Iron Oxide-Cobalt Nanocatalyst for O-tert-Boc Protection and O-Arylation of Phenols
Abstract
:1. Introduction
2. Results and Discussion
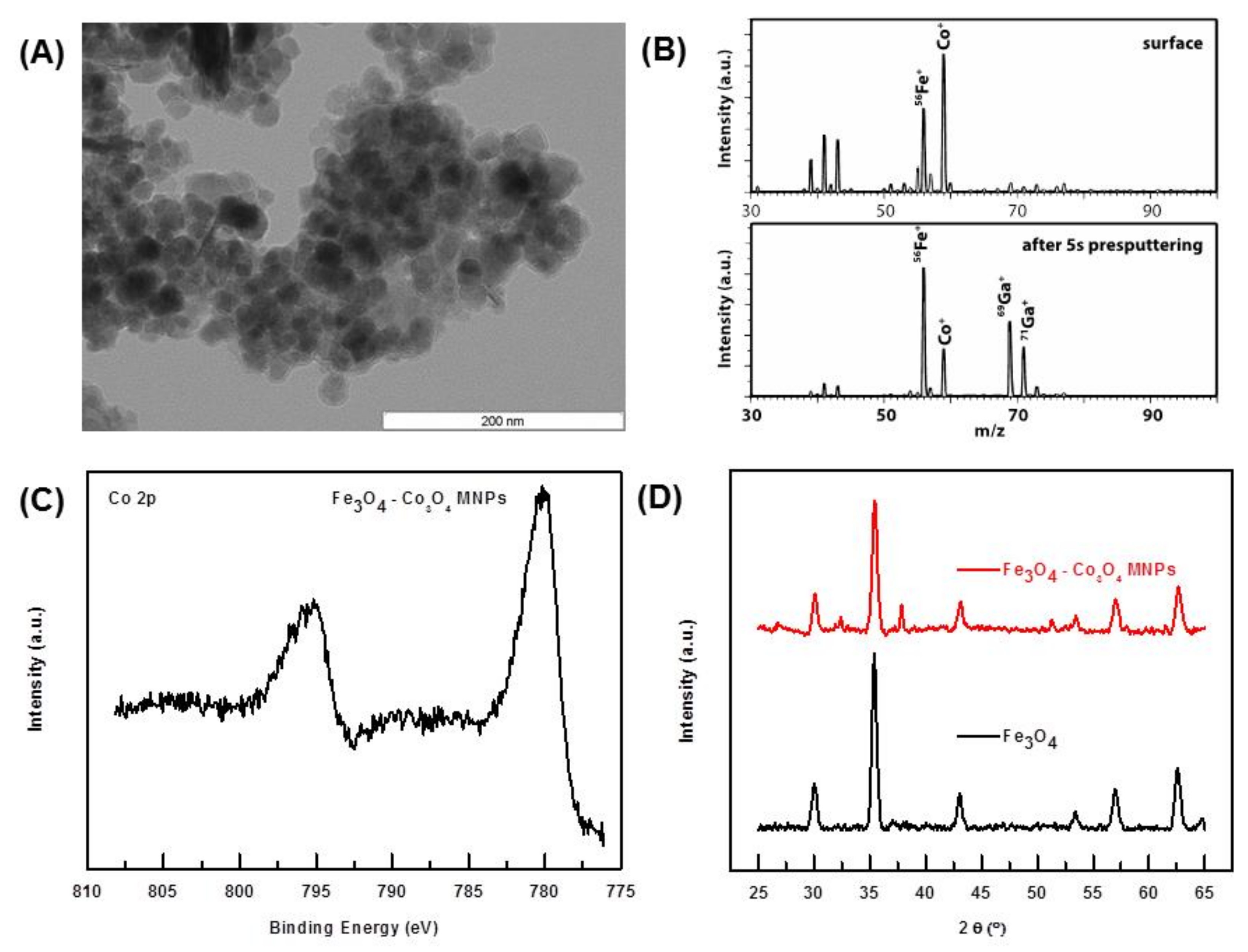
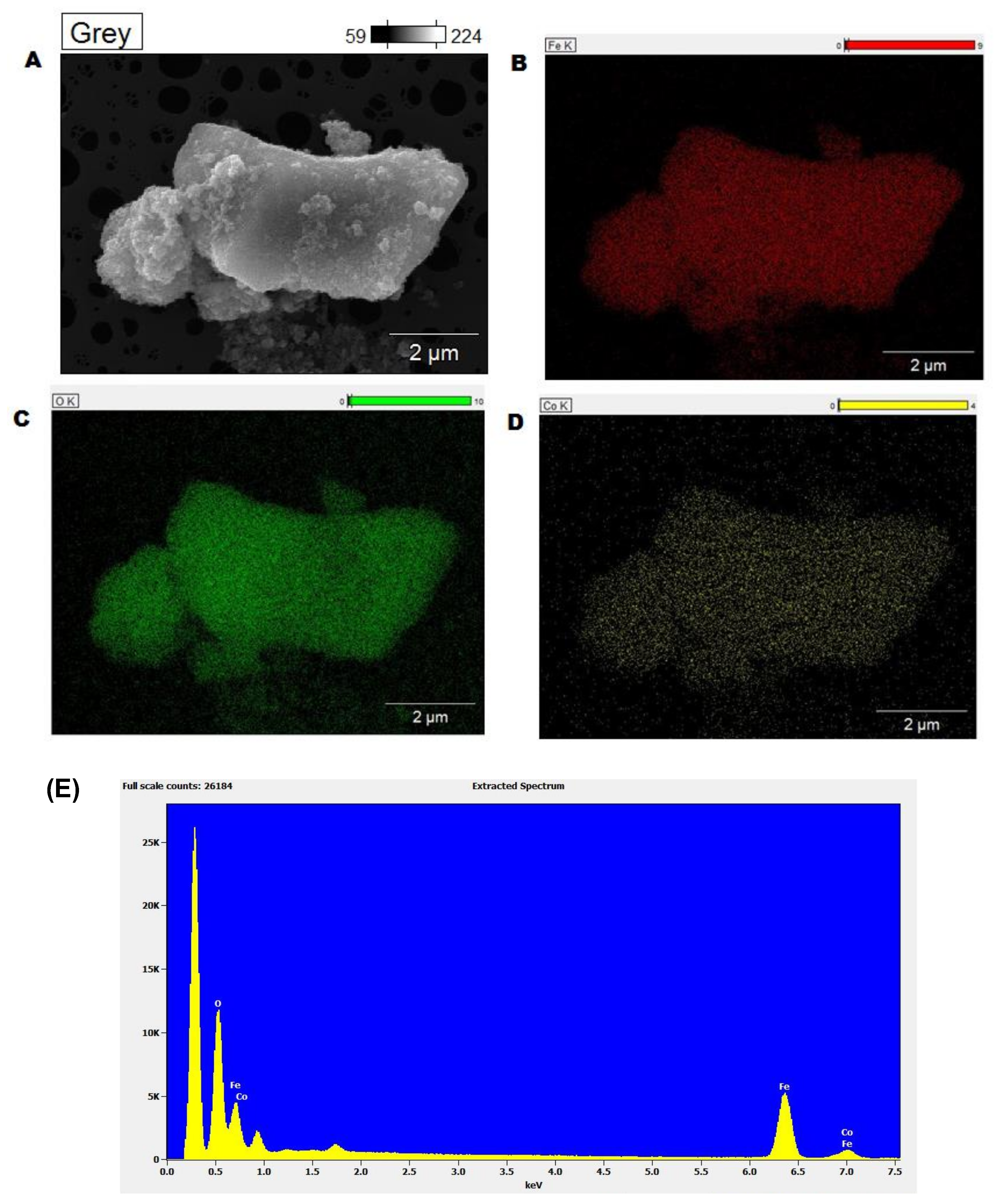
2.1. Characterization of the Catalyst
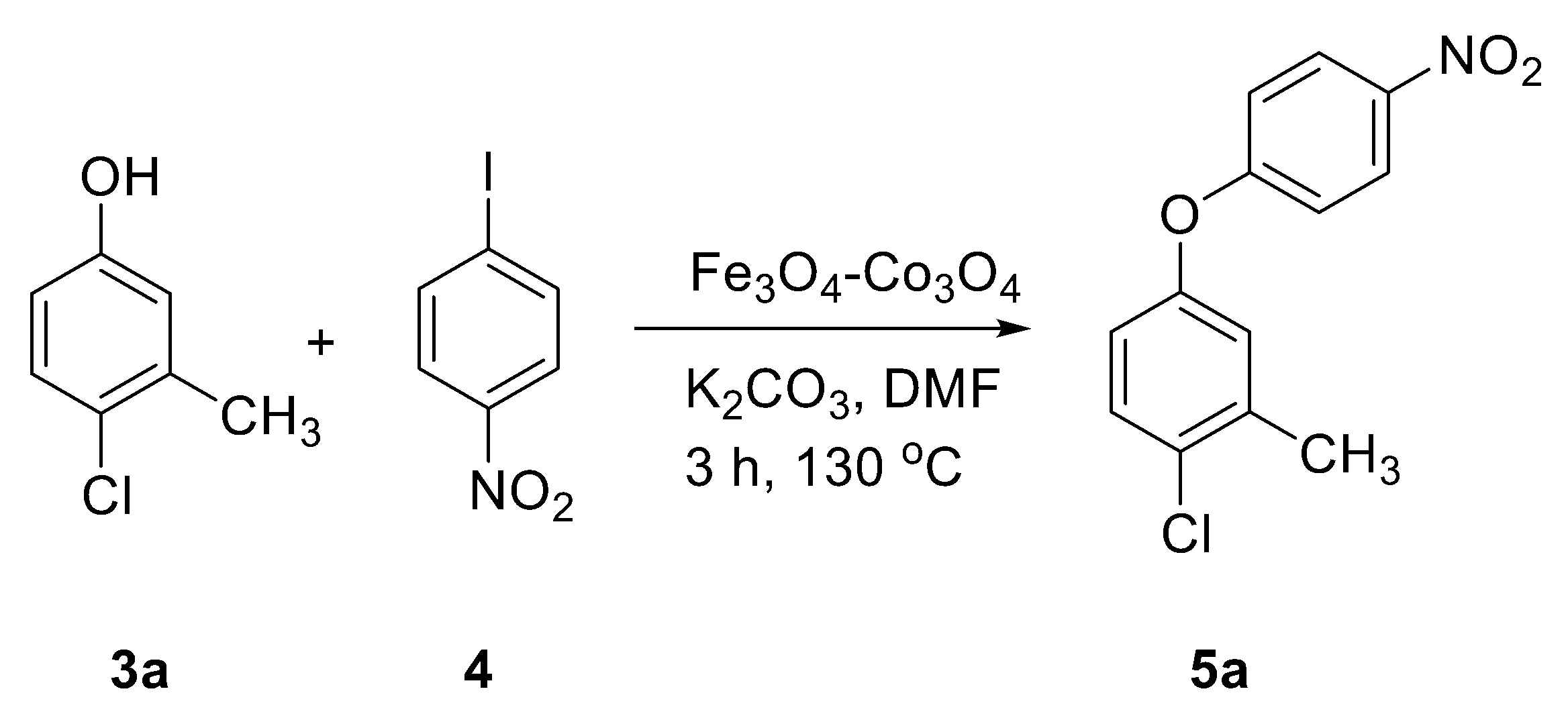
2.2. Catalytic Applications
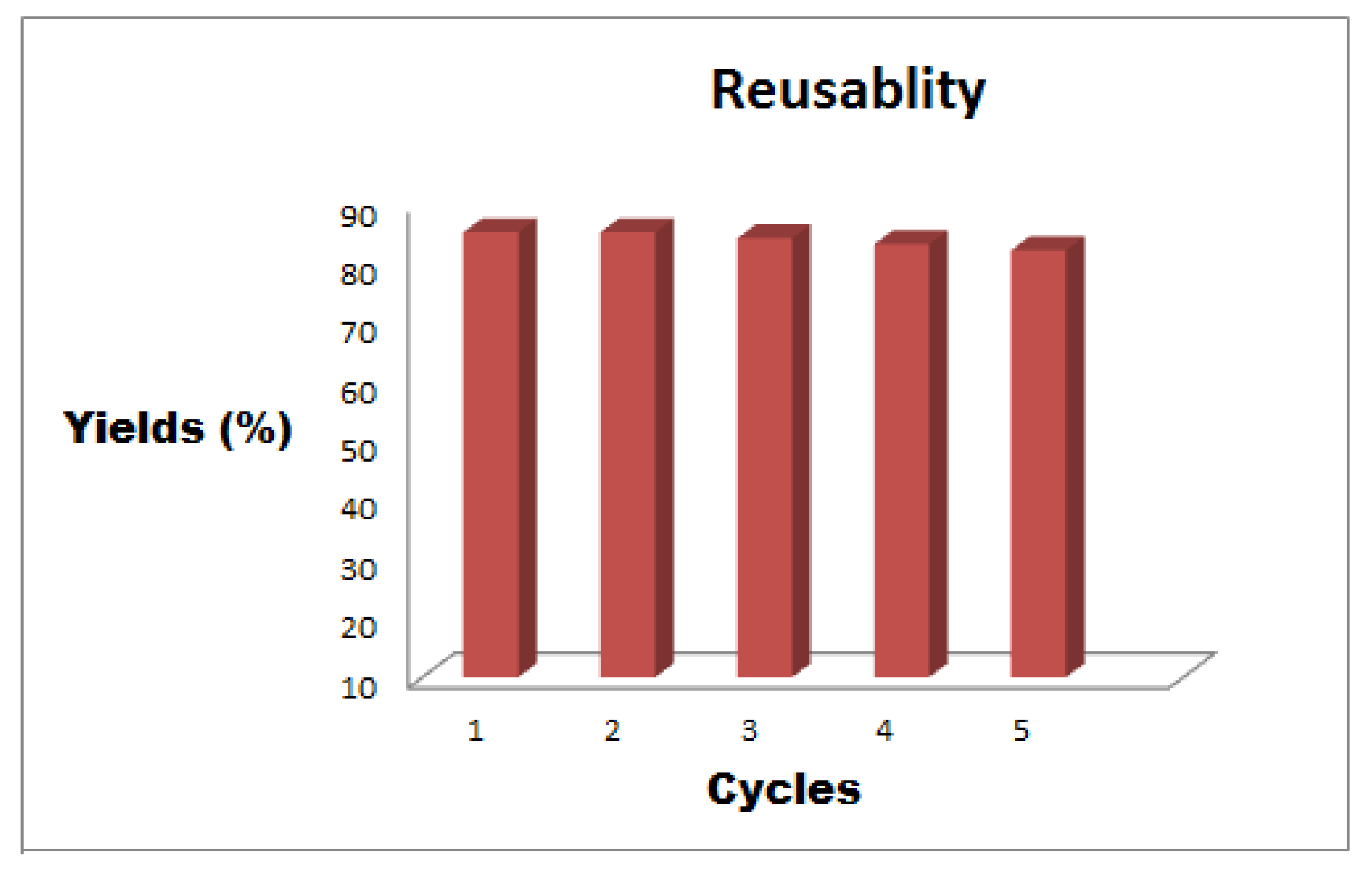
2.3. Recycling Study of Fe3O4-Co3O4 Nanocatalyst
3. Experimental Section
Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gawande, M.B.; Bonifacio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Benign by Design: Catalyst-Free In-Water, On-Water Green Chemical Methodologies in Organic Synthesis. Chem. Soc. Rev. 2013, 42, 5522–5551. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Bonifácio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Solvent-Free and Catalysts-Free Chemistry: A Benign Pathway to Sustainability. ChemSusChem 2014, 7, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, M.M.; Niknejad, N.; Veisi, H. A Mild and Green Method for the N-BOC Protection of Amines without Assistant of Catalyst Under Solvent-free Conditions. Lett. Org. Chem. 2013, 10, 121–125. [Google Scholar] [CrossRef]
- Anastas, P.T.; Bartlett, L.B.; Kirchhoff, M.M.; Williamson, T.C. The Role of Catalysis in the Design, Development and Implementation of Green Chemistry. Catal. Today 2000, 55, 11–22. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported Gold Nanoparticles as Catalysts for Organic Reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective Oxidation of Alcohols and Aldehydes over Supported Metal Nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Kunde, S.P.; Kanade, K.G.; Karale, B.K.; Akolkar, H.N.; Randhavane, P.V.; Shinde, S.T. Synthesis and Characterization of Nanostructured Cu-ZnO: An Efficient Catalyst for the Preparation of (E)-3-Styrylchromones. