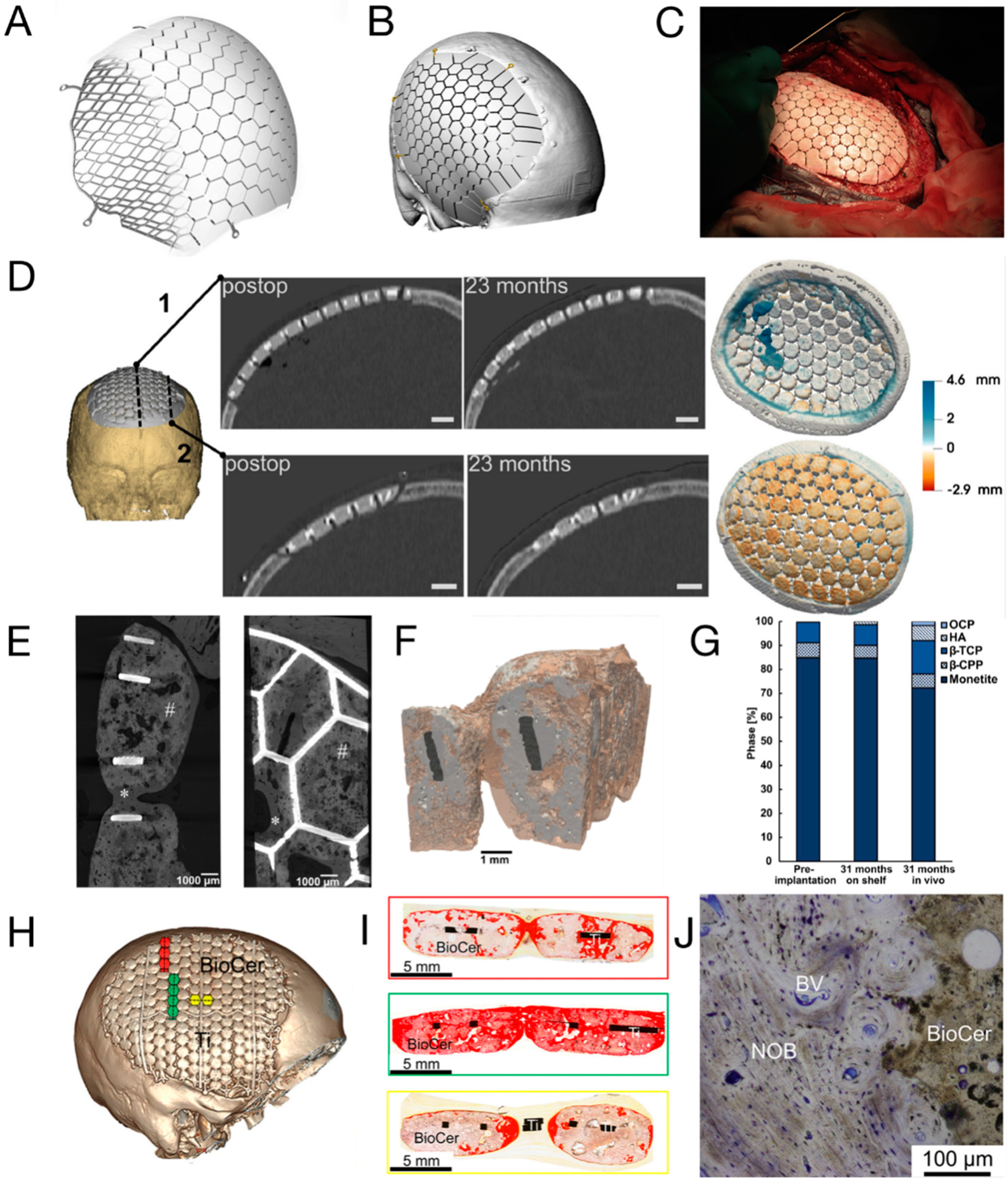
3.1. CaP/Ti Composites
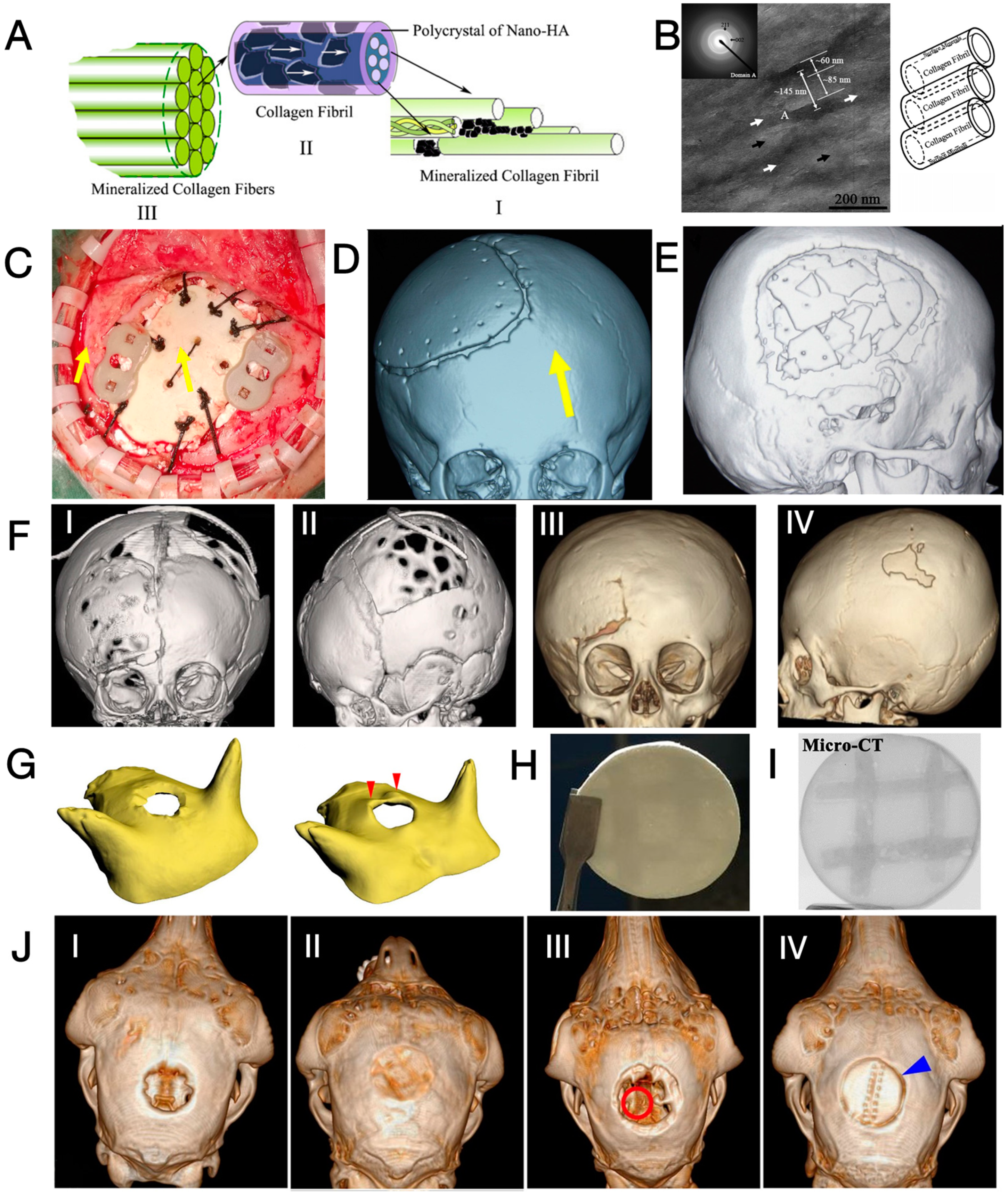
3.2. Mineralised Collagen
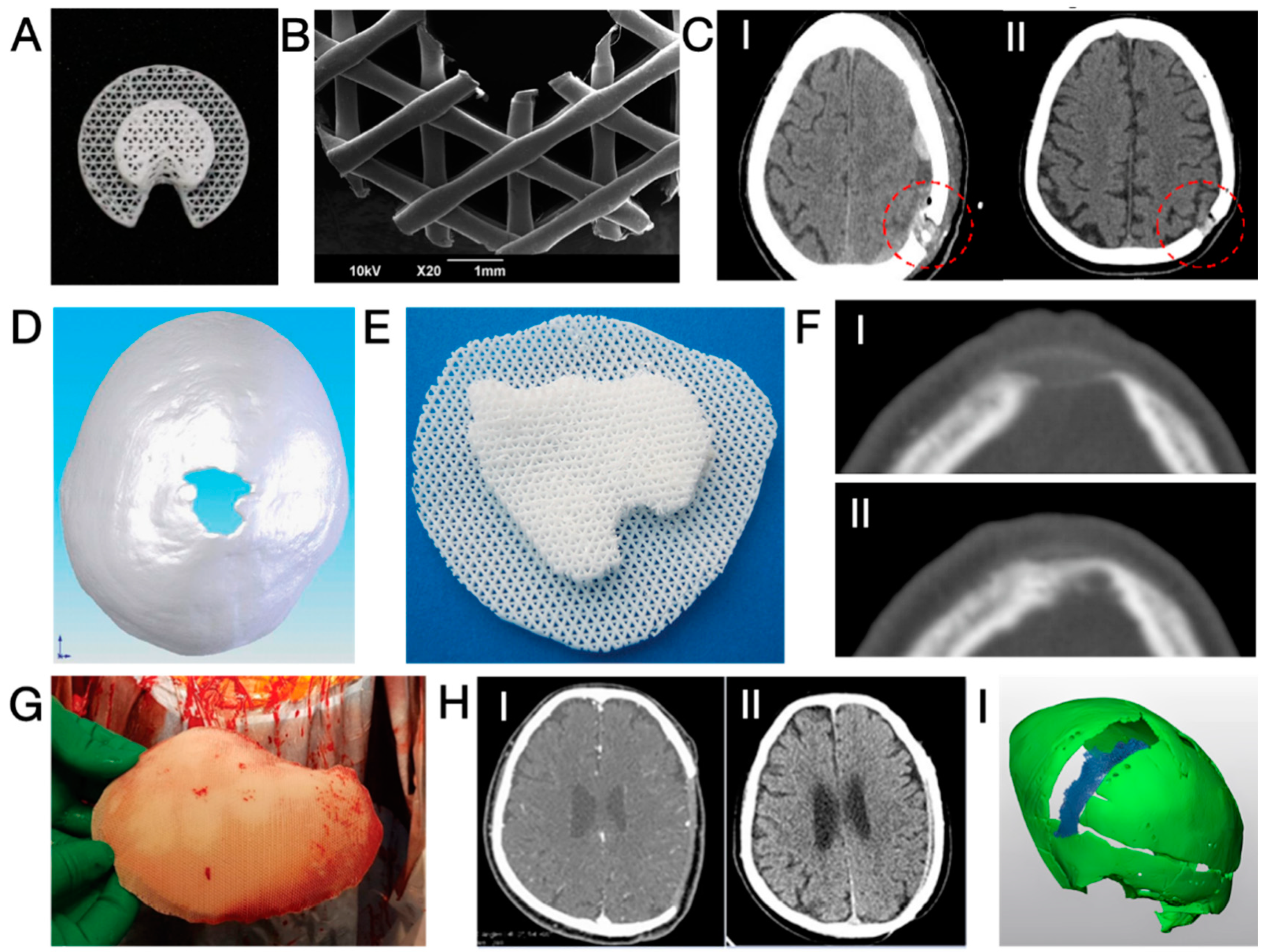
3.3. Three-dimensional-Printed PCL and Its Composites
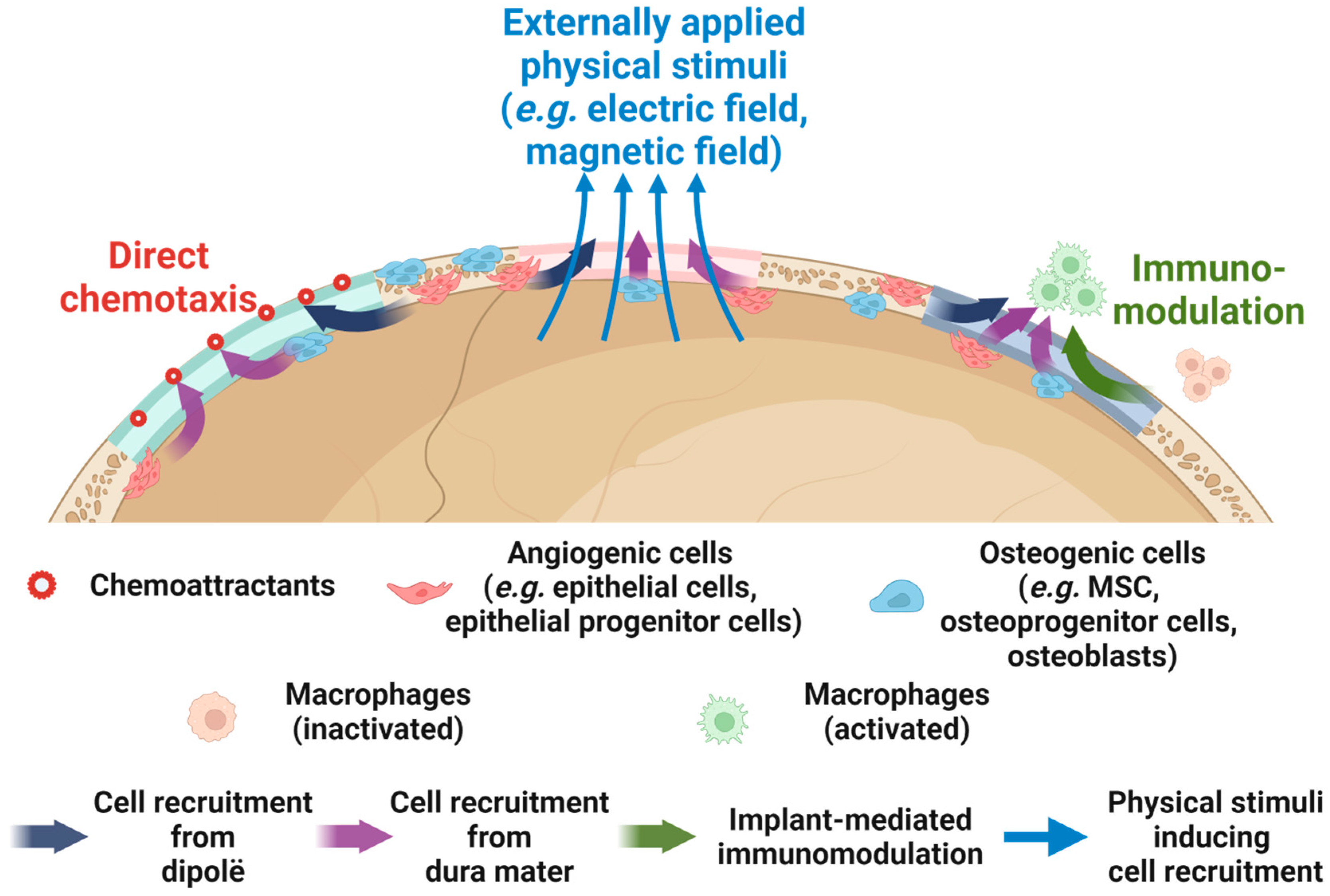
4. Perspectives on Enhancing the Regenerative Cranioplasty Implants
4.1. Enhancing the Osteogenic Potential on the Scalp Side
| Factors | Type of Factors | Carrier of the Factors | Animal Species And Size of Cranial Defects | Impact on Cell Recruitment | Ref. |
|---|---|---|---|---|---|
| IL-4 | Biological | Electrospun PLGA/HA scaffolds loaded with IL-4 and coated with carboxymethyl chitosan–collagen–hydrogel | SD rats D = 5 mm | The hydrogel coating impeded the IL-4 release in an early stage (Day 1–3) to maintain a moderate pro-inflammatory response that recruits BMSC. Afterwards, the hydrogel degraded and released trapped IL-4 to induce an anti-inflammatory response to upregulate cranium regeneration | [155] |
| BML-284, carboxymethyl chitosan | Biological | β-TCP with PDA coating | Rats D = 5 mm | Surface functionalisation with BML-284 and carboxymethyl chitosan promoted the M2 (anti-inflammatory) polarisation of macrophages and facilitated endogenous MSC recruitment to support cranium regeneration | [156] |
| HyA-DA | Biological | Collagen I/HA composite hydrogel | NZ rabbits D = 10 mm | The presence of HyA-DA at the Collagen I/HA interface activated the M2 polarisation of macrophages to induce the endogenous MSC recruitment and subsequent cranial defect repair | [157] |
| LepR-a | Biological | PLA with PDA coating and BMP-2 loaded hollow MnO2 nanoparticles | C57BL/6 mice D = 4 mm | Surface functionalisation with LepR, a surface marker for >90% of Prx1+ SSC, recruited SSC (in vitro and in vivo) through antibody-antigen reaction and contributed to enhanced cranium regeneration | [158] |
| SDF-1 | Biological | CSO/H NPs | Nude mice subcutaneous | SDF-1 released from nanoparticles induced in vitro and in vivo MSC recruitment, while BMP-2 (released subsequently) enhanced osteogenic differentiation of stem cells. | [148] |
| BMP-2 | Biological | Phosphate buffered saline (for injection) or collagen scaffold | C57BL/6 mice D = 5 mm | In vitro administration of BMP-2 enhanced the chemotactic migration of osteoblasts by 170–300%; Sequential release of SDF-1 (from scaffold) followed by BMP-2 (injection) dramatically enhanced the recruitment and osteogenic differentiation of BMSCs, leading to enhanced calvaria defect healing | [146,147] |
| FGF-2 | Biological | BMP-2 loaded HA/collagen scaffold with polyelectrolyte multilayer coating | Mice D = 3.5 mm | Sequential release of FGF-2 (from coating) followed by BMP-2 (from scaffold) greatly enhanced the recruitment of Sca-1+ progenitor cells and subsequent osteogenesis at the calvaria defects of mice | [159] |
| PDGF-B | Biological | Recombinant adenoviruses loaded in mesoporous glass–silk scaffolds | BALB/c mice 5 × 5 mm2 | Release of adenoviruses from the scaffold was able to infect MSC, PDLSC, and DPSC, leading to enhanced production of PDGF-B that subsequently improved the migration of these (undifferentiated) cells to support calvaria defect healing. | [160] |
| PFS (CPFSSTKT-NH2) peptide | Biological | SCP/SF scaffold | SD rats D = 5 mm | Functionalisation of SCP/SF with PFS peptide, a peptide with stem cell-homing ability, enhanced both in vitro and in vivo recruitment of MSC to promote cranium regeneration | [141] |
| Ground autologous bone | Biological | Bioprinted alginate–gelatin hydrogel scaffold | Beagle dogs 20 × 20 mm2 | Implantation of scaffolds containing ground autologous bone and transplanted BMSC into cranium defects of rats upregulated the expression of SDF-1 and consequently enhanced in situ recruitment of CD90+/CD105+ BMSC to support cranium regeneration | [143] |
| Lithium-doped BG | Chemical | PLGA | C57BL/6 mice D = 4 mm | Incorporation of Li in BG enhanced in vitro migration of BMSC under low/high glucose environment and contributed to superior regeneration of calvaria defect in mice with diabetes | [161] |
| Lanthanum-doped BG | Chemical | Chitosan | SD rats D = 5 mm | Increased loading of La-BG improved in vitro recruitment and subsequent expression of angiogenesis-related genes of HUVEC to improve cranium regeneration | [162] |
| Calcium ion (from CaSO4) | Chemical | Agarose/chitosan scaffold | BALB/c mice D = 5 mm | Calcium ions released from the scaffold enhanced in situ recruitment of Osx+ osteoprogenitor cells at the mice calvaria defect and subsequently resulted in more pronounced bone regeneration | [163] |
| Piezoelectric stimulus | Physical | PHA and PBT in CG | Rats Unknown size | PHA/CG/5%PBT hydrogel most effectively induced migration and M2 polarisation of RAW 264.7 cells (murine macrophages), which subsequently enhanced the in vitro recruitment of MC3T3-E1 (murine pre-osteoblasts) and HUVEC, and facilitated cranium regeneration | [164] |
| SrFe12O19 nanoparticles | Physical | Lanthanum-doped HA/CS scaffold | SD rats D = 5 mm | The incorporation of magnetic SrFe12O19 nanoparticles induced an incorporated magnetic field and promoted the recruitment of MSC to enhance cranium regeneration | [144] |
4.2. Proper Management of Surrounding Soft Tissue

4.3. Endochondral Ossification as an Alternative Ossification Mode
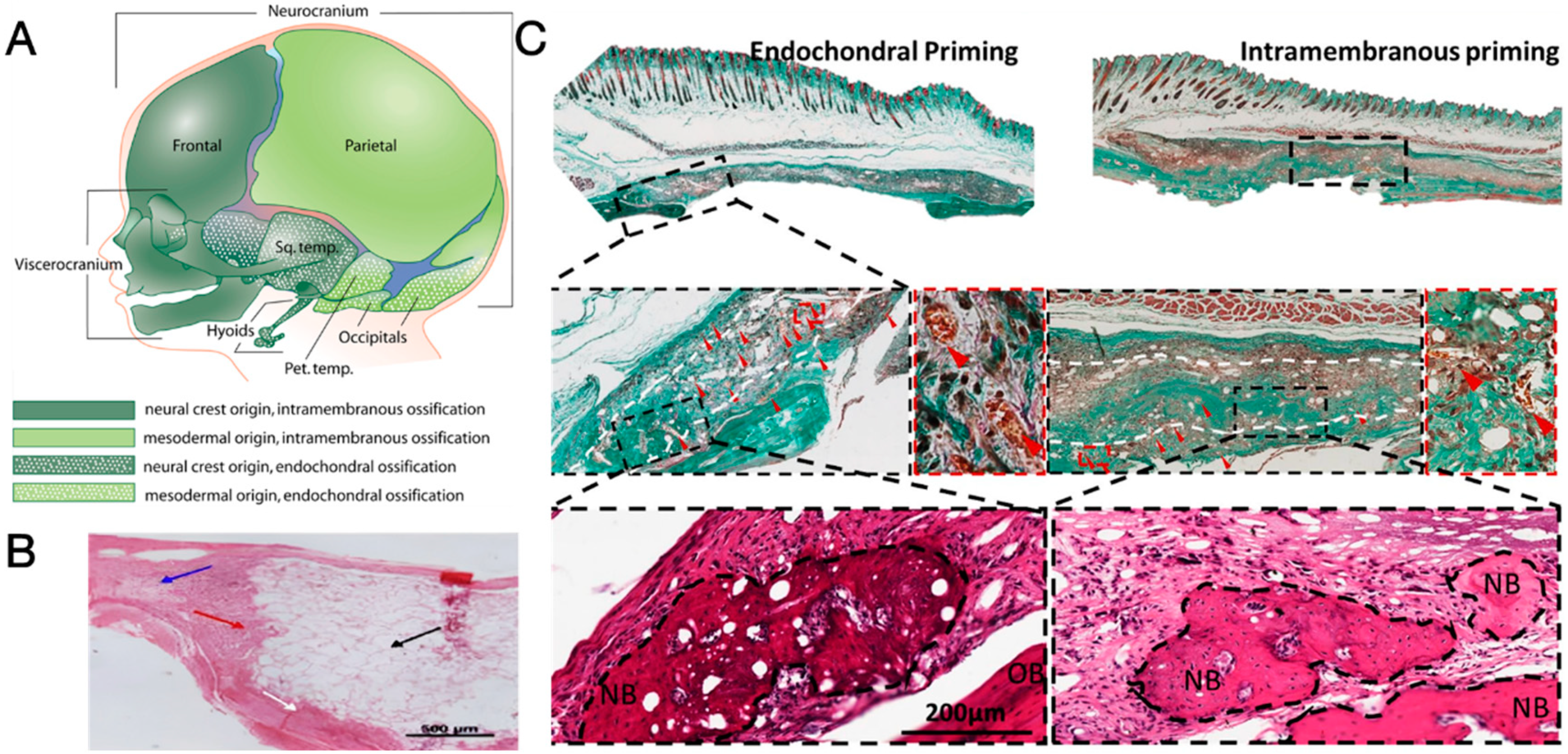
4.4. Consideration of the Local Mechanical Environment
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Piazza, M.; Grady, M.S. Cranioplasty. Neurosurg. Clin. N. Am. 2017, 28, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Jasey, N.; Ward, I.; Lequerica, A.; Chiaravalloti, N.D. The therapeutic value of cranioplasty in individuals with brain injury. Brain Inj. 2018, 32, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.K.; Tomar, K.; Thakral, A.; Rangan, N.M. Complications of Cranioplasty. J. Craniofac. Surg. 2018, 29, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Paliga, J.T.; Bartlett, S.P. Cranioplasty: Indications and advances. Curr. Opin. Otolaryngol. Head Neck Surg. 2013, 21, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Klieverik, V.M.; Miller, K.J.; Singhal, A.; Han, K.S.; Woerdeman, P.A. Cranioplasty after craniectomy in pediatric patients-a systematic review. Childs Nerv. Syst. 2019, 35, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.C.; Wang, Q.L.; Sun, P.; Zhan, S.H.; Guo, P.; Deng, W.S.; Dong, Q. Cryopreservation of Autologous Cranial Bone Flaps for Cranioplasty: A Large Sample Retrospective Study. World Neurosurg. 2018, 109, e853–e859. [Google Scholar] [CrossRef] [PubMed]
