An All-Solid-State Electrochromic Device Based on WO3–Nb2O5 Composite Films Prepared by Fast-Alternating Bipolar-Pulsed Reactive Magnetron Sputtering
Abstract
:1. Introduction
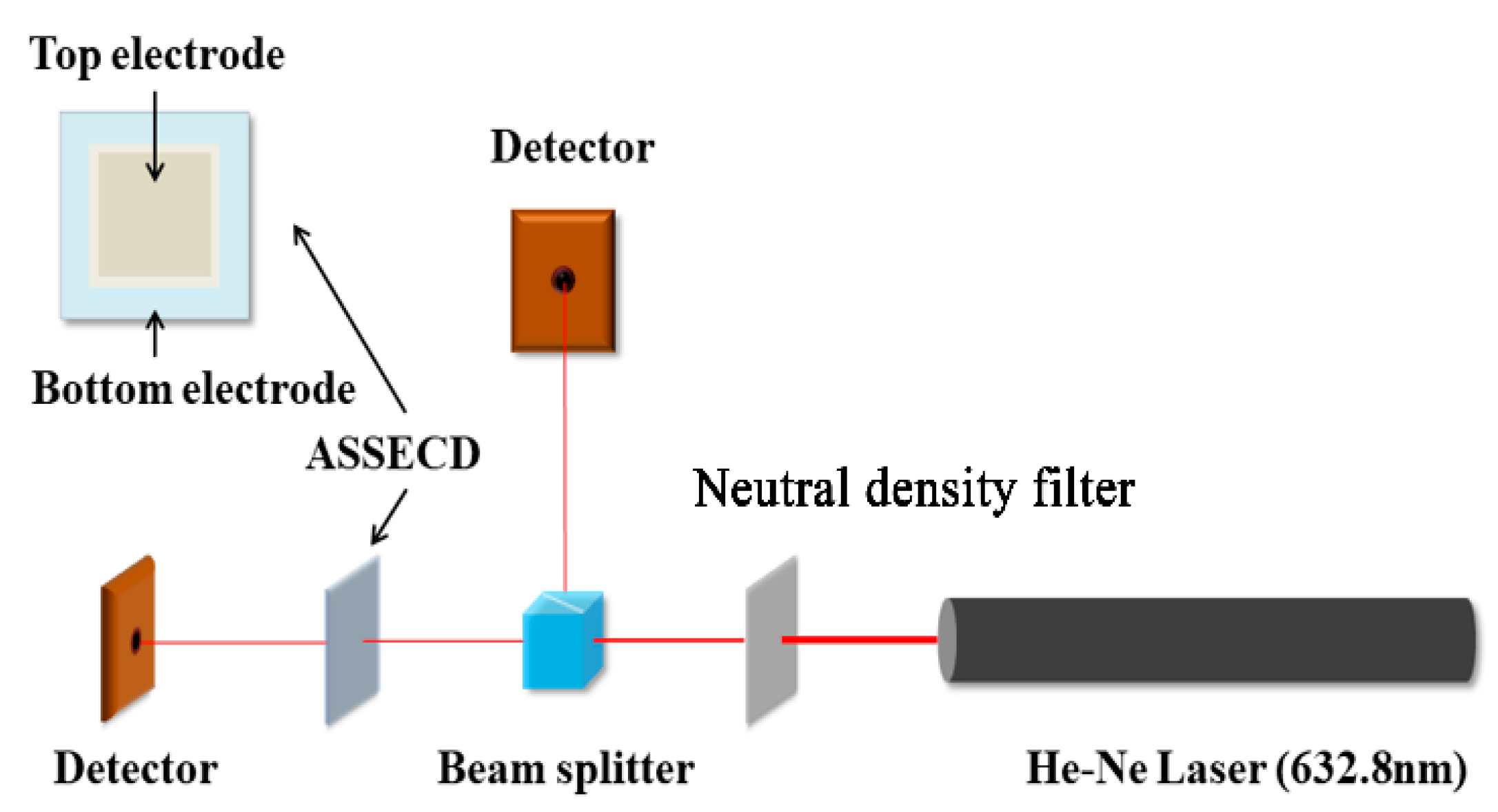
2. Experimental Procedures
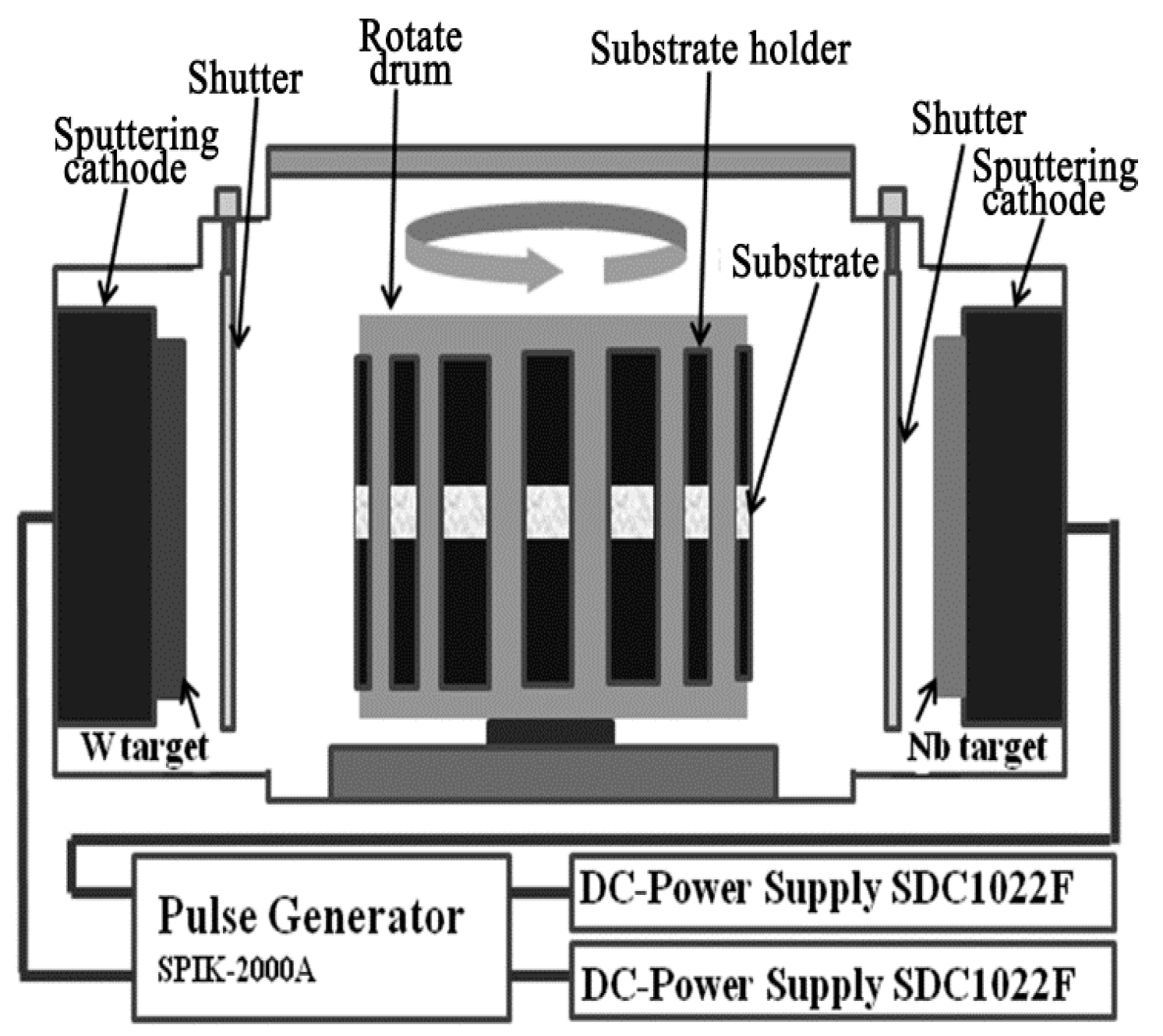
2.1. Film Deposition Process
2.2. Film Characterizations
3. Results and Discussion
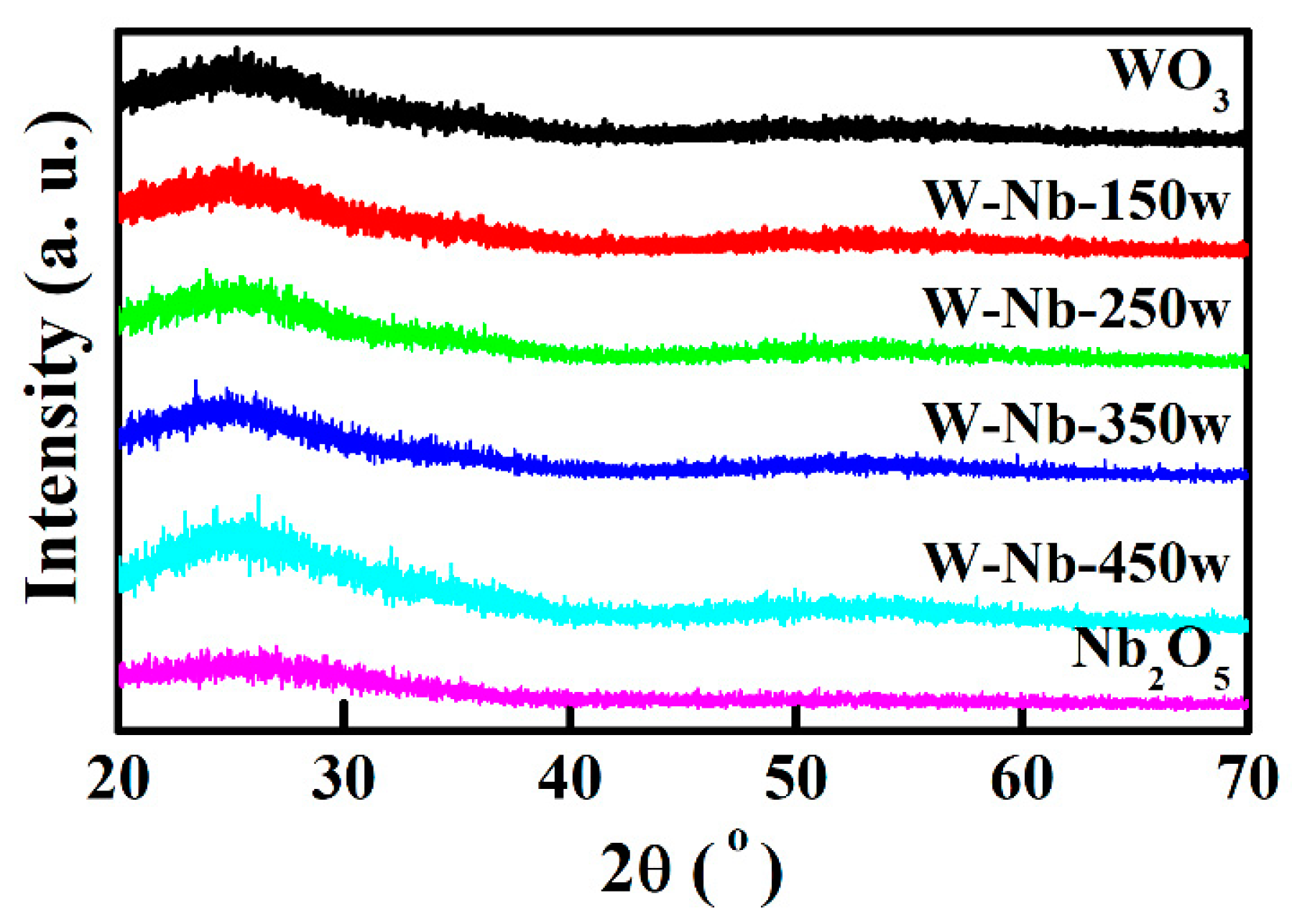
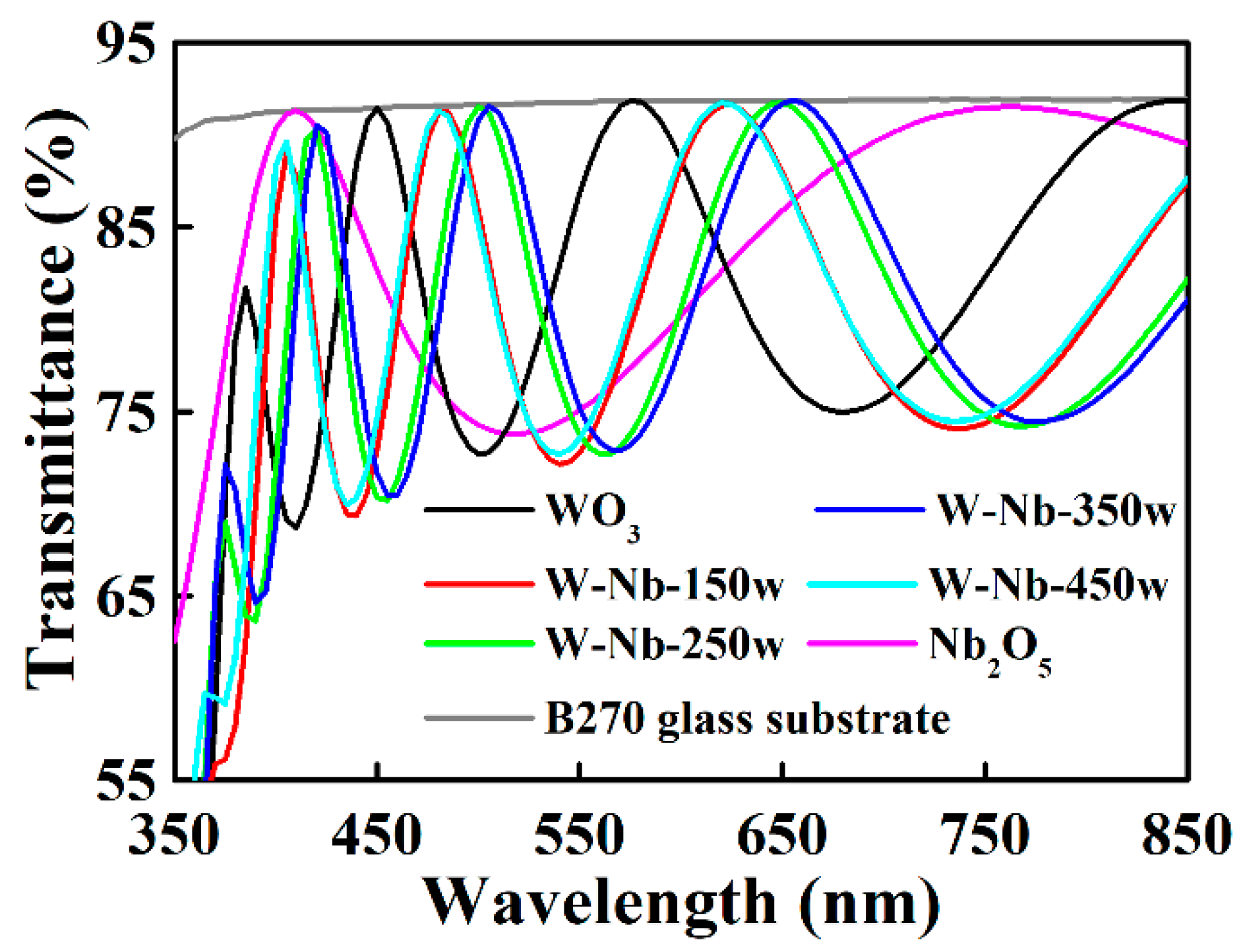
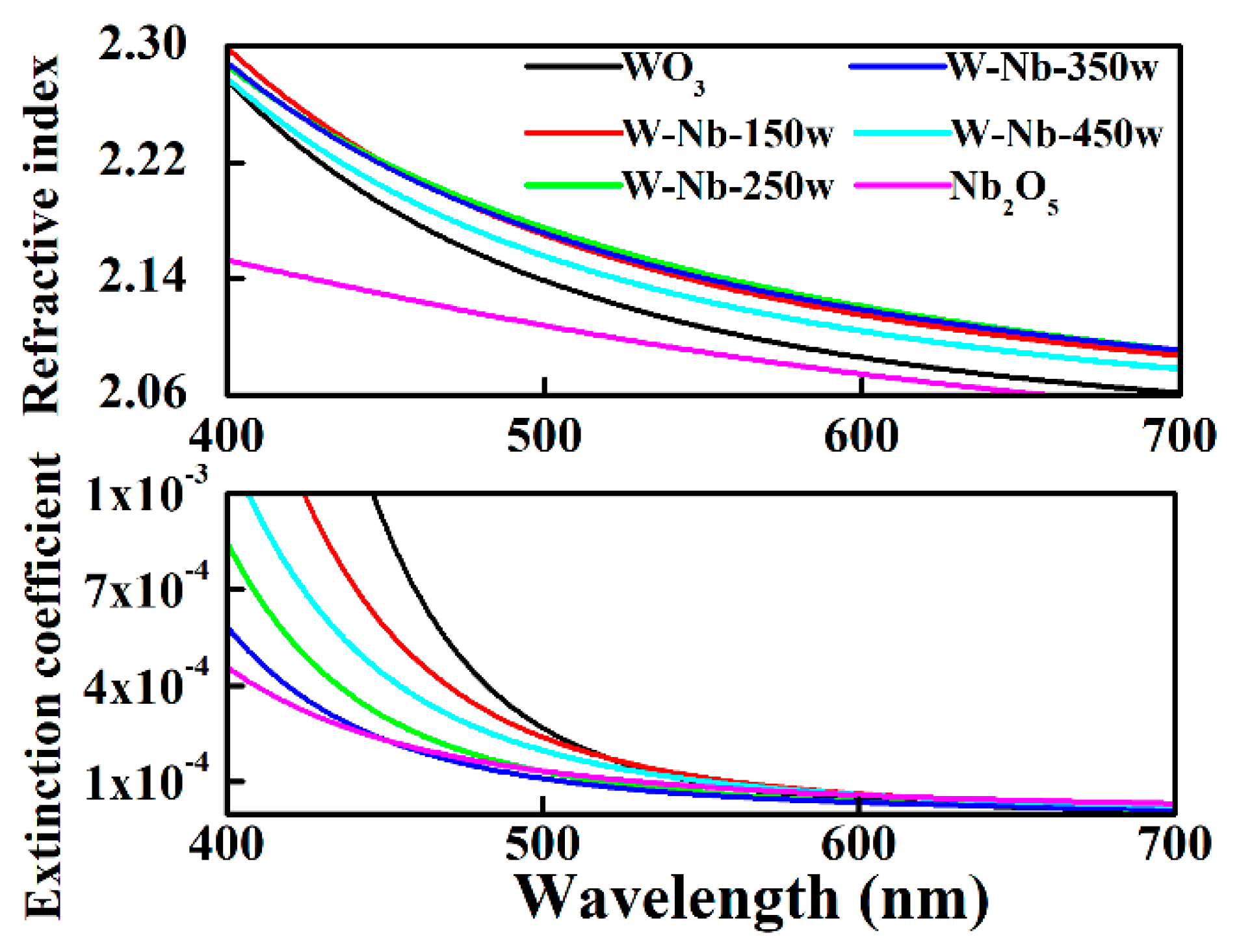
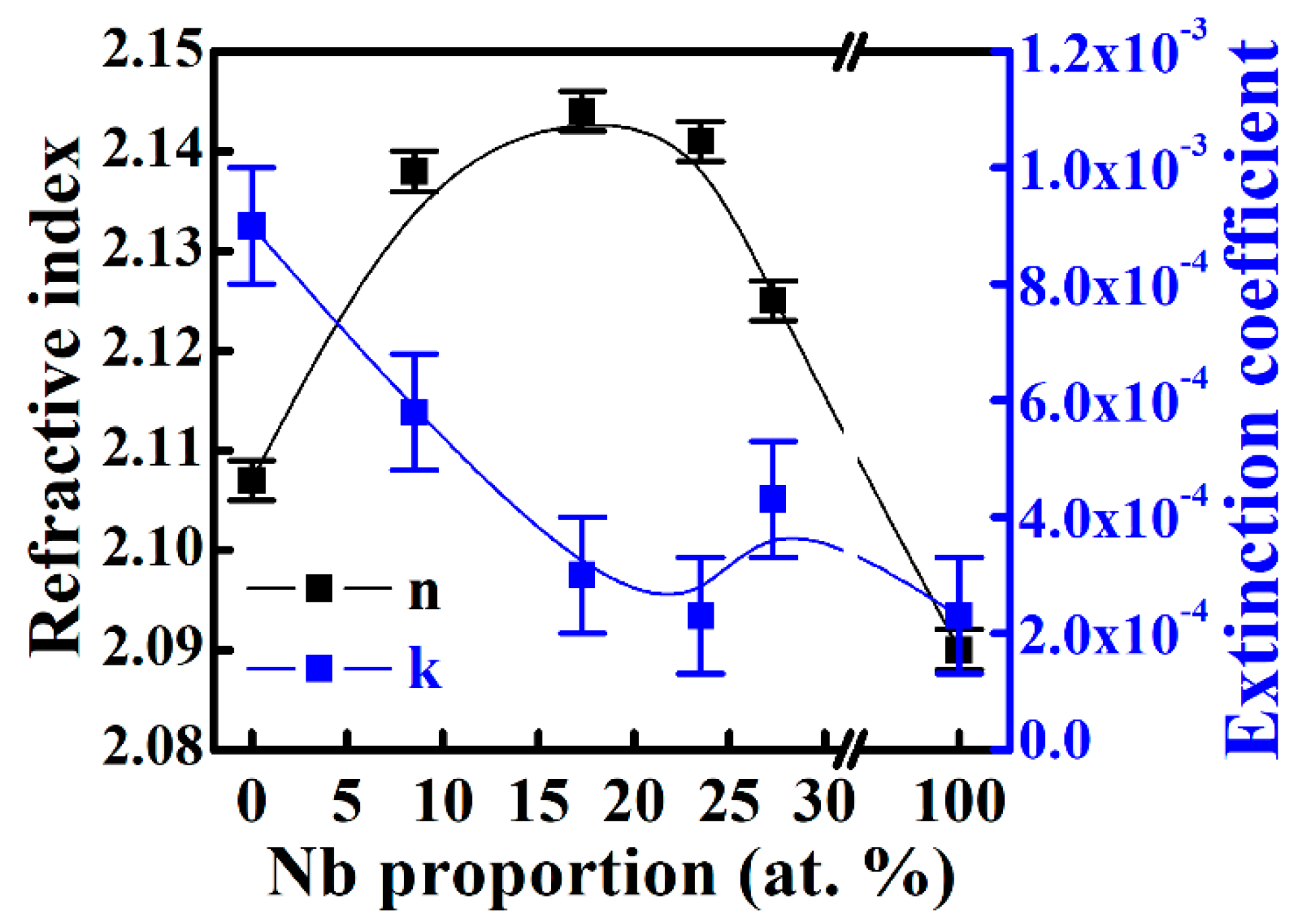
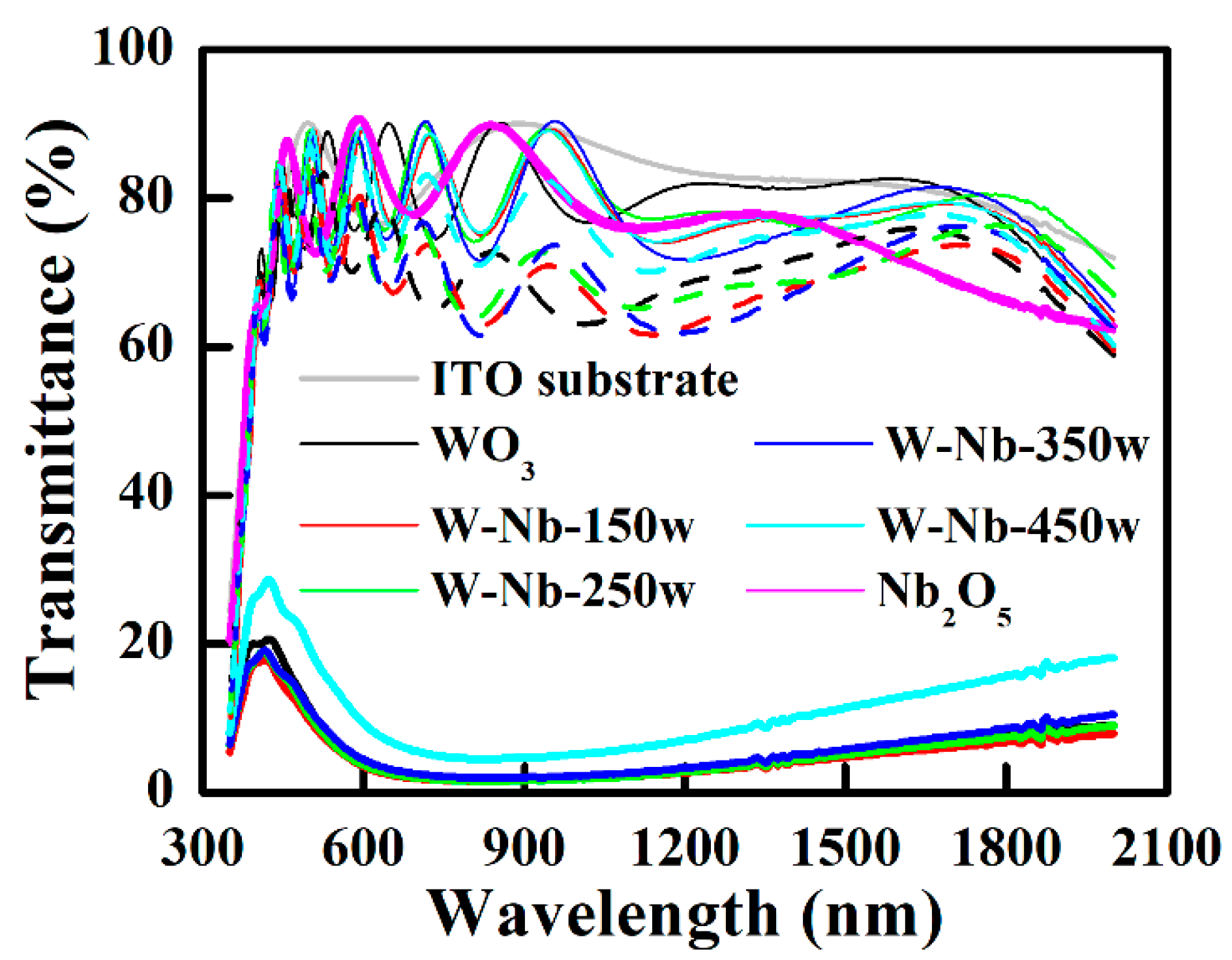
3.1. Electrochromic Films
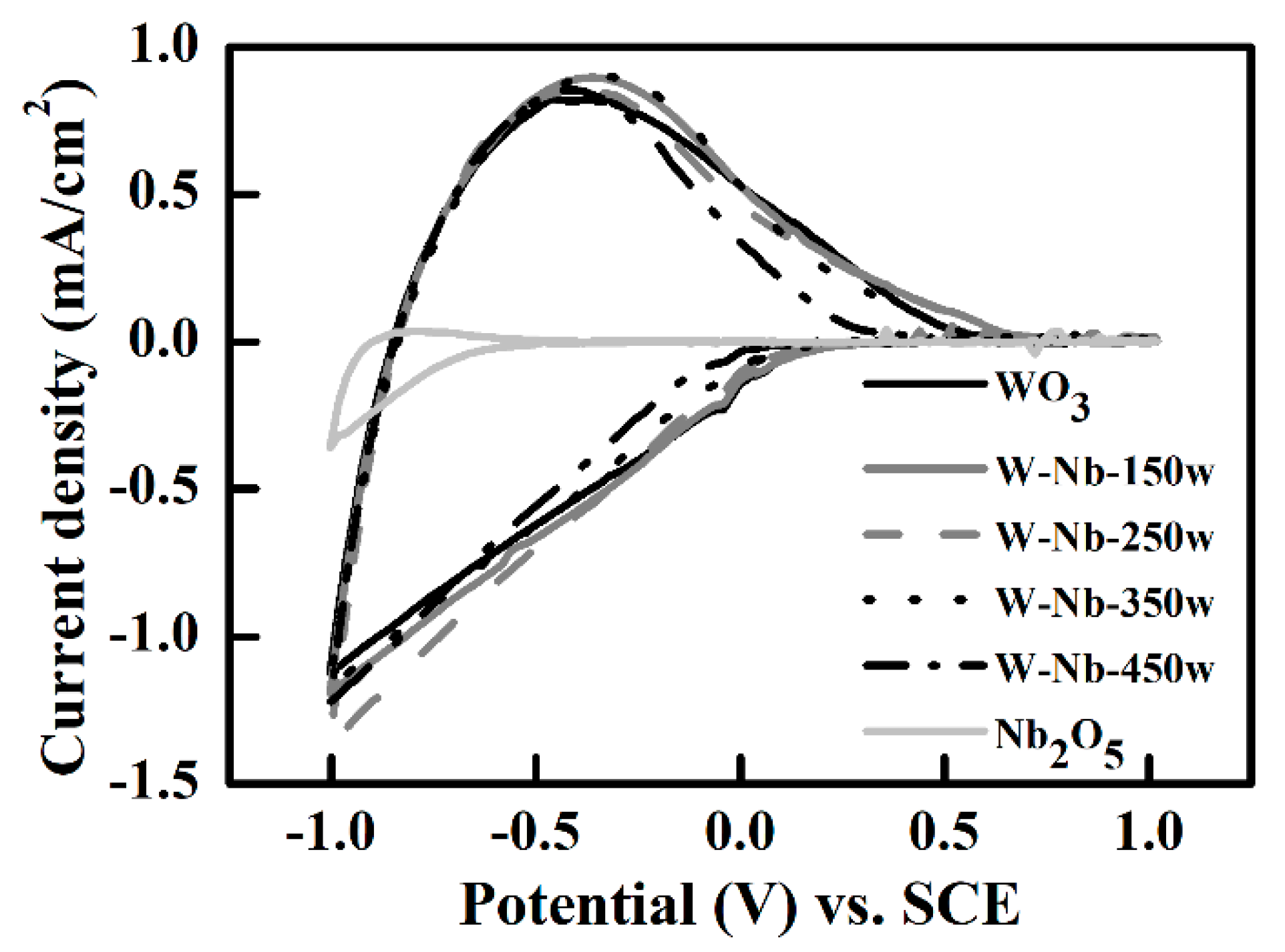
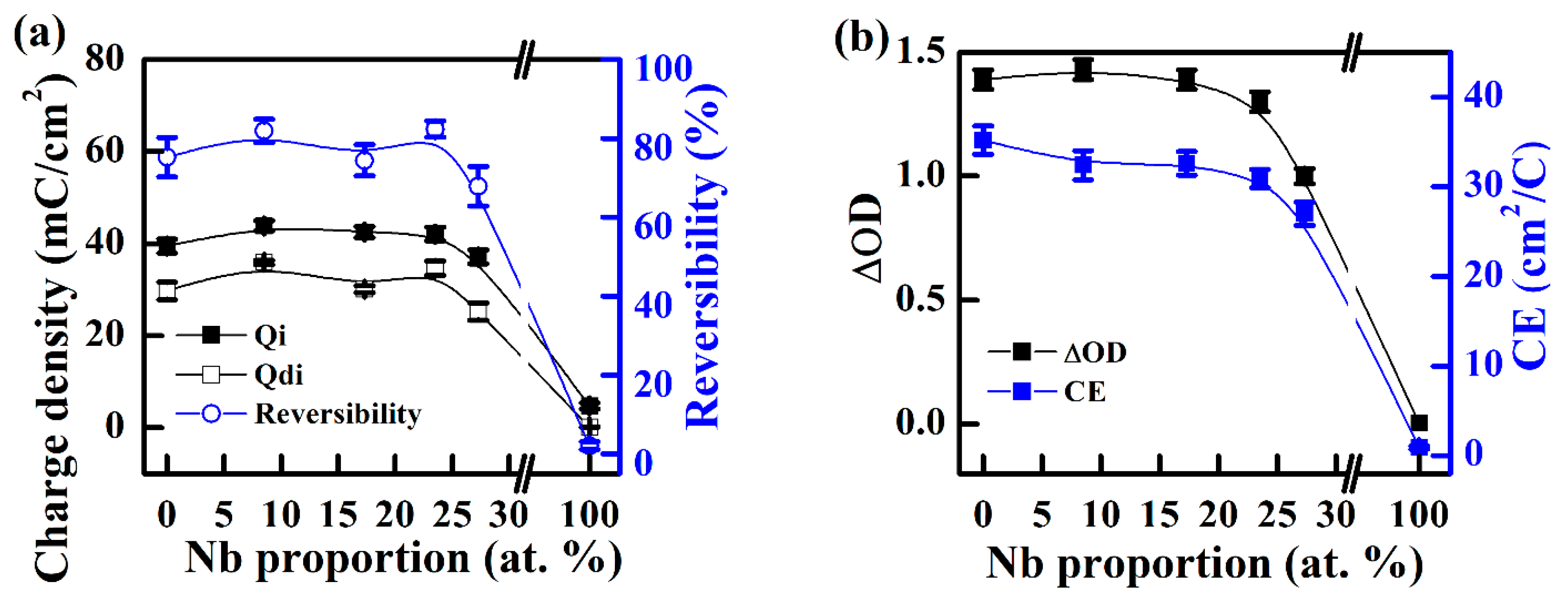
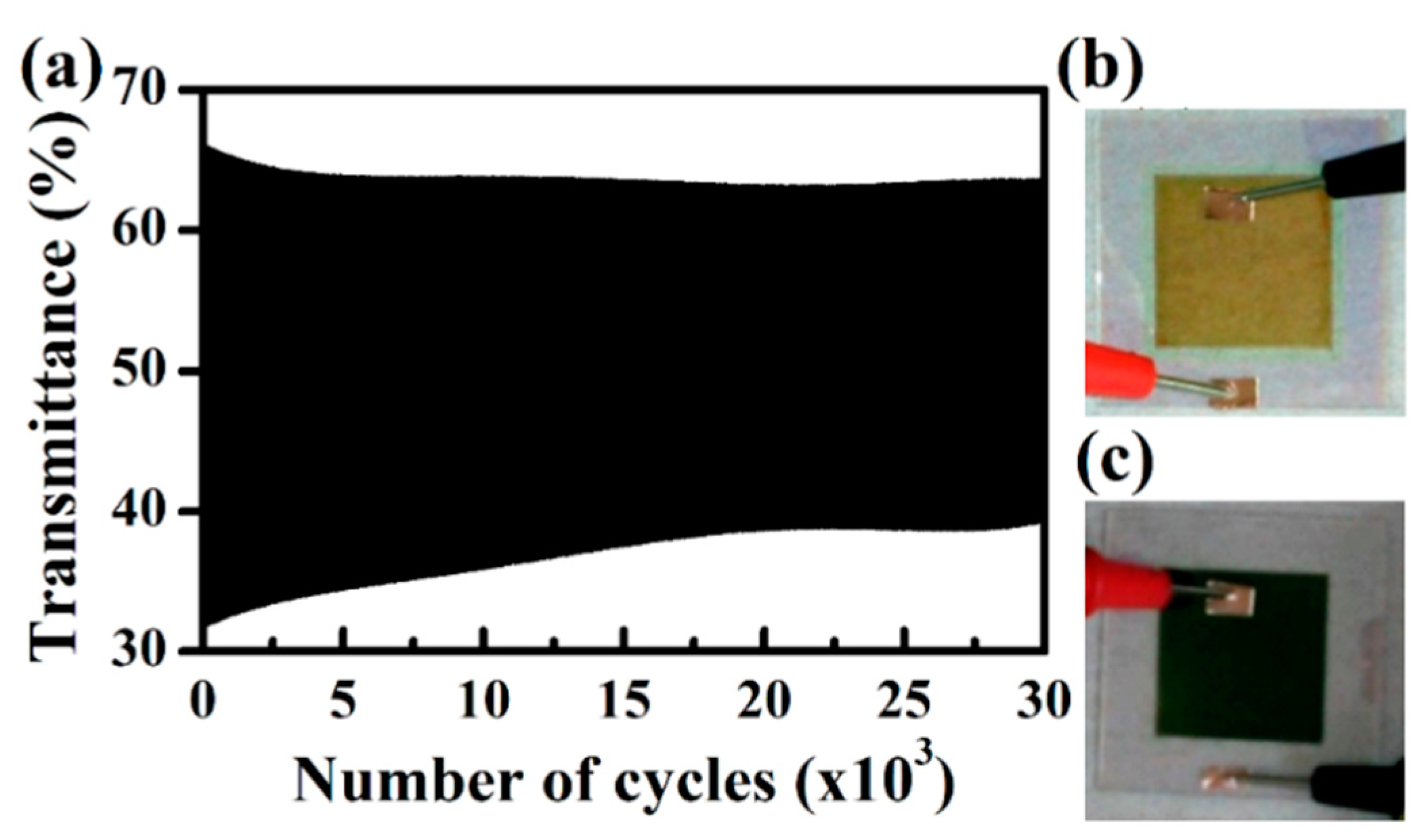
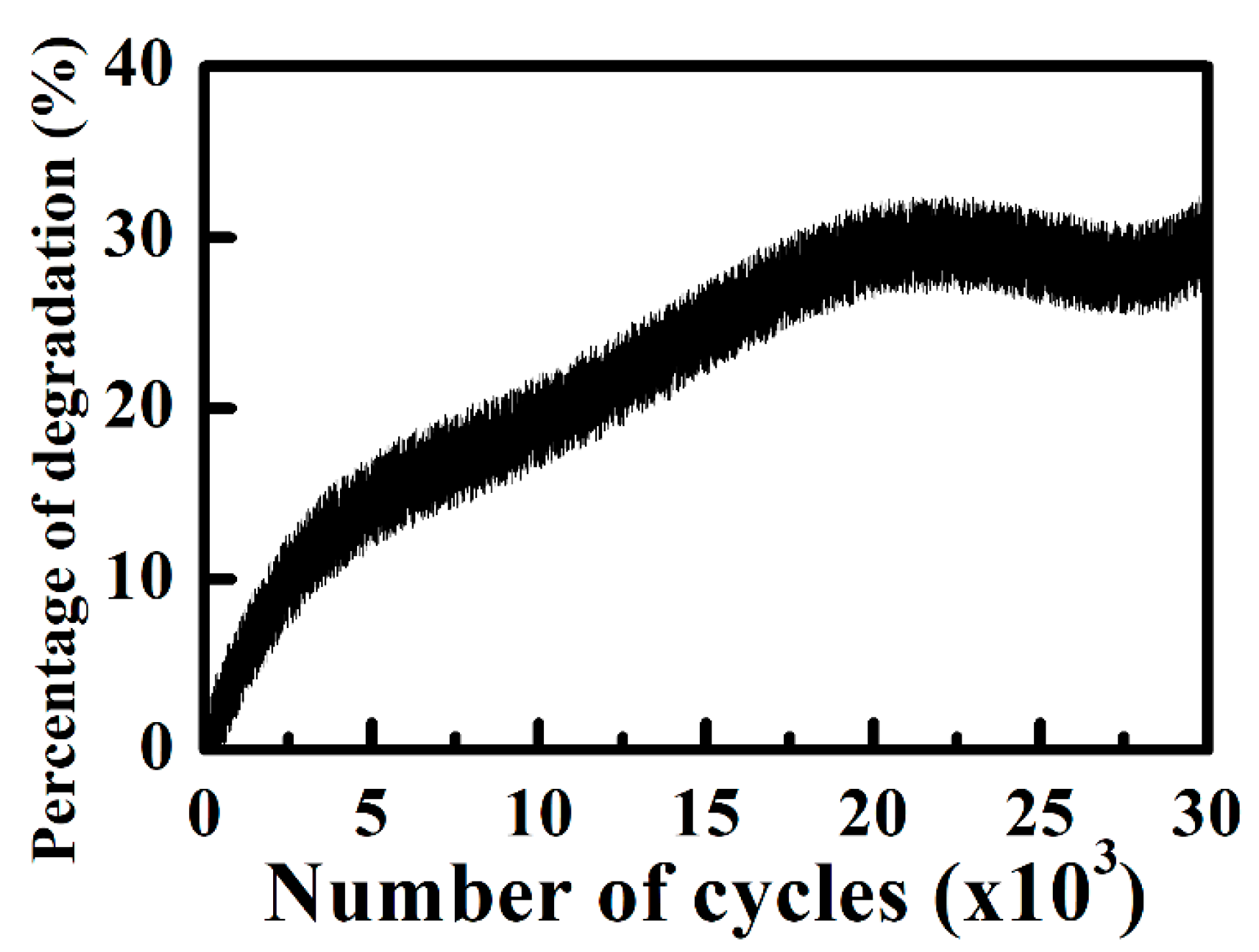
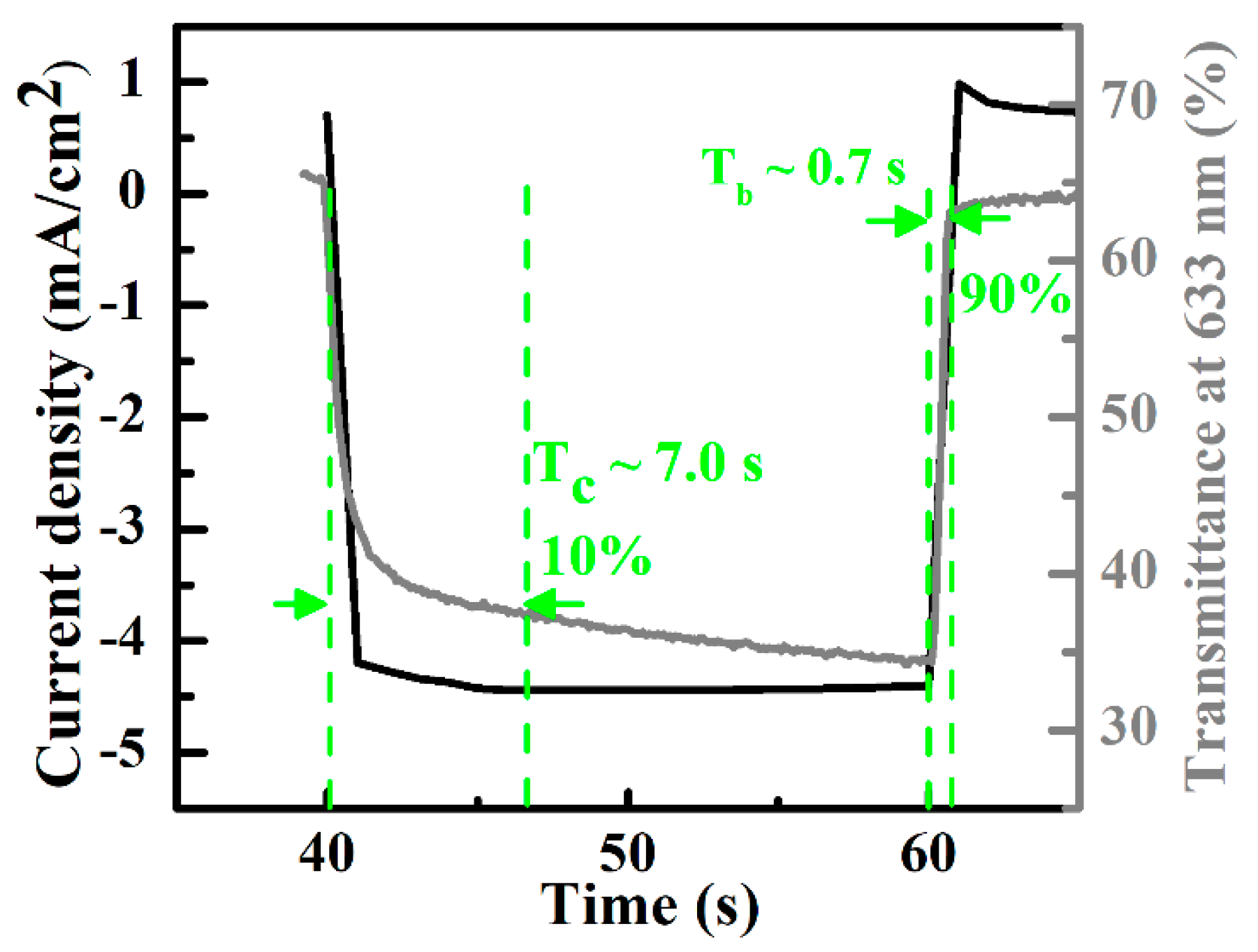
3.2. All-Solid-State Electrochromic Devices
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Granqvist, C.G.; Azens, A.; Isidorsson, J.; Kharrazi, M.; Kullman, L.; Lindstrtöm, T.; Niklasson, G.A.; Ribbing, C.G.; Rönnow, D.; Strømme Mattsson, M.; et al. Towards the smart window: Progress in electrochromics. J. Non-Cryst. Solids 1997, 218, 273–279. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromic tungsten oxide films: Review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 2000, 60, 201–262. [Google Scholar] [CrossRef]
- Granqvist, C.G. Oxide electrochromics: Why, how, and whither. Sol. Energy Mater. Sol. Cells 2008, 92, 203–208. [Google Scholar] [CrossRef]