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties and Applications toward Biology, Catalysis and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Kalidindi, S.B.; Jagirdar, B.R. Nanocatalysis and Prospects of Green Chemistry. ChemSusChem 2012, 5, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhou, X.; Shen, H.; Andoy, N.M.; Choudhary, E.; Han, K.-S.; Liu, G.; Meng, W. Single-Molecule Fluorescence Imaging of Nanocatalytic Processes. Chem. Soc. Rev. 2010, 39, 4560–4570. [Google Scholar] [CrossRef] [PubMed]
- Schatz, A.; Reiser, O.; Stark, W.J. Nanoparticles as Semi-Heterogeneous Catalyst Supports. Chem. Eur. J. 2010, 16, 8950–8967. [Google Scholar] [CrossRef] [PubMed]
- Schlogl, R.; Hamid, S.B.A. Nanocatalysis: Mature Science Revisited or Something Really New? Angew. Chem. Int. Ed. 2004, 43, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Murzin, D.Y. Nanokinetics for Nanocatalysis. Catal. Sci. Technol. 2011, 1, 380–384. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Magnetically Retrievable Catalysts for Organic Synthesis. Chem. Commun. 2013, 49, 752–770. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-Magnetite (Fe3O4) as a Support for Recyclable Catalysts in the Development of Sustainable Methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef] [PubMed]
- Jansat, S.; Picurelli, D.; Pelzer, K.; Philippot, K.; Gómez, M.; Muller, G.; Lecante, P.; Chaudret, B. Synthesis, Characterization and Catalytic Reactivity of Ruthenium Nanoparticles Stabilized by Chiral N-Donor Ligands. New J. Chem. 2006, 30, 115–122. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Han, D.; Gao, Q.; Li, C. Asymmetric Transfer Hydrogenation Using Recoverable Ruthenium Catalyst Immobilized in to Magnetic Mesoporous Silica. J. Mol. Catal. A Chem. 2009, 298, 31–35. [Google Scholar] [CrossRef]