- van de Vijfeijken, S.; Munker, T.; Spijker, R.; Karssemakers, L.H.E.; Vandertop, W.P.; Becking, A.G.; Ubbink, D.T.; CranioSafe, G. Autologous Bone Is Inferior to Alloplastic Cranioplasties: Safety of Autograft and Allograft Materials for Cranioplasties, a Systematic Review. World Neurosurg. 2018, 117, 443–452 e8. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, J.G.; Mahmooth, Z.; Rindler, R.S.; Allen, J.W.; Grossberg, J.A.; Pradilla, G.; Ahmad, F.U. Autologous Cranioplasty is Associated with Increased Reoperation Rate: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 116, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Wui, S.H.; Kim, K.M.; Ryu, Y.J.; Kim, I.; Lee, S.J.; Kim, J.; Kim, C.; Park, S. The Autoclaving of Autologous Bone is a Risk Factor for Surgical Site Infection After Cranioplasty. World Neurosurg. 2016, 91, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Pikis, S.; Goldstein, J.; Spektor, S. Potential neurotoxic effects of polymethylmethacrylate during cranioplasty. J. Clin. Neurosci. 2015, 22, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Jindal, P.; Chaitanya; Bharadwaja, S.S.S.; Rattra, S.; Pareek, D.; Gupta, V.; Breedon, P.; Reinwald, Y.; Juneja, M. Optimizing cranial implant and fixture design using different materials in cranioplasty. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2023, 237, 107–121. [Google Scholar] [CrossRef]
- Zanotti, B.; Verlicchi, A.; Indiani, S.; Scarparo, S.A.; Zingaretti, N.; Parodi, P.C. Spontaneous fractures in custom-made porous hydroxyapatite cranioplasty implants: Is fragility the only culprit? Acta Neurochir. 2015, 157, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, A.; Tanaka, H.; Iwamuro, H.; Takanashi, S.; Miyawaki, S.; Nakashima, M.; Nakaguchi, H.; Nagashima, T. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir. 2006, 148, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, T.; Yuan, Y.; Li, X.; Yu, H.; Guan, J. Evaluation of titanium mesh cranioplasty and polyetheretherketone cranioplasty: Protocol for a multicentre, assessor-blinded, randomised controlled trial. BMJ Open 2019, 9, e033997. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.N.; Addison, O.; Naji, N.; Seres, P.; Wilman, A.H.; Shepherd, D.E.T.; Grover, L.; Cox, S. Reducing MRI susceptibility artefacts in implants using additively manufactured porous Ti-6Al-4V structures. Acta Biomater. 2020, 107, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Jonkergouw, J.; van de Vijfeijken, S.E.; Nout, E.; Theys, T.; Van de Casteele, E.; Folkersma, H.; Depauw, P.R.; Becking, A.G. Outcome in patient-specific PEEK cranioplasty: A two-center cohort study of 40 implants. J. Cranio-Maxillofac. Surg. 2016, 44, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Ng, Z.Y.; Ang, W.J.; Nawaz, I. Computer-designed polyetheretherketone implants versus titanium mesh (+/- acrylic cement) in alloplastic cranioplasty: A retrospective single-surgeon, single-center study. J. Craniofac. Surg. 2014, 25, e185–e189. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Jiang, J.; Zhang, X. Effect of Reflection of Temporalis Muscle During Cranioplasty With Titanium Mesh After Standard Trauma Craniectomy. J. Craniofacial Surg. 2016, 27, 145–149. [Google Scholar] [CrossRef]
- Johnston, D.T.; Lohmeier, S.J.; Langdell, H.C.; Pyfer, B.J.; Komisarow, J.; Powers, D.B.; Erdmann, D. Current Concepts in Cranial Reconstruction: Review of Alloplastic Materials. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4466. [Google Scholar] [CrossRef] [PubMed]
- Lilly, G.L.; Santucci, N.M.; Petrisor, D.; Wax, M.K. Soft tissue coverage of cranial defects: An update. Plast. Aesthetic Res. 2021, 8, 35. [Google Scholar] [CrossRef]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue engineering and regenerative medicine: History, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues--state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Spector, J.A.; Glat, P.M. Hair-Bearing Scalp Reconstruction Using a Dermal Regeneration Template and Micrograft Hair Transplantation. Ann. Plast. Surg. 2007, 59, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Navsaria, H.A.; Ojeh, N.O.; Moiemen, N.; Griffiths, M.A.; Frame, J.D. Reepithelialization of a Full-Thickness Burn from Stem Cells of Hair Follicles Micrografted into a Tissue-Engineered Dermal Template (Integra). Plast. Reconstr. Surg. 2004, 113, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Omar, O.; Engstrand, T.; Kihlstrom Burenstam Linder, L.; Aberg, J.; Shah, F.A.; Palmquist, A.; Birgersson, U.; Elgali, I.; Pujari-Palmer, M.; Engqvist, H.; et al. In situ bone regeneration of large cranial defects using synthetic ceramic implants with a tailored composition and design. Proc. Natl. Acad. Sci. USA 2020, 117, 26660–26671. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, T.; Zhang, Y.; Zuo, H.; Zhao, Y.; Xue, C.; Luo, B.; Zhang, Q.; Zhu, J.; Wang, X.; et al. Repairing skull defects in children with nano-hap/collagen composites: A clinical report of thirteen cases. Transl. Neurosci. Clin. 2016, 2, 31–37. [Google Scholar] [CrossRef]
- Probst, F.A.; Hutmacher, D.W.; Muller, D.F.; Machens, H.G.; Schantz, J.T. Calvarial reconstruction by customized bioactive implant. Handchir. Mikrochir. Plast. Chir. 2010, 42, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Aalami, O.O.; Nacamuli, R.P.; Lenton, K.A.; Cowan, C.M.; Fang, T.D.; Fong, K.D.; Shi, Y.Y.; Song, H.M.; Sahar, D.E.; Longaker, M.T. Applications of a mouse model of calvarial healing: Differences in regenerative abilities of juveniles and adults. Plast. Reconstr. Surg. 2004, 114, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Aldawood, Z.A.; Mancinelli, L.; Geng, X.; Yeh, S.A.; Di Carlo, R.; Leite, T.; Gustafson, J.; Wilk, K.; Yozgatian, J.; Garakani, S.; et al. Expansion of the sagittal suture induces proliferation of skeletal stem cells and sustains endogenous calvarial bone regeneration. Proc. Natl. Acad. Sci. USA 2023, 120, e2120826120. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Itkin, T.; Mae, M.A.; Langen, U.H.; Betsholtz, C.; Lapidot, T.; Adams, R.H. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 2016, 532, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Shen, T.; Yu, Y.; Deng, S.; Mao, L.; Wang, J.; Liu, C. Generation of rhBMP-2-induced juvenile ossicles in aged mice. Biomaterials 2020, 258, 120284. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M.; et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Nakasaki, M.; Shih, Y.V.; Varghese, S. Effect of age on biomaterial-mediated in situ bone tissue regeneration. Acta Biomater. 2018, 78, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Aalami, O.O.; Nacamuli, R.P.; Salim, A.; Fong, K.D.; Lenton, K.A.; Song, H.M.; Fang, T.D.; Longaker, M.T. Differential transcriptional expression profiles of juvenile and adult calvarial bone. Plast. Reconstr. Surg. 2005, 115, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.D.; Schaffer, J.L.; Meazzini, M.C.; Zurakowski, D.; Nah, H.D.; Gerstenfeld, L.C. Developmental restriction of embryonic calvarial cell populations as characterized by their in vitro potential for chondrogenic differentiation. J. Bone Miner. Res. 1997, 12, 2024–2039. [Google Scholar] [CrossRef] [PubMed]
