Thiourea Derivative Metal Complexes: Spectroscopic, Anti-Microbial Evaluation, ADMET, Toxicity, and Molecular Docking Studies
Abstract
:1. Introduction
2. Results and Discussion
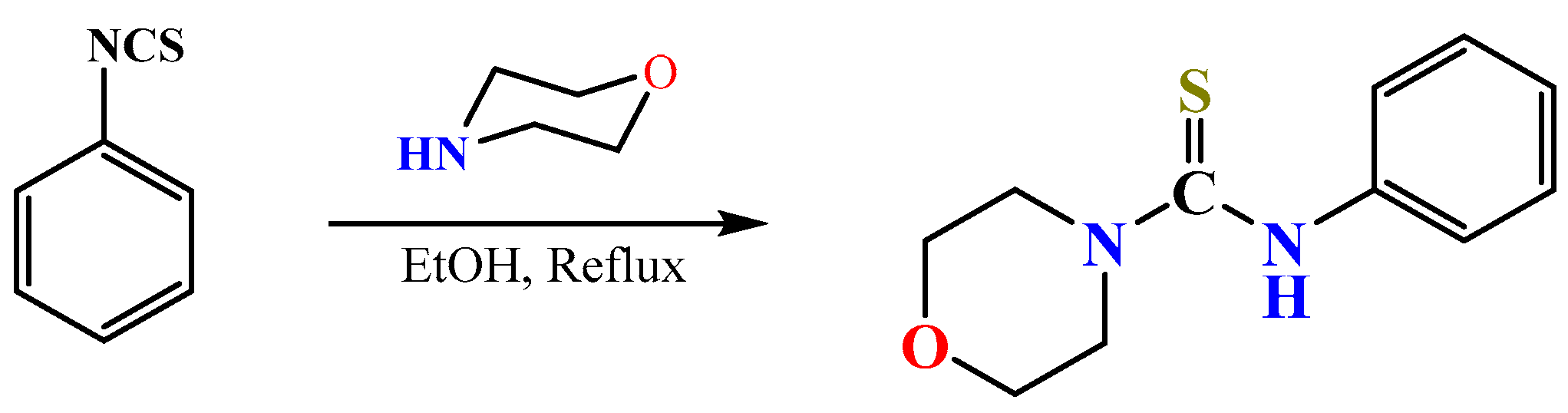