- Papaefthimiou, S.; Syrrakou, E.; Yianoulis, P. An alternative approach for the energy and environmental rating of advanced glazing: An electrochromic window case study. Energy Build. 2009, 41, 17–26. [Google Scholar] [CrossRef]
- Vernardou, D.; Psifis, K.; Louloudakis, D.; Papadimitropoulos, G.; Davazoglou, D.; Katsarakis, N.; Koudoumas, E. Low pressure CVD of electrochromic WO3 at 400 °C. J. Electrochem. Soc. 2015, 162, H579–H582. [Google Scholar] [CrossRef]
- Louloudakis, D.; Vernardou, D.; Papadimitropoulos, G.; Davazoglou, D.; Koudoumas, E. The effect of growth time and oxygen on the properties of electrochromic WO3 thin layers grown by LPCVD. Adv. Mater. Lett. 2018, 9, 578–584. [Google Scholar] [CrossRef]
- Baucke, F.G. Electrochromic mirrors with variable reflectance. Sol. Energy Mater. 1987, 16, 67–77. [Google Scholar] [CrossRef]
- Bange, K.; Gambke, T. Electrochromic materials for optical switching devices. Adv. Mater. 1990, 2, 10–16. [Google Scholar] [CrossRef]
- Corr, D.; Bach, U.; Fay, D.; Kinsella, M.; McAtamney, C.; O’Reilly, F.; Rao, S.N.; Stobie, N. Coloured electrochromic “paper-quality” displays based on modified mesoporous electrodes. Solid State Ionics 2003, 165, 315–321. [Google Scholar] [CrossRef]