- Alleyne, B.; Varghai, D.; Askeroglu, U.; Zwiebel, S.; Tobin, K.; Gosain, A.K. The Impact of Age Upon Healing: Absolute Quantification of Osteogenic Genes in Calvarial Critical-Sized Defects. J. Craniofac. Surg. 2016, 27, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Chen, Z.; Ishikawa, M.; Yue, X.; Kawanami, A.; Leahy, P.; Greenfield, E.M.; Murakami, S. Prx1 and 3.2kb Col1a1 promoters target distinct bone cell populations in transgenic mice. Bone 2014, 58, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Feng, J.; Ho, T.V.; Grimes, W.; Urata, M.; Chai, Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat. Cell Biol. 2015, 17, 386–396. [Google Scholar] [CrossRef]
- Wilk, K.; Yeh, S.A.; Mortensen, L.J.; Ghaffarigarakani, S.; Lombardo, C.M.; Bassir, S.H.; Aldawood, Z.A.; Lin, C.P.; Intini, G. Postnatal Calvarial Skeletal Stem Cells Expressing PRX1 Reside Exclusively in the Calvarial Sutures and Are Required for Bone Regeneration. Stem Cell Rep. 2017, 8, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Jeong, J.; Sheu, T.J.; Hsu, W. Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nat. Commun. 2016, 7, 10526. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gilbert, J.R.; Zhang, X.; Zhao, B.; Ker, D.F.E.; Cooper, G.M. Calvarial Versus Long Bone: Implications for Tailoring Skeletal Tissue Engineering. Tissue Eng. Part B Rev. 2020, 26, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Percival, C.J.; Richtsmeier, J.T. Angiogenesis and intramembranous osteogenesis. Dev. Dyn. 2013, 242, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.C.; Sumner, D.R. How faithfully does intramembranous bone regeneration recapitulate embryonic skeletal development? Dev. Dyn. 2021, 250, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Galea, G.L.; Zein, M.R.; Allen, S.; Francis-West, P. Making and shaping endochondral and intramembranous bones. Dev. Dyn. 2021, 250, 414–449. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Lassar, A.B. Fibroblast growth factor maintains chondrogenic potential of limb bud mesenchymal cells by modulating DNMT3A recruitment. Cell Rep. 2014, 8, 1419–1431. [Google Scholar] [CrossRef]
- Chen, G.; Xu, H.; Yao, Y.; Xu, T.; Yuan, M.; Zhang, X.; Lv, Z.; Wu, M. BMP Signaling in the Development and Regeneration of Cranium Bones and Maintenance of Calvarial Stem Cells. Front. Cell Dev. Biol. 2020, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Chu, H.T.; Le, T.D.; Le, T.A.; Duong, H.D.; Van Dong, H. Spontaneous cranial bone regeneration following craniectomy for traumatic brain injury in a pregnant woman: A case report. Int. J. Surg. Case Rep. 2021, 83, 105993. [Google Scholar] [CrossRef]
- Figueroa-Sanchez, J.A.; Ferrigno, A.S.; Moreno-Cuevas, J.; Gonzalez-Garza, M.T.; Jamall, S.; Martinez, H.R. Spontaneous Bone Regeneration After Large Craniectomy in Pediatric Patient. World Neurosurg. 2019, 127, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liang, M.; Su, L. Extensive skull ossification after decompressive craniectomy in an elderly patient: A case report and literature review. Medicine 2022, 101, e29015. [Google Scholar] [CrossRef] [PubMed]
- Guida, L.; Mazzoleni, F.; Bozzetti, A.; Sganzerla, E.; Giussani, C. Extensive Dural Ossification after Decompressive Posttraumatic Craniectomy: A Case Report and Review of the Literature. World Neurosurg. 2018, 120, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Thombre, B.D.; Prabhuraj, A.R. Spontaneous bone formation in a large craniectomy defect. Childs Nerv. Syst. 2018, 34, 1449–1450. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.; Chacko, A. Spontaneous Re-ossification of a Large Calvarial Defect in an Older Child. Turk. Neurosurg. 2008, 18, 407–408. [Google Scholar] [PubMed]
- Vega, R.A.; Hutchins, L. Heterotopic Ossification of the Calvarium Following Bilateral Craniectomies in Traumatic Brain Injury. Ochsner J. 2017, 17, 118–120. [Google Scholar] [PubMed]
- Gonzalez-Bonet, L.G. Spontaneous Cranial Bone Regeneration After a Craniectomy in an Adult. World Neurosurg. 2021, 147, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Lewin, S.; Aberg, J.; Neuhaus, D.; Engqvist, H.; Ferguson, S.J.; Ohman-Magi, C.; Helgason, B.; Persson, C. Mechanical behaviour of composite calcium phosphate-titanium cranial implants: Effects of loading rate and design. J. Mech. Behav. Biomed. Mater. 2020, 104, 103701. [Google Scholar] [CrossRef] [PubMed]
- Zaccaria, L.; Tharakan, S.J.; Altermatt, S. Hydroxyapatite ceramic implants for cranioplasty in children: A single-center experience. Childs Nerv. Syst. 2017, 33, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Gallinetti, S.; Linder, L.K.B.; Aberg, J.; Illies, C.; Engqvist, H.; Birgersson, U. Titanium reinforced calcium phosphate improves bone formation and osteointegration in ovine calvaria defects: A comparative 52 weeks study. Biomed. Mater. 2021, 16, 035031. [Google Scholar] [CrossRef] [PubMed]
- Sorek, S.; Miller, A.; Griepp, D.; Moawad, S.; Zanzerkia, R.; Rahme, R. Skull Reconstruction Using a Custom-Made, Three-Dimensional-Printed, Hydroxyapatite-Titanium Cranioplasty Implant: Largest Single-Center U.S. Experience. World Neurosurg. 2022, 167, e1387–e1394. [Google Scholar] [CrossRef] [PubMed]
- Lewin, S.; Fleps, I.; Åberg, J.; Ferguson, S.J.; Engqvist, H.; Öhman-Mägi, C.; Helgason, B.; Persson, C. Additively manufactured mesh-type titanium structures for cranial implants: E-PBF vs. L-PBF. Mater. Des. 2021, 197, 109207. [Google Scholar] [CrossRef]
- Kihlstrom Burenstam Linder, L.; Birgersson, U.; Lundgren, K.; Illies, C.; Engstrand, T. Patient-Specific Titanium-Reinforced Calcium Phosphate Implant for the Repair and Healing of Complex Cranial Defects. World Neurosurg. 2019, 122, e399–e407. [Google Scholar] [CrossRef]