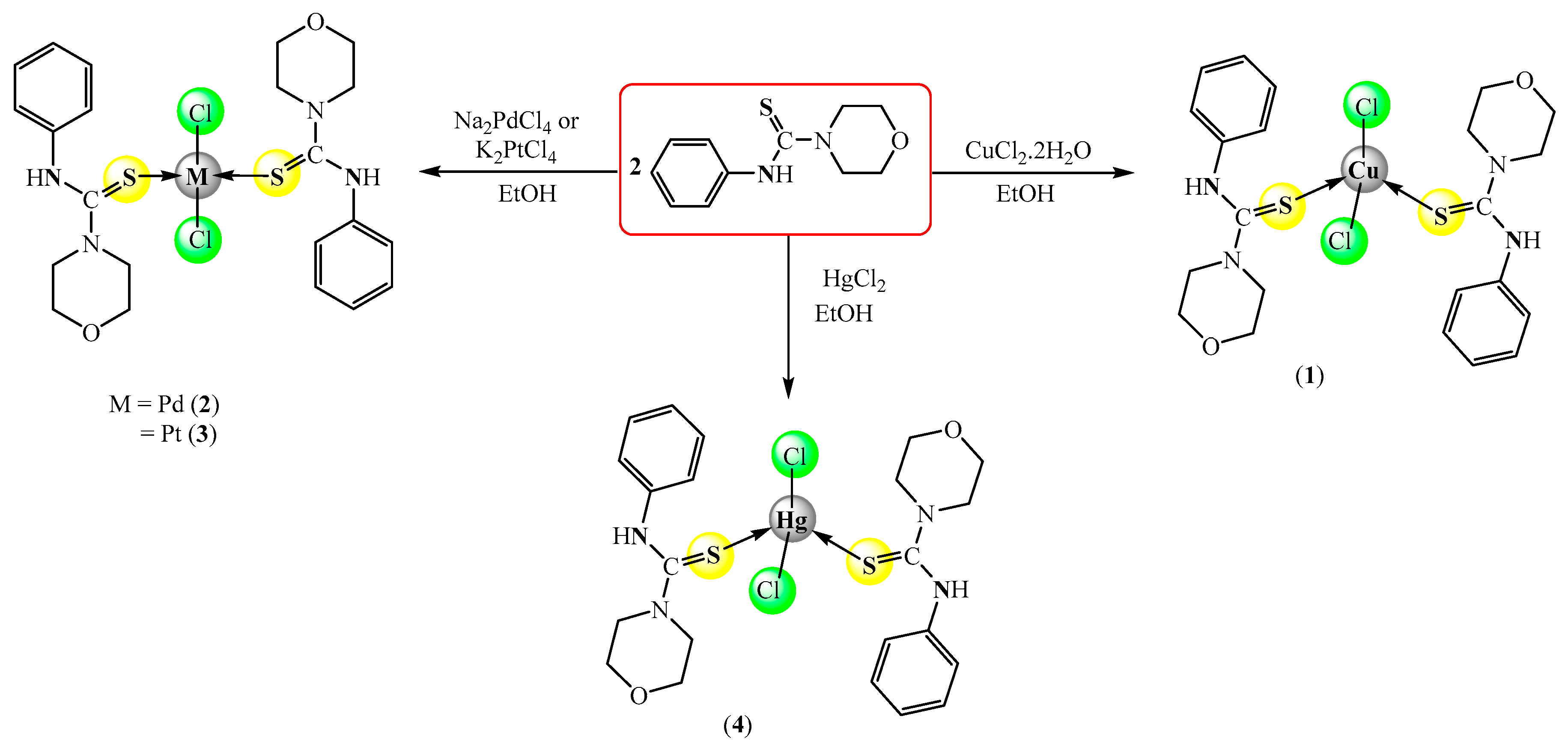
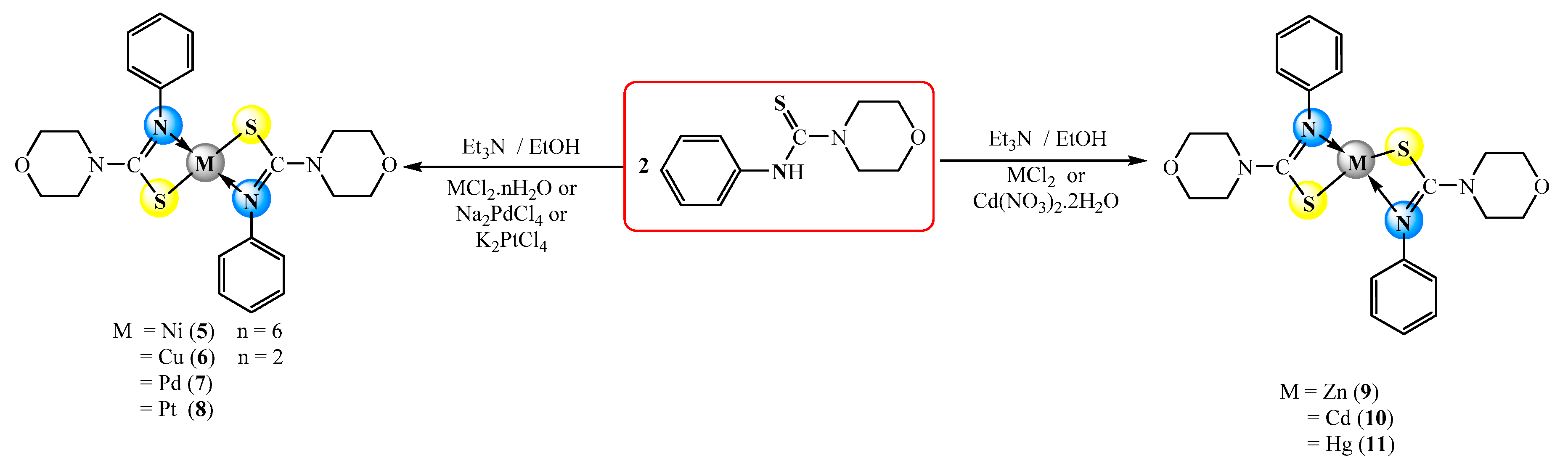
2.1. Synthesis