- Vlachopoulos, N.; Nissfolk, J.; Möller, M.; Briançon, A.; Corr, D.; Grave, C.; Leyland, N.; Mesmer, R.; Pichot, F.; Ryan, M.; et al. Electrochemical aspects of display technology based on nanostructured titanium dioxide with attached viologen chromophores. Electrochim. Acta 2008, 53, 4065–4071. [Google Scholar] [CrossRef]
- Argun, A.A.; Reynolds, J.R. Line patterning for flexible and laterally configured electrochromic devices. J. Mater. Chem. 2005, 15, 1793–1800. [Google Scholar] [CrossRef]
- Schlotter, P.; Pickelmann, L. The xerogel structure of thermally evaporated tungsten oxide layers. J. Electron. Mater. 1982, 11, 207–236. [Google Scholar] [CrossRef]
- Shiyanovskaya, I.V. Structure rearrangement and electrochromic properties of amorphous tungsten trioxide films. J. Non-Cryst. Solids 1995, 187, 420–424. [Google Scholar] [CrossRef]
- Tajima, K.; Hotta, H.; Yamada, Y.; Okada, M.; Yoshimura, K. Degradation studies of electrochromic all-solid-state switchable mirror glass under various constant temperature and relative humidity conditions. Sol. Energy Mater. Sol. Cells 2010, 94, 2411–2415. [Google Scholar] [CrossRef]
- Hashimoto, S.; Matsuoka, H. Lifetime of electrochromism of amorphous WO3-TiO2 thin films. J. Electrochem. Soc. 1991, 138, 2403–2408. [Google Scholar] [CrossRef]
- Ozer, N.; Lampert, C.M. Electrochromic performance of sol-gel deposited WO3-V2O5 films. Thin Solid Films 1999, 349, 205–211. [Google Scholar] [CrossRef]
- Da Costa, E.; Avellaneda, C.O.; Pawlicka, A. Alternative Nb2O5-TiO2 thin films for electrochromic devices. J. Mater. Sci. 2001, 36, 1407–1410. [Google Scholar] [CrossRef]