- Sundblom, J.; Xheka, F.; Casar-Borota, O.; Ryttlefors, M. Bone formation in custom-made cranioplasty: Evidence of early and sustained bone development in bioceramic calcium phosphate implants. Patient series. J. Neurosurg. Case Lessons 2021, 1, CASE20133. [Google Scholar] [CrossRef] [PubMed]
- Bloom, O.; Goddard, N.; Yannoulias, B.; Eccles, S. The successful use of a bespoke OssDsign cranial plate to reconstruct an occipital defect following excision of a recurrent epithelioid sarcoma. JPRAS Open 2020, 24, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Lewin, S.; Kihlstrom Burenstam Linder, L.; Birgersson, U.; Gallinetti, S.; Aberg, J.; Engqvist, H.; Persson, C.; Ohman-Magi, C. Monetite-based composite cranial implants demonstrate long-term clinical volumetric balance by concomitant bone formation and degradation. Acta Biomater. 2021, 128, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound-Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Gbureck, U.; Doillon, C.J.; Bassett, D.C.; van Blitterswijk, C.A.; Barralet, J.E. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials 2008, 29, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Pujari-Palmer, M.; Pujari-Palmer, S.; Lu, X.; Lind, T.; Melhus, H.; Engstrand, T.; Karlsson-Ott, M.; Engqvist, H. Pyrophosphate Stimulates Differentiation, Matrix Gene Expression and Alkaline Phosphatase Activity in Osteoblasts. PLoS ONE 2016, 11, e0163530. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, T.; Kihlstrom, L.; Neovius, E.; Skogh, A.C.; Lundgren, T.K.; Jacobsson, H.; Bohlin, J.; Aberg, J.; Engqvist, H. Development of a bioactive implant for repair and potential healing of cranial defects. J. Neurosurg. 2014, 120, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, T.; Kihlström, L.; Lundgren, K.; Trobos, M.; Engqvist, H.; Thomsen, P. Bioceramic Implant Induces Bone Healing of Cranial Defects. Plast. Reconstr. Surg.-Glob. Open 2015, 3, e491. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.; Sinha, S. OssDsign cranioplasty in children: A single-centre experience. Child’s Nerv. Syst. 2020, 36, 1773–1776. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Rafter, D.; Shok, G.; Murphy, S.; Kiaei, S.; Samadani, U. A retrospective descriptive study of cranioplasty failure rates and contributing factors in novel 3D printed calcium phosphate implants compared to traditional materials. 3D Print Med. 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Wesp, D.; Krenzlin, H.; Jankovic, D.; Ottenhausen, M.; Jagersberg, M.; Ringel, F.; Keric, N. Analysis of PMMA versus CaP titanium-enhanced implants for cranioplasty after decompressive craniectomy: A retrospective observational cohort study. Neurosurg. Rev. 2022, 45, 3647–3655. [Google Scholar] [CrossRef] [PubMed]
- AB, OssDsign. Improving Outcomes in Cranioplasty–Post-Market Surveillance Data from 1995 Cranioplasties Using Ossdsign® Cranial Psi. In Post-Market Surveillance Review; Ossdsign® Cranial PSI: Uppsala, Sweden, 2022. [Google Scholar]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- Cui, F.-Z.; Li, Y.; Ge, J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Liao, S.S.; Cui, F.Z.; Zhang, W.; Feng, Q.L. Hierarchically biomimetic bone scaffold materials: Nano-HA/collagen/PLA composite. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 69, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Koons, G.L.; Mikos, A.G.; Qiu, Z.; Song, T.; Cui, F.; Wang, X. Tuning pore features of mineralized collagen/PCL scaffolds for cranial bone regeneration in a rat model. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110186. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Itoh, S.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001, 22, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, L.; Cui, F.Z.; Qu, Y.; Lian, X.J.; Wang, X.M.; Wang, Y. Clinical Evaluation of Mineralized Collagen as a Bone Graft Substitute for Anterior Cervical Intersomatic Fusion. J. Biomater. Tissue Eng. 2012, 2, 170–176. [Google Scholar] [CrossRef]
- Kou, J.-M.; Fu, T.-Y.; Jia, X.-J.; Hou, J.-W.; Gao, C.; Ma, Y.-Z.; Qiu, Z.-Y.; Cui, F.-Z. Clinical Observations on Repair of Non-Infected Bone Nonunion by Using Mineralized Collagen Graft. J. Biomater. Tissue Eng. 2014, 4, 1107–1112. [Google Scholar] [CrossRef]
- Lian, K.; Lu, H.; Guo, X.; Cui, F.; Qiu, Z.; Xu, S. The mineralized collagen for the reconstruction of intra-articular calcaneal fractures with trabecular defects. Biomatter 2013, 3, e27250. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Qiu, Z.; Zhang, Y.; Zhang, Z.; Song, T.; Cui, F.; Zhang, Y.; Zhang, Z.; Song, T.; Cui, F. Biodegradable mineralized collagen plug for the reconstruction of craniotomy burr-holes: A report of three cases. Transl. Neurosci. Clin. 2015, 1, 3–9. [Google Scholar] [CrossRef]
- Lam, S.; Kuether, J.; Fong, A.; Reid, R. Cranioplasty for large-sized calvarial defects in the pediatric population: A review. Craniomaxillofac. Trauma Reconstr. 2015, 8, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, L.; Pan, X.; Xue, C.; Zhang, Q.; Zhang, Y. Investigation of Operative Skills and Cranioplasty Complications using Biomimetic Bone (Nano-Hap/Collagen Composites). Brain Sci. Adv. 2019, 4, 131–140. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Xue, C.; Pan, X.; Zhang, Q. Postoperative complications of pediatric biomimetic materials bone cranioplasty. Chin. J. Pediatr. Surg. 2020, 41, 607–612. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Miwa, T.; Yoshida, K.; Kishi, K. Assessment of Bioabsorbable Hydroxyapatite for Cranial Defect in Children. J. Craniofacial Surg. 2019, 30, e58–e60. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Miwa, T.; Sakamoto, Y.; Kishi, K.; Yoshida, K. Exchange Cranioplasty Using Bioabsorbable Hydroxyapatite and Collagen Complex After Removal of an Extensive Frontal Bone Tumor in an Infant. World Neurosurg. 2020, 142, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Z.; Yang, Y.; Mikos, A.G.; Qiu, Z.; Song, T.; Cui, F.; Wang, X.; Zhang, C. A high-strength mineralized collagen bone scaffold for large-sized cranial bone defect repair in sheep. Regen. Biomater. 2018, 5, 283–292. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Z.; Yang, Y.; Wang, S.; Zhao, Y.; Xiong, Y.; Yang, S.; Qiu, Z.; Song, T.; Zhang, C.; et al. Biphasic mineralized collagen-based composite scaffold for cranial bone regeneration in developing sheep. Regen. Biomater. 2022, 9, rbac004. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Zhao, Z.; Wang, X.; Mikos, A.G.; Qiu, Z.; Song, T.; Sun, X.; Zhao, L.; Zhang, C.; et al. Mineralized Collagen-Based Composite Bone Materials for Cranial Bone Regeneration in Developing Sheep. ACS Biomater. Sci. Eng. 2017, 3, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Li, S.; Mele, E.; Silberschmidt, V.V. Dry vs. wet: Properties and performance of collagen films. Part I. Mechanical behaviour and strain-rate effect. J. Mech. Behav. Biomed. Mater. 2020, 111, 103983. [Google Scholar] [CrossRef] [PubMed]