2.2. Spectroscopic Data
IR Spectra
2.3. NMR Spectra
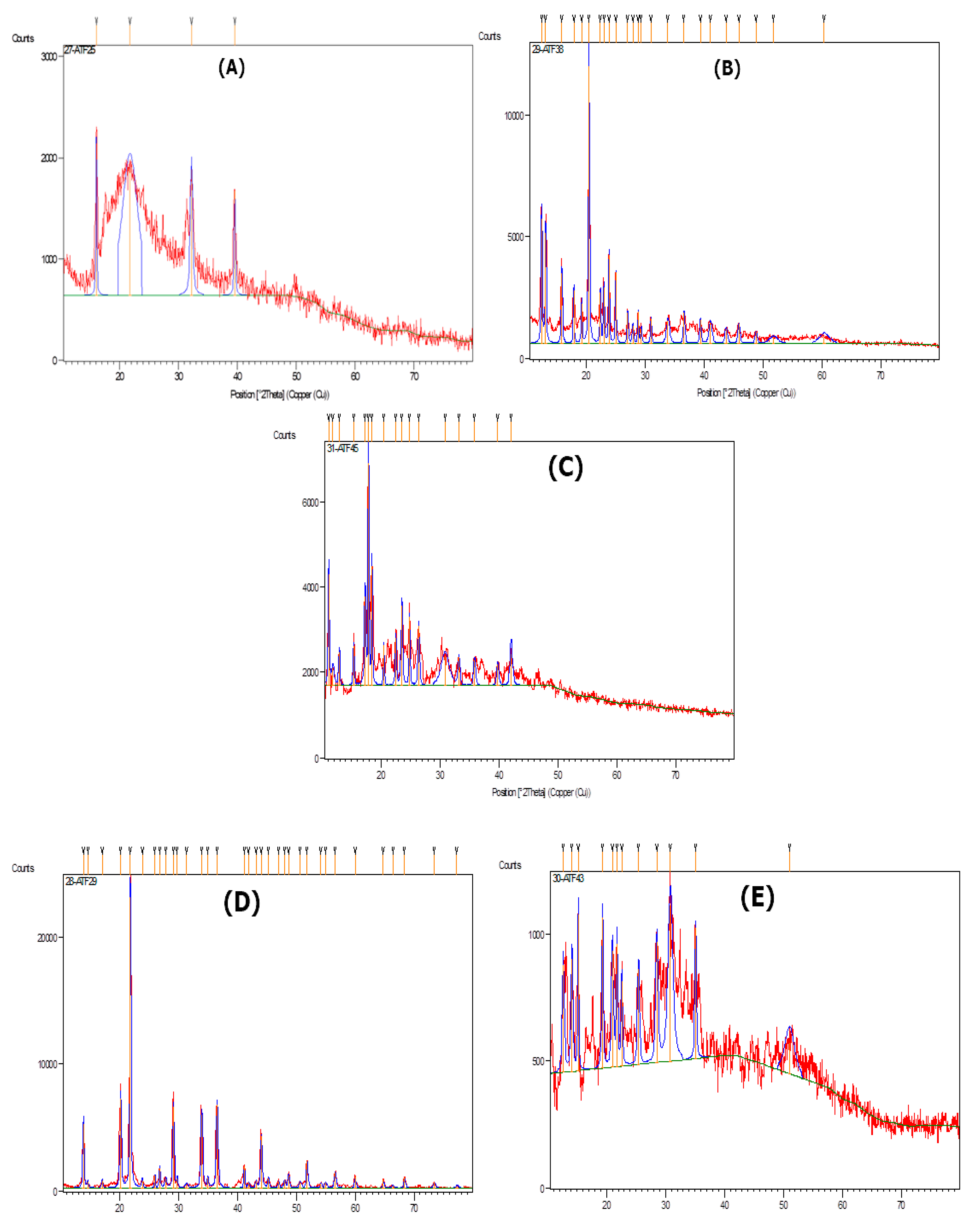
2.4. XRD and SEM Data