- Jansat, S.; Gómez, M.; Philippot, K.; Muller, G.; Guiu, E.; Claver, C.; Castillón, S.; Chaudret, B. A Case for Enantioselective Allylic Alkylation Catalyzed by Palladium Nanoparticles. J. Am. Chem. Soc. 2004, 126, 1592–1593. [Google Scholar] [CrossRef] [PubMed]
- Molvinger, K.; Lopez, M.; Court, J. Enantioselective Borane Reduction of Ketones with Oxazaborolidines Boron-bound to Nickel Boride Nanoparticles. Tetrahedron Lett. 1999, 40, 8375–8378. [Google Scholar] [CrossRef]
- Wang, B.G.; Ma, B.C.; Wang, Q.; Wang, W. Superparamagnetic Nanoparticle-Supported (S)-Diphenylprolinol Trimethylsilyl Ether as a Recyclable Catalyst for Asymmetric Michael Addition in Water. Adv. Synth. Catal. 2010, 352, 2923–2928. [Google Scholar] [CrossRef]
- Jin, M.J.; Lee, D.H. A Practical Heterogeneous Catalyst for the Suzuki, Sonogashira and Stille Coupling Reactions of Unreactive Aryl Chlorides. Angew. Chem. Int. Ed. 2010, 49, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, O.; Tekoriute, R.; Gunko, Y.K.; Connon, S.J. The First Magnetic Nanoparticle-Supported Chiral DMAP Analogue: Highly Enantioselective Acylation and Excellent Recyclability. Chem. Eur. J. 2009, 15, 5669–5673. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Q.; Wang, Y.; Qin, Y.; Chong, Y.; Yang, Q.; Li, G.; Zhang, L.; Li, W. One-pot Preparation of Magnetic N-Heterocyclic Carbene-Functionalized Silica Nanoparticles for the Suzuki–Miyaura Coupling of Aryl Chlorides: Improved Activity and Facile Catalyst Recovery. Green Chem. 2011, 13, 1352–1361. [Google Scholar] [CrossRef]
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 3rd ed.; Wiley: New York, NY, USA, 1999; p. 518, ISBN-100471160199. [Google Scholar]
- Basel, Y.; Hassner, A. Di-tert-butyl Dicarbonate and 4-(Dimethylamino)pyridine Revisited. Their Reactions with Amines and Alcohols. J. Org. Chem. 2000, 65, 6368–6380. [Google Scholar] [CrossRef] [PubMed]