- Preethi Soundarya, S.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. (Eds.) Material Extrusion. In Additive Manufacturing Technologies; Springer International Publishing: Cham, Switzerland, 2021; pp. 171–201. [Google Scholar]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. (Eds.) Introduction and Basic Principles. In Additive Manufacturing Technologies; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–21. [Google Scholar]
- Schantz, J.T.; Teoh, S.H.; Lim, T.C.; Endres, M.; Lam, C.X.; Hutmacher, D.W. Repair of calvarial defects with customized tissue-engineered bone grafts I. Evaluation of osteogenesis in a three-dimensional culture system. Tissue Eng. 2003, 9 (Suppl. S1), S113–S126. [Google Scholar] [CrossRef] [PubMed]
- Schantz, J.T.; Hutmacher, D.W.; Lam, C.X.; Brinkmann, M.; Wong, K.M.; Lim, T.C.; Chou, N.; Guldberg, R.E.; Teoh, S.H. Repair of calvarial defects with customised tissue-engineered bone grafts II. Evaluation of cellular efficiency and efficacy in vivo. Tissue Eng. 2003, 9 (Suppl. S1), S127–S139. [Google Scholar] [CrossRef] [PubMed]
- Rohner, D.; Hutmacher, D.W.; Cheng, T.K.; Oberholzer, M.; Hammer, B. In vivo efficacy of bone-marrow-coated polycaprolactone scaffolds for the reconstruction of orbital defects in the pig. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 66, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Schantz, J.T.; Lim, T.C.; Ning, C.; Teoh, S.H.; Tan, K.C.; Wang, S.C.; Hutmacher, D.W. Cranioplasty after trephination using a novel biodegradable burr hole cover: Technical case report. Neurosurgery 2006, 58, ONS-E176. [Google Scholar] [CrossRef] [PubMed]
- Low, S.W.; Ng, Y.J.; Yeo, T.T.; Chou, N. Use of Osteoplug™ polycaprolactone implants as novel burr-hole covers. Singap. Med. J. 2009, 50, 777–790. [Google Scholar]
- Yang, M.; Ng, H.J.; Nga, V.D.; Chou, N.; Yeo, T.T. Cranial reconstruction using a polycaprolactone implant after burr hole trephination. J. 3D Print. Med. 2020, 4, 9–16. [Google Scholar] [CrossRef]
- Toh, E.M.S.; Thenpandiyan, A.A.; Foo, A.S.C.; Zhang, J.J.Y.; Lim, M.J.R.; Goh, C.P.; Dinesh, N.; Vedicherla, S.V.; Yang, M.; Teo, K.; et al. Clinical Outcomes of 3D-Printed Bioresorbable Scaffolds for Bone Tissue Engineering-A Pilot Study on 126 Patients for Burrhole Covers in Subdural Hematoma. Biomedicines 2022, 10, 2702. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Villavicencio, J.B.; Agdamag, A.M.P. Tissue Engineering and Regenerative Medicine Cranioplasty Using Polycaprolactone-Tricalcium Phosphate: Management and Treatment Outcomes. Neurosurg. Open 2021, 2, okab027. [Google Scholar] [CrossRef]
- Koo, H.T.; Oh, J.; Heo, C.Y. Cranioplasty Using Three-Dimensional-Printed Polycaprolactone Implant and Free Latissimus Dorsi Musculocutaneous Flap in a Patient with Repeated Wound Problem following Titanium Cranioplasty. Arch. Plast. Surg. 2022, 49, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Matheus, I.; Phua, Y. Three-Dimensional Printed Polycaprolactone Mesh in Pediatric Cranial Vault Remodeling Surgery. J. Craniofac. Surg. 2023, 34, 1403–1406. [Google Scholar] [CrossRef]
- Castrisos, G.; Gonzalez Matheus, I.; Sparks, D.; Lowe, M.; Ward, N.; Sehu, M.; Wille, M.L.; Phua, Y.; Medeiros Savi, F.; Hutmacher, D.; et al. Regenerative matching axial vascularisation of absorbable 3D-printed scaffold for large bone defects: A first in human series. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 2108–2118. [Google Scholar] [CrossRef] [PubMed]
- Herrera, N.; Olsén, P.; Berglund, L.A. Strongly Improved Mechanical Properties of Thermoplastic Biocomposites by PCL Grafting inside Holocellulose Wood Fibers. ACS Sustain. Chem. Eng. 2020, 8, 11977–11985. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, W.; Wen, C.; Pan, H.; Wang, T.; Darvell, B.W.; Lu, W.W.; Huang, W. Bone regeneration: Importance of local pH—Strontium-doped borosilicate scaffold. J. Mater. Chem. 2012, 22, 8662–8670. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Sun, W.-J.; Kothari, S.; Sun, C.C. The relationship among tensile strength, Young’s modulus, and indentation hardness of pharmaceutical compacts. Powder Technol. 2018, 331, 1–6. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, K.; Ma, Y.; Yu, Z.; Chiou, B.-S.; Jia, M.; Chen, M.; Zhong, F. Collagen films with improved wet state mechanical properties by mineralization. Food Hydrocoll. 2023, 139, 108579. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Martin, R.I.; Brown, P.W. Mechanical properties of hydroxyapatite formed at physiological temperature. J. Mater.Sci. Mater. Med. 1995, 6, 138–143. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Dobelin, N. beta-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.H.; Fogle, J.L.; Melvin, J.W.; Haynes, R.R.; Roberts, V.L.; Alem, N.M. Mechanical properties on cranial bone. J. Biomech. 1970, 3, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.K.; Santoro, T.D.; Song, L.S.; Capel, C.C.; Sudhakar, P.V.; Matloub, H.S. Osteogenesis in calvarial defects: Contribution of the dura, the pericranium, and the surrounding bone in adult versus infant animals. Plast. Reconstr. Surg. 2003, 112, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.K.; Gosain, S.A.; Sweeney, W.M.; Song, L.S.; Amarante, M.T.J. Regulation of osteogenesis and survival within bone grafts to the calvaria: The effect of the dura versus the pericranium. Plast. Reconstr. Surg. 2011, 128, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hopper, R.A.; Zhang, J.R.; Fourasier, V.L.; Morova-Protzner, I.; Protzner, K.F.; Pang, C.Y.; Forrest, C.R. Effect of isolation of periosteum and dura on the healing of rabbit calvarial inlay bone grafts. Plast. Reconstr. Surg. 2001, 107, 454–462. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Chen, Q.; Bao, C.; Zhao, L.; Zhu, Z.; Xu, H.H.K. Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs. J. Tissue Eng. Regen. Med. 2018, 12, 191–203. [Google Scholar] [CrossRef]