2.5. Biological Studies
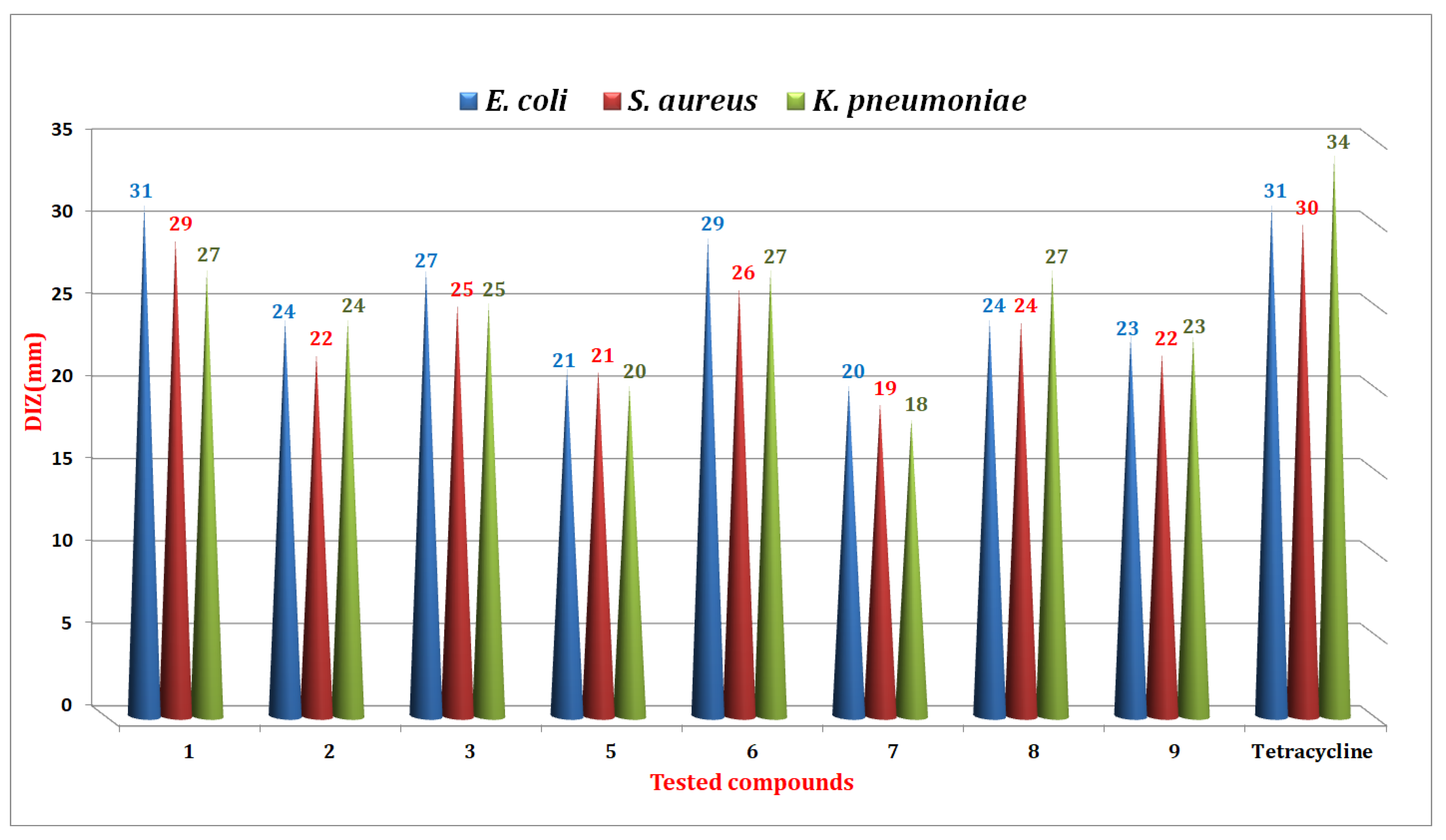
2.5.1. Antibacterial Activity