- Houlihan, F.; Bouchard, F.; Frechet, J.M.; Willson, C.G. Phase Transfer Catalysis in the tert-Butyloxycarbonylation of Alcohols, Phenols, Enols and Thiols with di-tert-Butyl Dicarbonate. Can. J. Chem. 1985, 63, 153–162. [Google Scholar] [CrossRef]
- Hegarty, A.F. Comprehensive Organic Chemistry; Sutherland, I.O., Ed.; Pergamon: London, UK, 1979; Volume 2, p. 1067. [Google Scholar]
- Tundo, P.; Rossi, L.; Loris, A. Dimethyl Carbonate as an Ambident Electrophile. J. Org. Chem. 2005, 70, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Veldurthy, B.; Figueras, F. An Efficient Synthesis of Organic Carbonates: Atom Economic Protocol with a New Catalytic System. Chem. Commun. 2004, 6, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Bratt, M.O.; Taylor, P.C. Synthesis of Carbonates and Related Compounds from Carbon Dioxide via Methane Sulfonyl Carbonates. J. Org. Chem. 2003, 68, 5439–5444. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.G.; Sivaram, S. Organic Carbonates. Chem. Rev. 1996, 96, 951–976. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.P.; Salvatore, R.N.; Jung, K.W. Perspectives on Alkyl Carbonates in Organic Synthesis. Tetrahedron 2000, 56, 8207–8237. [Google Scholar] [CrossRef]
- Zhu, J. SNAr Based Macrocyclization via Biaryl Ether Formation: Application in Natural Product Synthesis. Synlett 1997, 2, 133–144. [Google Scholar] [CrossRef]
- Boger, D.L.; Patane, M.A.; Zhou, J. Total Synthesis of Bouvardin, O-Methylbouvardin, and O-Methyl-N9-desmethylbouvardin. J. Am. Chem. Soc. 1994, 116, 8544–8556. [Google Scholar] [CrossRef]
- Huffman, L.M.; Stahl, S.S. Carbon-Nitrogen Bond Formation Involving Well-Defined Aryl-Copper (III) Complexes. J. Am. Chem. Soc. 2008, 130, 9196–9197. [Google Scholar] [CrossRef] [PubMed]