- Rougier, A.; Blyr, A.; Garcia, J.; Zhang, Q.; Impey, S.A. Electrochromic W–M–O (M = V, Nb) sol-gel thin films: A way to neutral colour. Sol. Energy Mater. Sol. Cells 2002, 71, 343–357. [Google Scholar] [CrossRef]
- Ivanova, T.; Gesheva, K.A.; Kalitzova, M.; Marsen, B.; Cole, B.; Miller, E.L. Electrochromic behavior of Mo/W oxides related to their surface morphology and intercalation process parameters. Mater. Sci. Eng. B 2007, 142, 126–134. [Google Scholar] [CrossRef]
- Arvizu, M.A.; Triana, C.A.; Stefanov, B.I.; Granqvust, C.G.; Niklasson, G.A. Electrochromism in sputtering-deposited W–Ti oxide films: Durability enhancement due to Ti. Sol. Energy Mater. Sol. Cells 2014, 125, 184–189. [Google Scholar] [CrossRef]
- Pehlivan, E.; Tepehan, F.Z.; Tepehan, G.G. Comparison of optical, structural and electrochromic properties of undoped and WO3-doped Nb2O5 thin films. Solid State Ionics 2003, 165, 105–110. [Google Scholar] [CrossRef]
- Bathe, S.R.; Patil, P.S. Influence of Nb doping on the electrochromic properties of WO3 films. J. Phys. D Appl. Phys. 2007, 40, 7423–7431. [Google Scholar] [CrossRef]
- Wang, C.K.; Sahu, D.; Wang, S.C.; Huang, J.L. Electrochromic Nb-doped WO3 films: Effects of post annealing. Ceram. Int. 2012, 38, 2829–2833. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Li, Q.; Luo, H. An all-solid-state inorganic electrochromic display of WO3 and NiO film with LiNbO3 ion conductor. IEEE Electron Device Lett. 2000, 21, 215–217. [Google Scholar] [CrossRef]