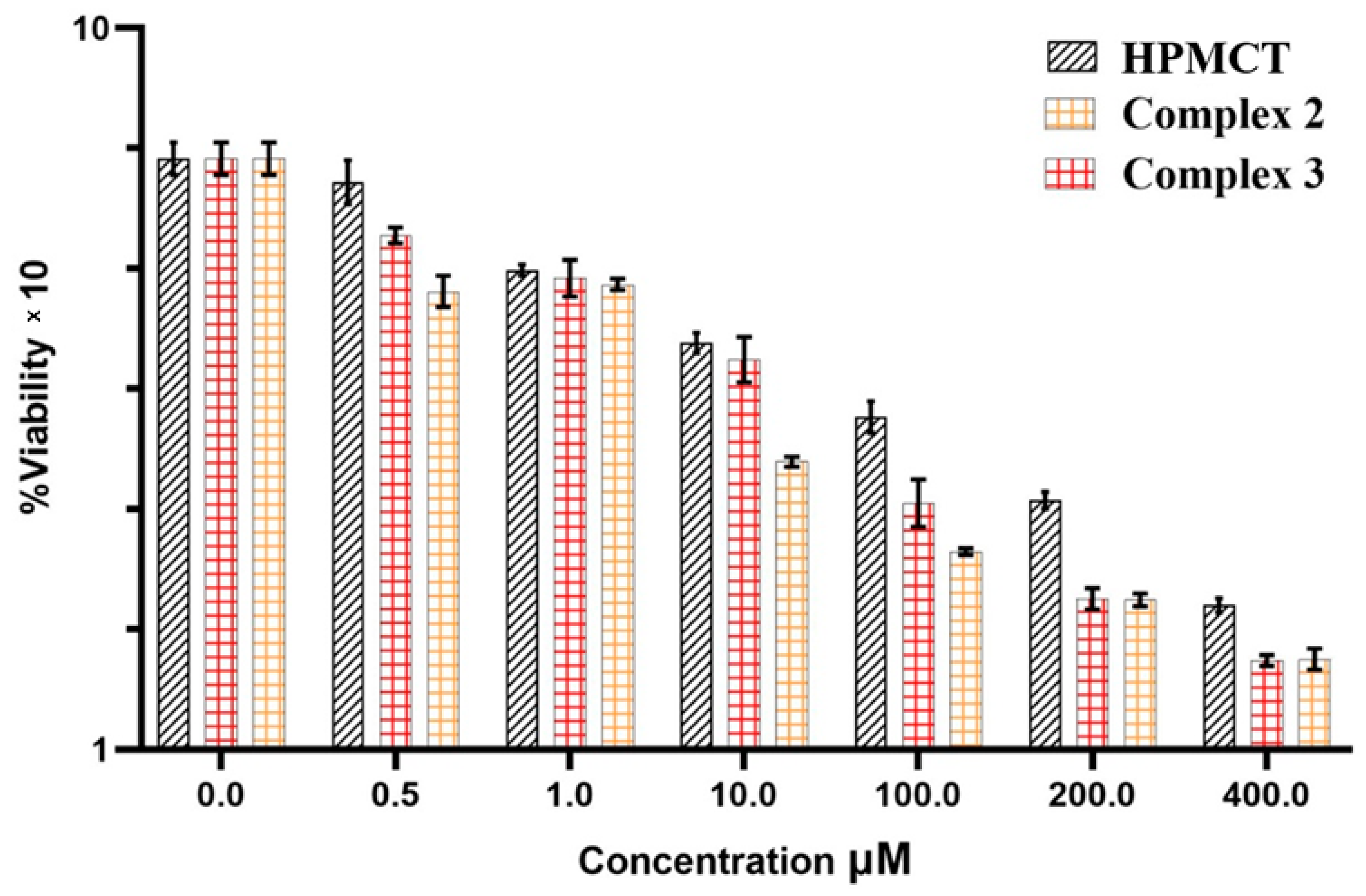
2.5.2. Anti-Cancer Activity
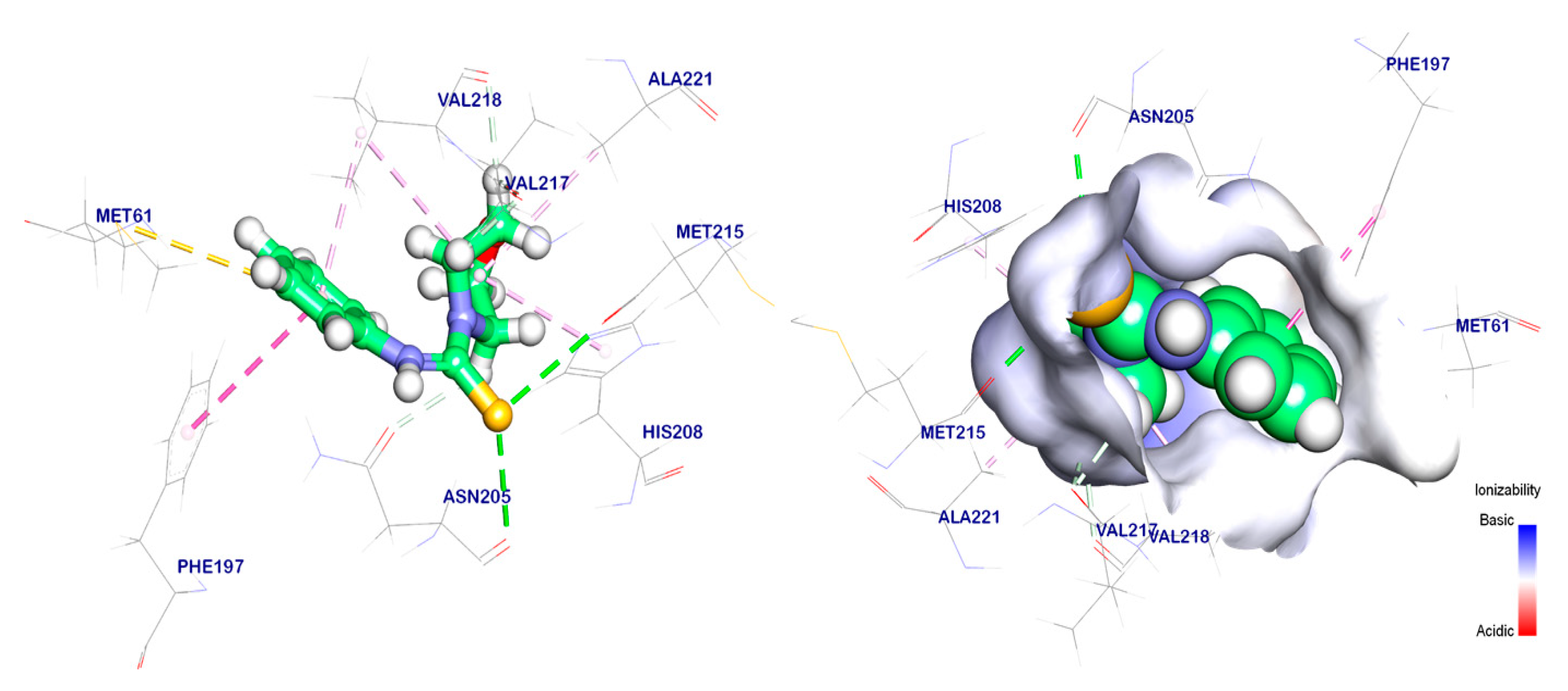