- Torraca, K.E.; Huang, X.; Parrish, C.A.; Buchwald, S.L. An Efficient Intermolecular Palladium-Catalyzed Synthesis of Aryl Ethers. J. Am. Chem. Soc. 2001, 123, 10770–10771. [Google Scholar] [CrossRef] [PubMed]
- Desmarets, C.; Schneider, R.; Fort, Y. Nickel(0)/Dihydroimidazol-2-ylidene Complex Catalyzed Coupling of Aryl Chlorides and Amines. J. Org. Chem. 2002, 67, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Babu, S.; Karvembu, R. Room Temperature Ullmann Type C–O and C–S Cross Coupling of Aryl Halides with Phenol/Thiophenol Catalyzed by CuO Nanoparticles. Tetrahedron Lett. 2013, 54, 1677–1680. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S. An Efficient and Expeditious Fmoc Protection of Amines and Amino Acids in Aqueous Media. Green Chem. 2011, 13, 3355–3359. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.N.; Bankar, S.R.; Mahske, G.R.; Kadam, S.S.; Murade, D.K.; Bhorkade, S.B.; Rathi, A.K.; Bundaleski, N.; Teodoro, O.M.N.D.; Zboril, R.; et al. Iron Oxide-Supported Copper Oxide Nanoparticles (Nanocat-Fe-CuO): Magnetically Recyclable Catalysts for the Synthesis of Pyrazole Derivatives, 4-Methoxyaniline and Ullmann-type Condensation Reactions. ACS Sustain. Chem. Eng. 2014, 2, 1699–1706. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Branco, P.S.; Rathi, A.; Pandey, R.K. Mixed Metal MgO–ZrO2 Nanoparticle-Catalyzed O-tert-Boc Protection of Alcohols and Phenols under Solvent-Free Conditions. Appl. Organometal. Chem. 2012, 26, 395–400. [Google Scholar] [CrossRef]
- Gawande, M.B.; Pandey, R.K.; Jayaram, R.V. Role of Mixed Metal Oxides in Catalysis Science-Versatile Applications in Organic Synthesis. Catal. Sci. Technol. 2012, 2, 1113–1125. [Google Scholar] [CrossRef]