- Zigdon-Giladi, H.; Bick, T.; Morgan, E.F.; Lewinson, D.; Machtei, E.E. Peripheral blood-derived endothelial progenitor cells enhance vertical bone formation. Clin. Implant Dent. Relat. Res. 2015, 17, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, N.; Hattori, K.; Suzawa, Y.; Iwai, S.; Matsumoto, T.; Tadokoro, M.; Nakano, T.; Akashi, M.; Ohgushi, H.; Yura, Y. Mesenchymal stromal cells improve the osteogenic capabilities of mineralized agarose gels in a rat full-thickness cranial defect model. J. Tissue Eng. Regen. Med. 2013, 7, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zigdon-Giladi, H.; Lewinson, D.; Bick, T.; Machtei, E.E. Mesenchymal stem cells combined with barrier domes enhance vertical bone formation. J. Clin. Periodontol. 2013, 40, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Liu, B.; Sun, J.; Li, W.; Cui, L. Bone regeneration in a canine cranial model using allogeneic adipose derived stem cells and coral scaffold. Biomaterials 2013, 34, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Tajima, S.; Tobita, M.; Orbay, H.; Hyakusoku, H.; Mizuno, H. Direct and indirect effects of a combination of adipose-derived stem cells and platelet-rich plasma on bone regeneration. Tissue Eng. Part A 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Li, K.D.; Wang, Y.; Sun, Q.; Li, M.S.; Chen, J.L.; Liu, L. Rabbit umbilical cord mesenchymal stem cells: A new option for tissue engineering. J. Gene Med. 2021, 23, e3282. [Google Scholar] [CrossRef] [PubMed]
- Khoobi, M.M.; Naddaf, H.; Hoveizi, E.; Mohammadi, T. Silymarin effect on experimental bone defect repair in rat following implantation of the electrospun PLA/carbon nanotubes scaffold associated with Wharton’s jelly mesenchymal stem cells. J. Biomed. Mater. Res. A 2020, 108, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; Xing, F.; Yang, Y.; Gong, M.; Liu, G.; Wu, S.; Luo, R.; Duan, X.; Liu, M.; et al. Graphene oxide-modified silk fibroin/nanohydroxyapatite scaffold loaded with urine-derived stem cells for immunomodulation and bone regeneration. Stem. Cell Res. Ther. 2021, 12, 591. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.; Zhao, L.; Weir, M.D.; Sun, J.; Chen, W.; Man, Y.; Xu, H.H. Bone tissue engineering via human induced pluripotent, umbilical cord and bone marrow mesenchymal stem cells in rat cranium. Acta Biomater. 2015, 18, 236–248. [Google Scholar] [CrossRef]
- Xie, J.; Peng, C.; Zhao, Q.; Wang, X.; Yuan, H.; Yang, L.; Li, K.; Lou, X.; Zhang, Y. Osteogenic differentiation and bone regeneration of iPSC-MSCs supported by a biomimetic nanofibrous scaffold. Acta Biomater. 2016, 29, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Sandor, G.K.; Numminen, J.; Wolff, J.; Thesleff, T.; Miettinen, A.; Tuovinen, V.J.; Mannerstrom, B.; Patrikoski, M.; Seppanen, R.; Miettinen, S.; et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem. Cells Transl. Med. 2014, 3, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Thesleff, T.; Lehtimaki, K.; Niskakangas, T.; Huovinen, S.; Mannerstrom, B.; Miettinen, S.; Seppanen-Kaijansinkko, R.; Ohman, J. Cranioplasty with Adipose-Derived Stem Cells, Beta-Tricalcium Phosphate Granules and Supporting Mesh: Six-Year Clinical Follow-Up Results. Stem. Cells Transl. Med. 2017, 6, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Thesleff, T.; Lehtimaki, K.; Niskakangas, T.; Mannerstrom, B.; Miettinen, S.; Suuronen, R.; Ohman, J. Cranioplasty with adipose-derived stem cells and biomaterial: A novel method for cranial reconstruction. Neurosurgery 2011, 68, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.A.; Kop, A.M.; Nilasaroya, A.; Sturm, M.; Shaw, K.; Honeybul, S. Cranial reconstruction using allogeneic mesenchymal stromal cells: A phase 1 first-in-human trial. J. Tissue Eng. Regen. Med. 2018, 12, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Boukhechba, F.; Balaguer, T.; Bouvet-Gerbettaz, S.; Michiels, J.F.; Bouler, J.M.; Carle, G.F.; Scimeca, J.C.; Rochet, N. Fate of bone marrow stromal cells in a syngenic model of bone formation. Tissue Eng. Part A 2011, 17, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Suvarnapathaki, S.; Wu, X.; Zhang, T.; Nguyen, M.A.; Goulopoulos, A.A.; Wu, B.; Camci-Unal, G. Oxygen generating scaffolds regenerate critical size bone defects. Bioact. Mater. 2022, 13, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Rong, Z.; Wu, G.; Wang, Y.; Tan, Z.; Zheng, J.; Jin, Y.; Liang, Z.; Liu, C.; Guo, J.; et al. Gelatin-CaO2/SAP/PLGA composite scaffold enhances the reparation of critical-sized cranial defects by promoting seed cell survival. Appl. Mater. Today 2021, 22, 100960. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Zhang, R.; He, Y.; Tao, B.; Wu, J.; Hu, X.; Li, X.; Xia, Z.; Cai, K. Multifunctional silicon calcium phosphate composite scaffolds promote stem cell recruitment and bone regeneration. J. Mater. Chem. B 2022, 10, 5218–5230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, W.; Xie, C.; Wu, X.; Ren, Q.; Wang, F.; Shen, X.; Hong, Y.; Wu, H.; Liao, Y.; et al. Msx1(+) stem cells recruited by bioactive tissue engineering graft for bone regeneration. Nat. Commun. 2022, 13, 5211. [Google Scholar] [CrossRef]
- Huan, Y.; Zhou, D.; Wu, X.; He, X.; Chen, H.; Li, S.; Jia, B.; Dou, Y.; Fei, X.; Wu, S.; et al. 3D bioprinted autologous bone particle scaffolds for cranioplasty promote bone regeneration with both implanted and native BMSCs. Biofabrication 2023, 15, acbe21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tang, Y.; Ke, Q.; Yin, W.; Zhang, C.; Guo, Y.; Guan, J. Magnetic lanthanum-doped hydroxyapatite/chitosan scaffolds with endogenous stem cell-recruiting and immunomodulatory properties for bone regeneration. J. Mater. Chem. B 2020, 8, 5280–5292. [Google Scholar] [CrossRef]
- Lind, M.; Eriksen, E.F.; Bunger, C. Bone morphogenetic protein-2 but not bone morphogenetic protein-4 and -6 stimulates chemotactic migration of human osteoblasts, human marrow osteoblasts, and U2-OS cells. Bone 1996, 18, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Roderer, G.; Gunther, K.P.; Brenner, R.E. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J. Cell Biochem. 2002, 87, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.D.; Lee, J.T.; Koh, J.T.; Jung, H.M.; Lee, H.J.; Kwon, T.G. Sequential Treatment with SDF-1 and BMP-2 Potentiates Bone Formation in Calvarial Defects. Tissue Eng. Part A 2015, 21, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, Y.; Chen, X.; Zeng, C.; Hu, Q.; Yin, W.; Li, W.; Xie, H.; Zhang, B.; Huang, X.; et al. Nanoparticle-modified chitosan-agarose-gelatin scaffold for sustained release of SDF-1 and BMP-2. Int. J. Nanomed. 2018, 13, 7395–7408. [Google Scholar] [CrossRef] [PubMed]