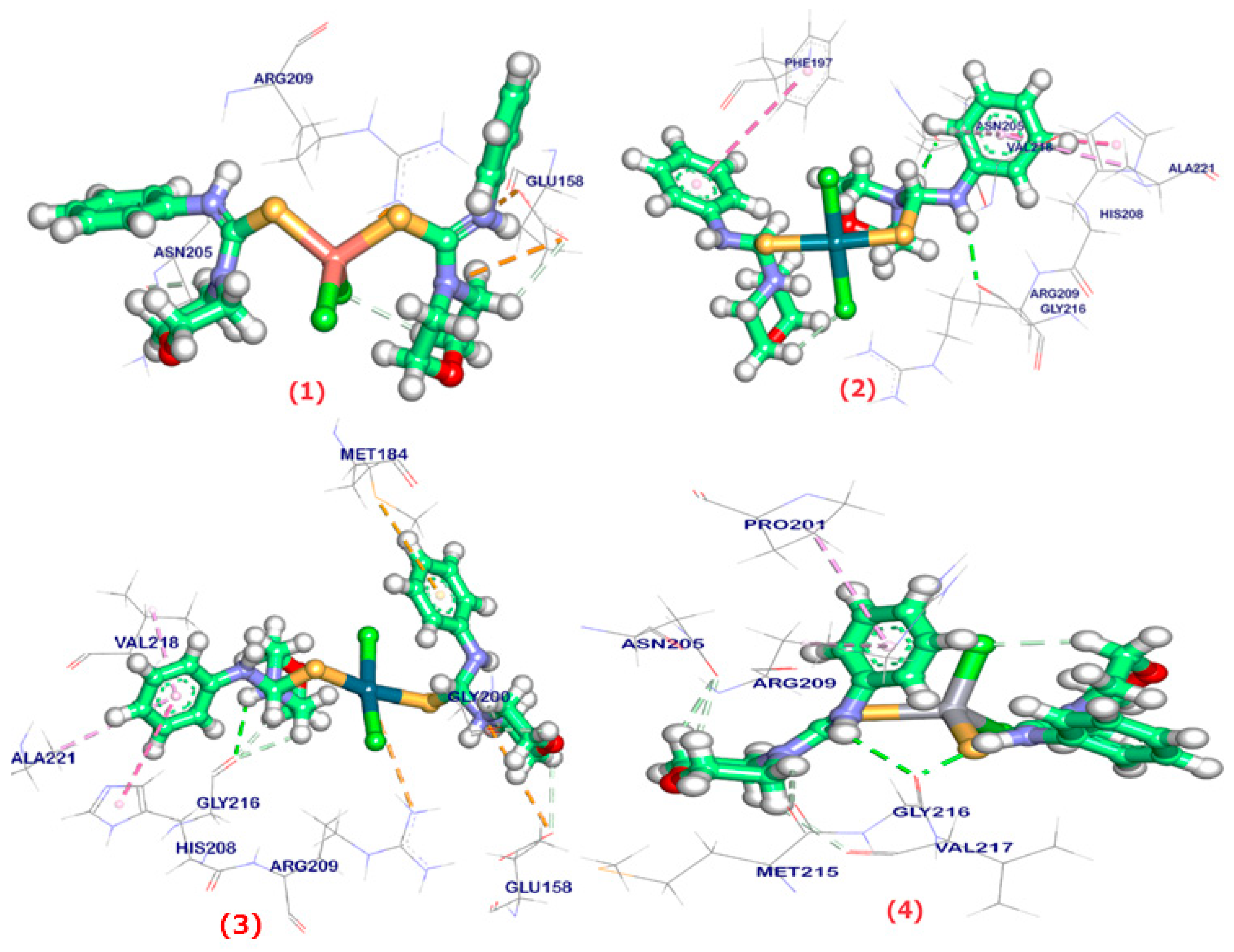
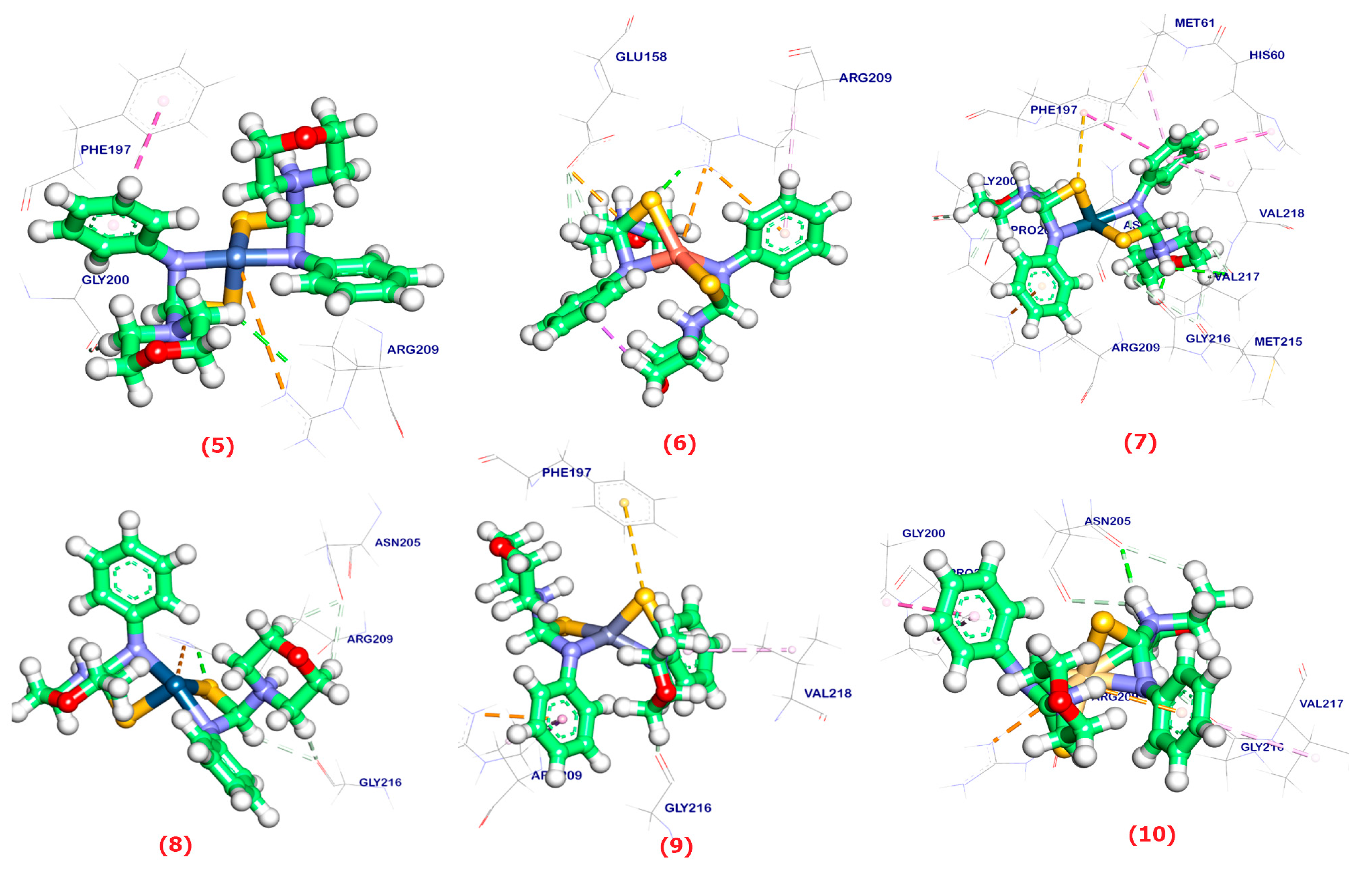
2.6. Molecular Docking