- Sonavane, S.U.; Gawande, M.B.; Deshpande, S.S.; Venkataraman, A.; Jayaram, R.V. Chemoselective Transfer Hydrogenation Reactions Over Nanosized γ-Fe2O3 Catalyst Prepared by Novel Combustion Route. Catal. Commun. 2007, 8, 1803–1806. [Google Scholar] [CrossRef]
- Gade, V.B.; Rathi, A.K.; Bhalekar, S.B.; Tucek, J.; Tomanec, O.; Varma, R.S.; Zboril, R.; Shelke, S.N.; Gawande, M.B. Iron-Oxide-Supported UltrasmallZnO Nanoparticles: Applications for Transesterification, Amidation and O-Acylation Reactions. ACS Sustain. Chem. Eng. 2017, 5, 3314–3320. [Google Scholar] [CrossRef]
- Gawande, M.B.; Rathi, A.; Nogueira, I.D.; Ghumman, C.A.A.; Bundaleski, N.; Teodoro, O.M.N.D.; Branco, P.S. A Recyclable Ferrite-Co Magnetic Nanocatalyst for the Oxidation of Alcohols to Carbonyl Compounds. ChemPlusChem 2012, 77, 865–871. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Nogueira, I.D.; Ghumman, C.A.A.; Bundaleski, N.; Santos, A.; Teodoro, O.M.N.D.; Luque, R. Catalytic Applications of a Versatile Magnetically Separable Fe-Mo (Nanocat-Fe-Mo) Nanocatalyst. Green Chem. 2013, 15, 682–689. [Google Scholar] [CrossRef]
- Gawande, M.B.; Rathi, A.K.; Nogueira, I.D.; Varma, R.S.; Branco, P.S. Magnetite-Supported Sulfonic Acid: A Retrievable Nanocatalyst For the Ritter Reaction and Multicomponent Reactions. Green Chem. 2013, 15, 1895–1899. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, W.; Huang, T.; Li, M.; Wang, W.; Liu, Y.; Mao, C.; Meng, F.; Wang, M.; Cheng, M.; et al. Facile scalable synthesis of Co3O4/carbon nanotube hybrids as superior anode materials for lithium-ion batteries. Mater. Res. Bull. 2013, 48, 4419–4423. [Google Scholar] [CrossRef]
- Chuang, T.J.; Brundle, C.R.; Rice, D.W. Interpretation of the X-ray Photoemission Spectra of Cobalt Oxides and Cobalt Oxide Surfaces. Surf. Sci. 1976, 59, 413–429. [Google Scholar] [CrossRef]