- Arnander, C.; Westermark, A.; Veltheim, R.; Docherty-Skogh, A.C.; Hilborn, J.; Engstrand, T. Three-dimensional technology and bone morphogenetic protein in frontal bone reconstruction. J. Craniofac. Surg. 2006, 17, 275–279. [Google Scholar] [CrossRef]
- Kohan, E.; Roostaeian, J.; Yuan, J.T.; Fan, K.L.; Federico, C.; Kawamoto, H.; Bradley, J.P. Customized Bilaminar Resorbable Mesh With BMP-2 Promotes Cranial Bone Defect Healing. Ann. Plast. Surg. 2015, 74, 603–608. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Xu, X.; Liao, L.; Tian, W. Strategies of Prevascularization in Tissue Engineering and Regeneration of Craniofacial Tissues. Tissue Eng. Part B Rev. 2022, 28, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Roux, B.M.; Akar, B.; Zhou, W.; Stojkova, K.; Barrera, B.; Brankov, J.; Brey, E.M. Preformed Vascular Networks Survive and Enhance Vascularization in Critical Sized Cranial Defects. Tissue Eng. Part A 2018, 24, 1603–1615. [Google Scholar] [CrossRef]
- Roux, B.M.; Vaicik, M.K.; Shrestha, B.; Montelongo, S.; Stojkova, K.; Yang, F.; Guda, T.; Cinar, A.; Brey, E.M. Induced Pluripotent Stem Cell-Derived Endothelial Networks Accelerate Vascularization But Not Bone Regeneration. Tissue Eng. Part A 2021, 27, 940–961. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, L.; Tang, Y.; Xi, K.; Tang, J.; Xu, Y.; Xu, J.; Lu, J.; Guo, K.; Gu, Y.; et al. Spatiotemporal Regulation of the Bone Immune Microenvironment via Dam-Like Biphasic Bionic Periosteum for Bone Regeneration. Adv. Healthc. Mater. 2023, 12, e2201661. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, F.; Liu, H.; Wu, P.; Yang, Z.; Zhang, Z.; Su, J.; Cai, L.; Zhang, Y. Bioinspired sandwich-like hybrid surface functionalized scaffold capable of regulating osteogenesis, angiogenesis, and osteoclastogenesis for robust bone regeneration. Mater. Today Bio. 2022, 17, 100458. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Wang, P.; Lu, G.; Tong, L.; Liu, Q.; Chen, Y.; Lin, J.; Luo, E.; Liang, J.; et al. Dopamine-Integrated Nanointerface between Fibrillar Matrix and Hydrophilic Nanohydroxyapatite Regulates Immune Microenvironment to Boost Endogenous Bone Regeneration. Adv. Funct. Mater. 2023, 33, 2212738. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Pan, J.; Li, J.; Zhang, K.; Duan, W.; Liang, H.; Chen, K.; Geng, D.; Shi, Q.; et al. Nanoscaled Bionic Periosteum Orchestrating the Osteogenic Microenvironment for Sequential Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 36823–36836. [Google Scholar] [CrossRef] [PubMed]
- Gronowicz, G.; Jacobs, E.; Peng, T.; Zhu, L.; Hurley, M.; Kuhn, L.T. Calvarial Bone Regeneration Is Enhanced by Sequential Delivery of FGF-2 and BMP-2 from Layer-by-Layer Coatings with a Biomimetic Calcium Phosphate Barrier Layer. Tissue Eng. Part A 2017, 23, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Y.; Wu, C.; Miron, R.J.; Cheng, X. Platelet-derived growth factor BB gene-released scaffolds: Biosynthesis and characterization. J. Tissue Eng. Regen. Med. 2016, 10, E372–E381. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, L.; Wang, Y.; Lin, K.; Liu, J. Lithium-containing bioactive glasses enhanced 3D-printed PLGA scaffolds for bone regeneration in diabetes. Compos. Part B Eng. 2022, 230, 109550. [Google Scholar] [CrossRef]
- Zhu, D.; Lu, B.; Yang, Q.; Yu, H.; Liu, P.; Yin, J.; Chen, Y.; Huang, Y.; Ke, Q.; Zhang, C.; et al. Lanthanum-doped mesoporous bioglasses/chitosan composite scaffolds enhance synchronous osteogenesis and angiogenesis for augmented osseous regeneration. Chem. Eng. J. 2021, 405, 127077. [Google Scholar] [CrossRef]
- Aquino-Martinez, R.; Angelo, A.P.; Pujol, F.V. Calcium-containing scaffolds induce bone regeneration by regulating mesenchymal stem cell differentiation and migration. Stem. Cell Res. Ther. 2017, 8, 265. [Google Scholar] [CrossRef]
- Wu, P.; Shen, L.; Liu, H.F.; Zou, X.H.; Zhao, J.; Huang, Y.; Zhu, Y.F.; Li, Z.Y.; Xu, C.; Luo, L.H.; et al. The marriage of immunomodulatory, angiogenic, and osteogenic capabilities in a piezoelectric hydrogel tissue engineering scaffold for military medicine. Mil. Med. Res. 2023, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Koga, N. Soft Tissue of the Scalp and Temporal Regions. In Anatomy for Plastic Surgery of the Face, Head, and Neck; Watanabe, K., Shoja, M.M., Loukas, M., Tubbs, R.S., Eds.; Thieme Medical Publishers: New York, NY, USA, 2016; pp. 33–39. [Google Scholar]
- Bakker, X.R.; Nicolai, J.P. Ectopic bone formation after temporal muscle transposition for facial paralysis. Plast. Reconstr. Surg. 2000, 105, 2079–2081. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, K.; Holan, C.; Choudhary, N.; Curry, E.; Harshbarger, R. A Comprehensive Approach To Alloplastic Cranioplasty: Novel Implant Design And Refinement of Soft Tissue Management. Face 2021, 3, 43–52. [Google Scholar] [CrossRef]
- Liu, R.; Chen, S.; Huang, P.; Liu, G.; Luo, P.; Li, Z.; Xiao, Y.; Chen, Z.; Chen, Z. Immunomodulation-Based Strategy for Improving Soft Tissue and Metal Implant Integration and Its Implications in the Development of Metal Soft Tissue Materials. Adv. Funct. Mater. 2020, 30, 1910672. [Google Scholar] [CrossRef]
- Zanotti, B.; Zingaretti, N.; Almesberger, D.; Verlicchi, A.; Stefini, R.; Ragonese, M.; Guarneri, G.F.; Parodi, P.C. Enhancing dermal and bone regeneration in calvarial defect surgery. Indian J. Plast. Surg. 2014, 47, 325–332. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.B.; Barnett, S.; Madden, C.; Welch, B.; Mickey, B.; Rozen, S. Computed-tomography modeled polyether ether ketone (PEEK) implants in revision cranioplasty. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.N.; Ko, Y.-G.; Yeo, T.; Kim, E.J.; Kwon, O.K.; Kwon, O.H. Guided Regeneration of Rabbit Calvarial Defects Using Silk Fibroin Nanofiber–Poly(glycolic acid) Hybrid Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 5266–5272. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Fama, C.; Kaye, G.J.; Flores, R.; Lopez, C.D.; Bekisz, J.M.; Torroni, A.; Tovar, N.; Coelho, P.G.; Witek, L. Three-Dimensionally-Printed Bioactive Ceramic Scaffolds: Construct Effects on Bone Regeneration. J. Craniofac. Surg. 2021, 32, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Veldeman, M.; Geiger, M.; Clusmann, H. How I do it-the posterior question mark incision for decompressive hemicraniectomy. Acta Neurochir. 2021, 163, 1447–1450. [Google Scholar] [CrossRef] [PubMed]