- Atak, G.; Coşkun, Ö. LiNbO3 thin film for all-solid-state electrochromic devices. Opt. Mater. 2018, 81, 160–167. [Google Scholar] [CrossRef]
- Coşkun, Ö.D.; Atak, G. The effects of lithiation process on the performance of all-solid-state electrochromic devices. Thin Solid Films 2018, 662, 13–20. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, G.; Xiao, Y.; Gao, F.; Wang, M.; Wang, Q.; Wang, S.; Zuo, H.; Diao, X. An all-thin-film inorganic electrochromic device monolithically fabricated on flexible PET/ITO substrate by magnetron sputtering. Mater. Lett. 2015, 142, 232–234. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, G.; Chen, Q.; Guo, J.; Xiao, Y.; Delplancke-Ogletree, M.P.; Reniers, F.; Diao, X. Charge-transfer kinetics and cyclic properties of inorganic all-solid-state electrochromic device with remarkably improve optical memory. Sol. Energy Mater. Sol. Cells 2018, 174, 545–553. [Google Scholar] [CrossRef]
- Tang, C.-J.; Ye, J.-M.; Yang, Y.-T.; He, J.-L. Large-area flexible monolithic ITO/WO3/Nb2O5/NiVOx/ITO electrochromic devices prepared by using magnetron sputter deposition. Opt. Mater. 2016, 55, 83–89. [Google Scholar] [CrossRef]
- Manifacier, J.C.; Gasiot, J.; Fillard, J.P. A simple method for the determination of the optical constants n, k and the thickness of the weakly absorbing thin film. J. Phys. E Sci. Instrum. 1976, 9, 1002. [Google Scholar] [CrossRef]