- Gautier, J.L.; Rios, E.; Gracia, M.; Marco, J.F.; Gancedo, J.R. Characterization by X-ray Photoelectron Spectroscopy of Thin MnxCo3−xO4 (1 ≥ x ≥ 0) Spinel Films Prepared by Low-Temperature Spray Pyrolysis. Thin Solid Films 1997, 311, 51–57. [Google Scholar] [CrossRef]
- Shen, Z.X.; Allen, J.W.; Lindberg, P.A.P.; Dessau, D.S.; Wells, B.O.; Borg, A.; Ellis, W.; Kang, J.S.; Oh, S.J.; Lindau, I.; et al. Photoemission Study of CoO. Phys. Rev. B 1990, 42, 1817–1828. [Google Scholar] [CrossRef]
- Van Elp, J.; Wieland, J.L.; Eskes, H.; Kuiper, P.; Sawatzky, G.A.; de Groot, F.M.F.; Turner, T.S. Electronic Structure of CoO, Li-Doped CoO, and LiCoO2. Phys. Rev. B. 1991, 44, 6090–6103. [Google Scholar] [CrossRef]
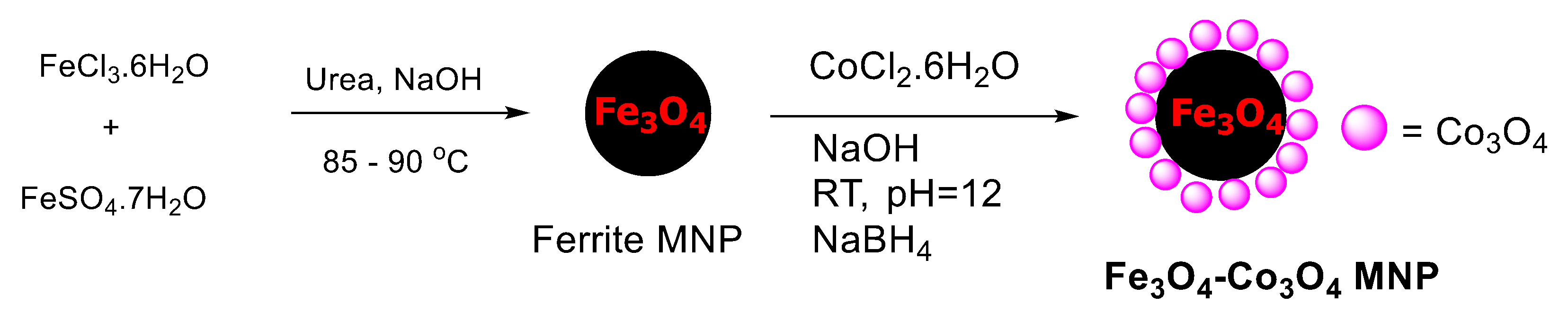
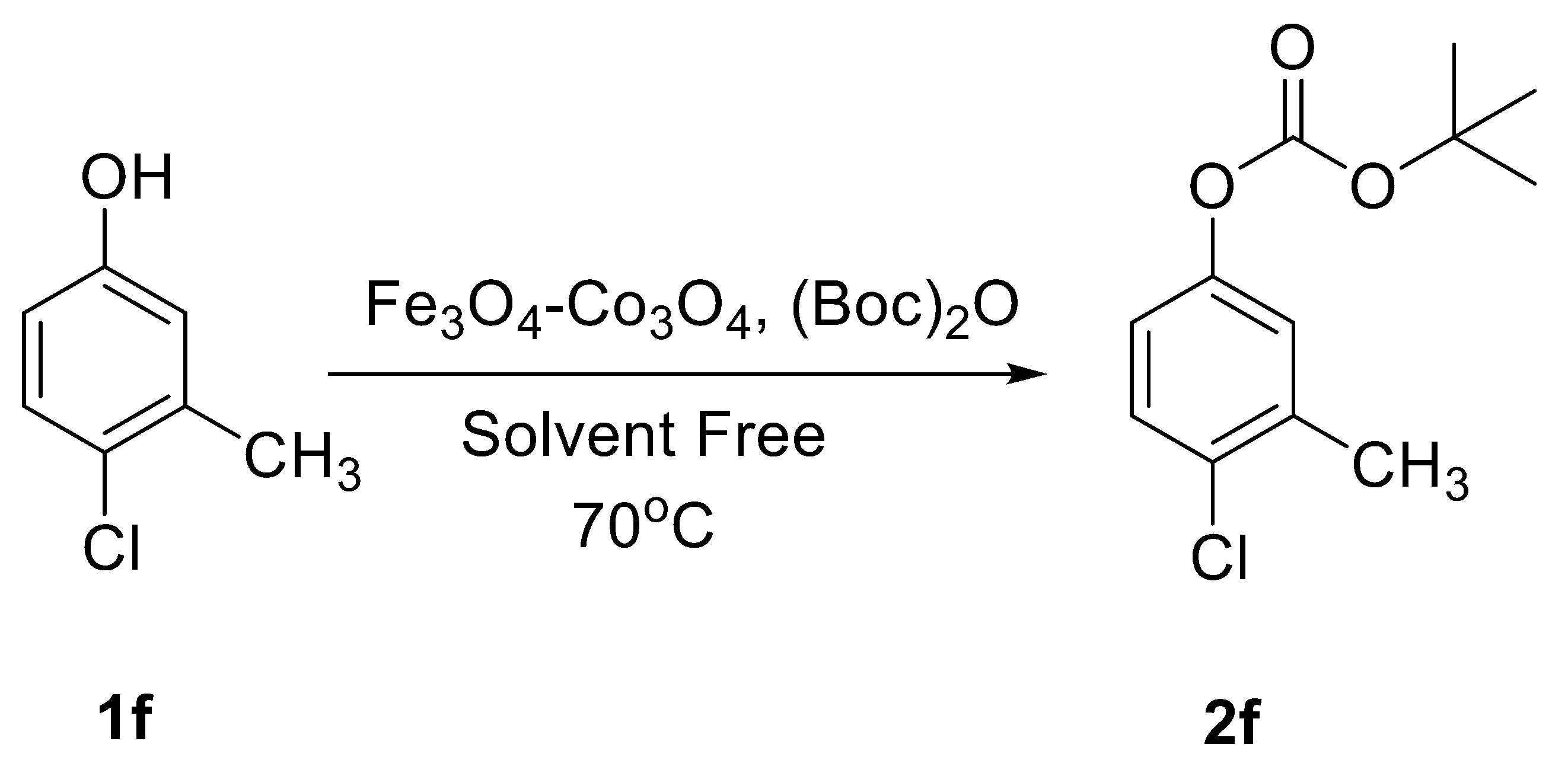
| No. | Catalyst | Temperature (°C) | Time (h) | Yield b (2f, %) | TON | TOF (h−1) |
|---|---|---|---|---|---|---|
| 1 | -- | RT | 16 | NR | -- | -- |
| 2 | -- | 70 | 16 | Trace | -- | -- |
| 3 | Fe3O4-Co3O4 MNPs (10 mol %) | RT | 16 | 88 | 108.3 | 6.7 |
| 4 | Fe3O4-Co3O4 MNPs (10 mol %) | 70 | 3 | 94 | 115.7 | 38.5 |
| 5 | Fe3O4-Co3O4 MNPs (5 mol %) | 70 | 3 | 72 | 177.3 | 59.1 |
| 6 | Fe3O4 MNPs (10 mol %) | 70 | 3 | 56 | 5.6 | 1.8 |
| 7 | Co3O4NPs | 70 | 3 | 61 | 6.1 | 2 |
| No. | Phenol | Product | Time (h) | Yield b (%) |
|---|---|---|---|---|
| 1 |  1a | 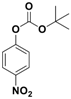 2a | 2.5 | 95 |
| 2 |  1b | 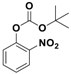 2b | 3 | 92 |
| 3 |  1c |  2c | 3.5 | 92 |
| 4 | 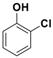 1d | 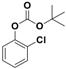 2d | 4 | 87 |
| 5 | 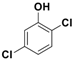 1e | 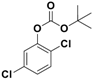 2e | 3 | 91 |
| 6 |  1f | 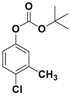 2f | 3 | 94 |
| 7 |  1g | 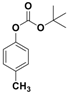 2g | 3.5 | 93 |
| 8 |  1h | 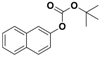 2h | 4 | 85 |