- Baucher, G.; Bernard, F.; Graillon, T.; Dufour, H. Interfascial approach for pterional craniotomy: Technique and adjustments to prevent cosmetic complications. Acta Neurochir. 2019, 161, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Alberius, P.; Johnell, O. Repair of intra-membranous bone fractures and defects in rats. Immunolocalization of bone and cartilage proteins and proteoglycans. J. Cranio-Maxillofac. Surg. 1991, 19, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Matsiko, A.; Kelly, D.J.; Gleeson, J.P.; O’Brien, F.J. An Endochondral Ossification-Based Approach to Bone Repair: Chondrogenically Primed Mesenchymal Stem Cell-Laden Scaffolds Support Greater Repair of Critical-Sized Cranial Defects Than Osteogenically Stimulated Constructs In Vivo. Tissue Eng. Part A 2016, 22, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, G.M.; Vinardell, T.; Thompson, E.M.; Daly, A.C.; Matsiko, A.; O’Brien, F.J.; Kelly, D.J. Chondrogenically primed mesenchymal stem cell-seeded alginate hydrogels promote early bone formation in critically-sized defects. Eur. Polym. J. 2015, 72, 464–472. [Google Scholar] [CrossRef]
- Gerber, H.P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Carlevaro, M.F.; Cermelli, S.; Cancedda, R.; Descalzi Cancedda, F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: Auto-paracrine role during endochondral bone formation. J. Cell Sci. 2000, 113 Pt 1, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kanou, M.; Ueno, T.; Kagawa, T.; Fujii, T.; Sakata, Y.; Ishida, N.; Fukunaga, J.; Sugahara, T. Osteogenic potential of primed periosteum graft in the rat calvarial model. Ann. Plast. Surg. 2005, 54, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Ueno, T.; Kagawa, T.; Sakata, Y.; Sugahara, T. Comparison of bone formation ingrafted periosteum harvested from tibia and calvaria. Microsc. Res. Tech. 2006, 69, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Nussenbaum, B.; Rutherford, R.B.; Krebsbach, P.H. Bone regeneration in cranial defects previously treated with radiation. Laryngoscope 2005, 115, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Kruijt Spanjer, E.C.; Bittermann, G.K.P.; van Hooijdonk, I.E.M.; Rosenberg, A.; Gawlitta, D. Taking the endochondral route to craniomaxillofacial bone regeneration: A logical approach? J. Cranio-Maxillofac. Surg. 2017, 45, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Lyons, F.G.; Al-Munajjed, A.A.; Kieran, S.M.; Toner, M.E.; Murphy, C.M.; Duffy, G.P.; O’Brien, F.J. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials 2010, 31, 9232–9243. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Brennan, M.A.; Browe, D.C.; Renaud, A.; De Lima, J.; Kelly, D.J.; McNamara, L.M.; Layrolle, P. A Developmental Engineering-Based Approach to Bone Repair: Endochondral Priming Enhances Vascularization and New Bone Formation in a Critical Size Defect. Front Bioeng. Biotechnol. 2020, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Lewitz, M.; Salma, A.; Welzel Saravia, H.; Sakellaropoulou, I.; Sarkis, H.M.; Ewelt, C.; Fortmann, T.; Wilbers, E.; Schipmann, S.; Suero Molina, E.; et al. Load-Bearing Capacity and Design Advantages of a Custom-Made, Thin Pure-Titanium Cranioplasty (CranioTop). J. Craniofac. Surg. 2021, 32, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Tse, H.H.; Zwienenberg-Lee, M.; Smith, M.; Zovickian, J. The combined use of hydroxyapatite and bioresorbable plates to repair cranial defects in children. J. Neurosurg. 2005, 102, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Losee, J.E.; Karmacharya, J.; Gannon, F.H.; Slemp, A.E.; Ong, G.; Hunenko, O.; Gorden, A.D.; Bartlett, S.P.; Kirschner, R.E. Reconstruction of the immature craniofacial skeleton with a carbonated calcium phosphate bone cement: Interaction with bioresorbable mesh. J. Craniofac. Surg. 2003, 14, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shi, Q.; Shui, H.; Wang, P.; Chen, Q.; Li, Z. Degradation of 3D-Printed Porous Polylactic Acid Scaffolds Under Mechanical Stimulus. Front Bioeng. Biotechnol. 2021, 9, 691834. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chu, Z.; Li, X.; Ding, X.; Guo, M.; Zhao, H.; Yao, J.; Wang, L.; Cai, Q.; Fan, Y. The effect of mechanical loads on the degradation of aliphatic biodegradable polyesters. Regen. Biomater. 2017, 4, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, S.; Soballe, K.; Josephsen, K.; Hansen, E.S.; Bunger, C. Role of different loading conditions on resorption of hydroxyapatite coating evaluated by histomorphometric and stereological methods. J. Orthop. Res. 1996, 14, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Wagshul, M.E.; Eide, P.K.; Madsen, J.R. The pulsating brain: A review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 2011, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Milani-Nejad, N.; Janssen, P.M. Small and large animal models in cardiac contraction research: Advantages and disadvantages. Pharmacol. Ther. 2014, 141, 235–249. [Google Scholar] [CrossRef] [PubMed]
| Category | Biodegradability | Mechanical Properties of Major Components | Bone-Bonding and Bone Regeneration | Highlights of Reviewed Clinical Studies |
|---|---|---|---|---|
| CaP/Ti | Partly biodegradable | Ti: ~900 MPa (UTS) [111] 105–125 GPa (YM)[111] CaP (Monetite *, dense): ~445 MPa (UTS) [112] ~377.5 GPa (YM) [112] | Yes |
|
| Mineralised collagen | Fully biodegradable | Collagen (bulk): 2–90 MPa (UTS) [92,113] <2 GPa (YM) [114] HA: 308–509 MPa (CS) [115] 42.2–81.4 GPa (CM) [115] | Yes |
|
| Three-dimensional-printed PCL and β-TCP/PCL composites | Fully biodegradable | PCL: ~28.7 MPa (UTS) [109] 0.25 GPa (YM-Tension)[109] β-TCP: 1–10 GPa (UTS-Theoretical) [116] ~110 GPa (YM) [116] | No (PCL) Yes (β-TCP/PCL) |
|
| Human calvaria | - | 43–79 MPa (UTS) [117] 11.7–15.0 GPa (YM) [117] | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L. Biomaterials for Regenerative Cranioplasty: Current State of Clinical Application and Future Challenges. J. Funct. Biomater. 2024, 15, 84. https://doi.org/10.3390/jfb15040084
He L. Biomaterials for Regenerative Cranioplasty: Current State of Clinical Application and Future Challenges. Journal of Functional Biomaterials. 2024; 15(4):84. https://doi.org/10.3390/jfb15040084
Chicago/Turabian StyleHe, Lizhe. 2024. "Biomaterials for Regenerative Cranioplasty: Current State of Clinical Application and Future Challenges" Journal of Functional Biomaterials 15, no. 4: 84. https://doi.org/10.3390/jfb15040084
APA StyleHe, L. (2024). Biomaterials for Regenerative Cranioplasty: Current State of Clinical Application and Future Challenges. Journal of Functional Biomaterials, 15(4), 84. https://doi.org/10.3390/jfb15040084