- Özkan, E.; Tepehan, F.Z. Optical and structural characteristics of sol-gel-deposited tungsten oxide and vanadium-doped tungsten oxide films. Sol. Energy Mater. Sol. Cells 2001, 68, 265–277. [Google Scholar] [CrossRef]
- Washizu, E.; Yamamoto, A.; Abe, Y.; Kawamura, M.; Sasaki, K. Optical and electrochromic properties of RF reactively sputtered WO3 films. Solid State Ionics 2003, 165, 175–180. [Google Scholar] [CrossRef]
- Subrahmanyam, A.; Karuppasamy, A. Optical and electrochromic properties of oxygen sputtered tungsten oxide (WO3) thin films. Sol. Energy Mater. Sol. Cells 2007, 91, 266–274. [Google Scholar] [CrossRef]
- Karuppasamy, A.; Subrahmanyam, A. Studies on electrochromic smart windows based on titanium doped WO3 thin films. Thin Solid Films 2007, 516, 175–178. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Cao, H. Effects of film density on electrochromic tungsten oxide thin films deposited by reactive dc-pulsed magnetron sputtering. J. Alloy. Compd. 2010, 504, S418–S421. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Berggren, L.; Larsson, A.L. Electrochromic tungsten oxide: The role of defects. Sol. Energy Mater. Sol. Cells 2004, 84, 315–328. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Kim, Y.S.; Jang, B.U.; Im, H.; Jung, W.; Kim, D.Y.; Kim, H. Effects of oxygen stoichiometry on electrochromic properties in amorphous tungsten oxide films. Thin Solid Films 2012, 520, 5367–5371. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Hu, X. Structure, morphological and electrochromic properties of Nb2O5 films deposited by reactive sputtering. Sol. Energy Mater. Sol. Cells 2003, 77, 155–162. [Google Scholar] [CrossRef]
- Bathe, S.R.; Patil, P.S. Electrochromic characteristics of fibrous reticulated WO3 thin films prepared by pulsed spray pyrolysis technique. Sol. Energy Mater. Sol. Cells 2007, 91, 1097–1101. [Google Scholar] [CrossRef]
- Ye, J.-M.; Lin, Y.-P.; Yang, Y.-T.; Chang, J.-T.; He, J.-L. Electrochromic properties of Ni(V)Ox films deposited via reactive magnetron sputtering with a 8V–92Ni alloy target. Thin Solid Films 2010, 519, 1578–1582. [Google Scholar] [CrossRef]





| Samples | P (mtorr) | fAr (sccm) | fO₂ (sccm) | Psp (W) | Vdischarge (V) | d (nm) | |
|---|---|---|---|---|---|---|---|
| WO3 | 10 | 50 | PID | (DC) | 250 | 550 | 411 |
| W-Nb-150w | 10 | 50 | PID | (Bipolar) | W 250 | W 636 | 442 |
| Nb 150 | Nb 522 | ||||||
| W-Nb-250w | 10 | 50 | PID | (Bipolar) | W 250 | W 620 | 462 |
| Nb 250 | Nb 565 | ||||||
| W-Nb-350w | 10 | 50 | PID | (Bipolar) | W 250 | W 625 | 467 |
| Nb 350 | Nb 588 | ||||||
| W-Nb-450w | 10 | 50 | PID | (Bipolar) | W 250 | W 610 | 443 |
| Nb 450 | Nb 625 | ||||||
| Nb2O5 | 10 | 50 | PID | (DC) | 500 | 580 | 186 |