| No. | Catalyst | Solvent | Base | Time (h) | Yield b (5a, %) | TON | TOF (h−1) |
|---|---|---|---|---|---|---|---|
| 1 | -- | Toluene | K3PO4 | 6 | 10 | -- | -- |
| 2 | -- | DMF | K3PO4 | 6 | 18 | -- | -- |
| 3 | -- | DMF | K2CO3 | 6 | 22 | -- | -- |
| 4 | Fe3O4-Co3O4 (10 mol %) | DMF | K3PO4 | 3 | 72 | 88.6 | 29.5 |
| 5 | Fe3O4-Co3O4 (10 mol %) | DMF | K2CO3 | 3 | 85 | 104.6 | 34.8 |
| 6 | Fe3O4-Co3O4 (5 mol %) | DMF | K2CO3 | 3 | 62 | 152.7 | 50.9 |
| 7 | Fe3O4 | DMF | K2CO3 | 3 | 58 | 5.8 | 1.9 |
| 8 | Co3O4 | DMF | K2CO3 | 3 | 67 | 6.7 | 2.2 |
| No. | Phenol | Product | Time (h) | Yield b (%) |
|---|---|---|---|---|
| 1 |  3a |  5a | 3 | 85 |
| 2 | 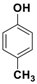 3b |  5b | 3 | 89 |
| 3 |  3c | 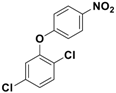 5c | 4 | 91 |
| 4 |  3d |  5d | 3.5 | 87 |
| 5 | 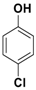 3e | 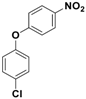 5e | 3 | 88 |
| 6 | 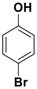 3f |  5f | 3 | 91 |
| 7 |  3g |  5g | 3.5 | 94 |
| 8 |  3h | 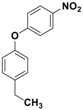 5h | 3 | 90 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gade, V.B.; Goswami, A.; Varma, R.S.; Shelke, S.N.; Gawande, M.B. Iron Oxide-Cobalt Nanocatalyst for O-tert-Boc Protection and O-Arylation of Phenols. Nanomaterials 2018, 8, 246. https://doi.org/10.3390/nano8040246
Gade VB, Goswami A, Varma RS, Shelke SN, Gawande MB. Iron Oxide-Cobalt Nanocatalyst for O-tert-Boc Protection and O-Arylation of Phenols. Nanomaterials. 2018; 8(4):246. https://doi.org/10.3390/nano8040246
Chicago/Turabian StyleGade, Vilas B., Anandarup Goswami, Rajender S. Varma, Sharad N. Shelke, and Manoj B. Gawande. 2018. "Iron Oxide-Cobalt Nanocatalyst for O-tert-Boc Protection and O-Arylation of Phenols" Nanomaterials 8, no. 4: 246. https://doi.org/10.3390/nano8040246