| ITO | 3 | 40 | 1 | (DC) | 300 | 355 | 230 |
| Thin Film | P (mtorr) | fAr (sccm) | fO₂ (sccm) | Psp (W) | Vdischarge (V) | d (nm) | |
|---|---|---|---|---|---|---|---|
| ITO | 3 | 40 | 1 | (DC) | 300 | 355 | 230 |
| W-Nb-350w | 10 | 50 | PID | (Bipolar) | W 250 | W 625 | 350 |
| Nb 350 | Nb 588 | ||||||
| Nb2O5 | 10 | 50 | PID | (DC) | 500 | 580 | 230 |
| NiVOx | 10 | 125 | 6 | (DC) | 500 | 400 | 200 |
| ITO | 3 | 40 | 1 | (DC) | 300 | 355 | 230 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.-J.; He, J.-L.; Jaing, C.-C.; Liang, C.-J.; Chou, C.-H.; Han, C.-Y.; Tien, C.-L. An All-Solid-State Electrochromic Device Based on WO3–Nb2O5 Composite Films Prepared by Fast-Alternating Bipolar-Pulsed Reactive Magnetron Sputtering. Coatings 2019, 9, 9. https://doi.org/10.3390/coatings9010009
Tang C-J, He J-L, Jaing C-C, Liang C-J, Chou C-H, Han C-Y, Tien C-L. An All-Solid-State Electrochromic Device Based on WO3–Nb2O5 Composite Films Prepared by Fast-Alternating Bipolar-Pulsed Reactive Magnetron Sputtering. Coatings. 2019; 9(1):9. https://doi.org/10.3390/coatings9010009
Chicago/Turabian StyleTang, Chien-Jen, Ju-Liang He, Cheng-Chung Jaing, Chen-Jui Liang, Ching-Hung Chou, Chien-Yuan Han, and Chuen-Lin Tien. 2019. "An All-Solid-State Electrochromic Device Based on WO3–Nb2O5 Composite Films Prepared by Fast-Alternating Bipolar-Pulsed Reactive Magnetron Sputtering" Coatings 9, no. 1: 9. https://doi.org/10.3390/coatings9010009