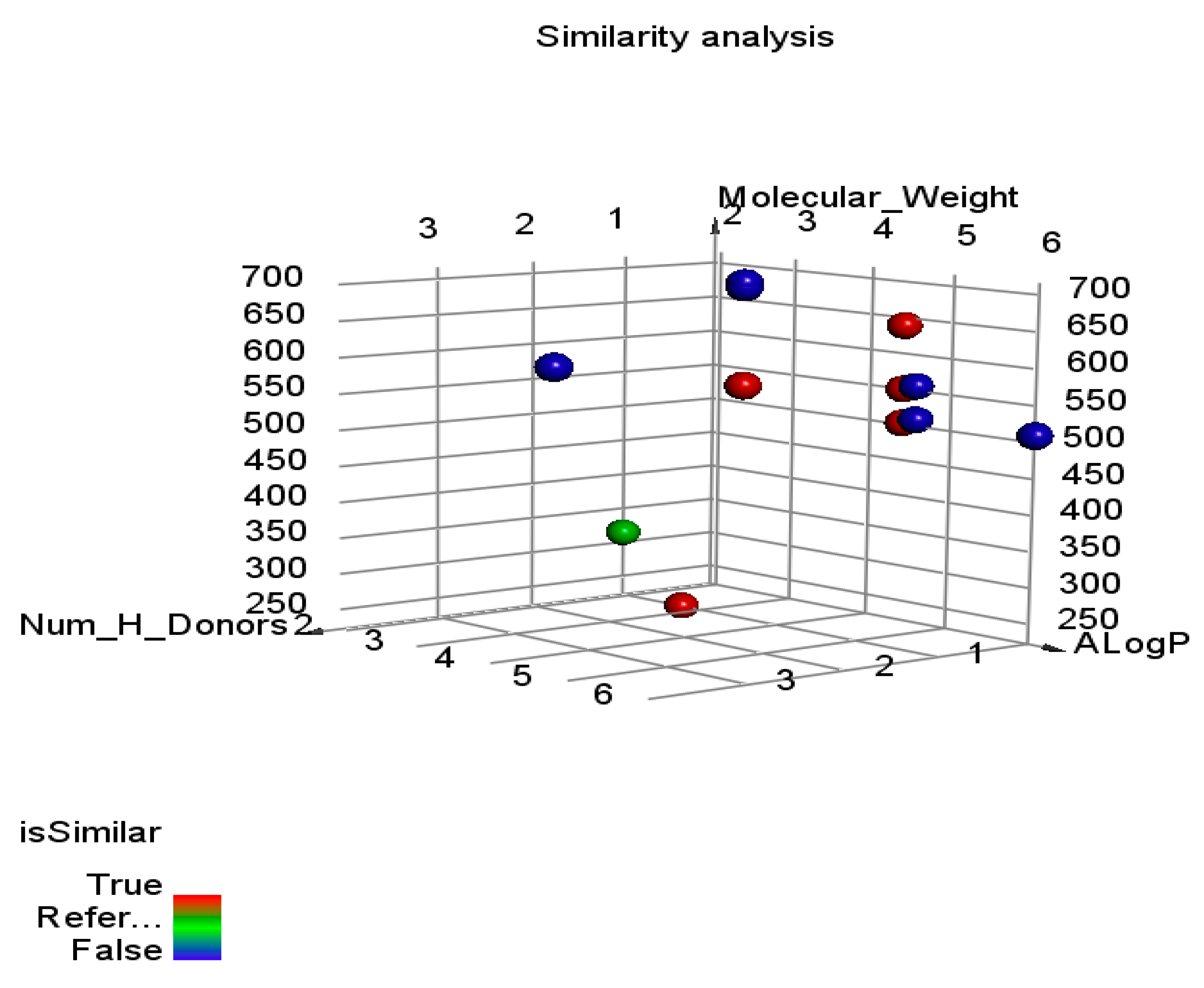
Molecular Similarity
2.7. ADMET Studies
2.8. Toxic Effective
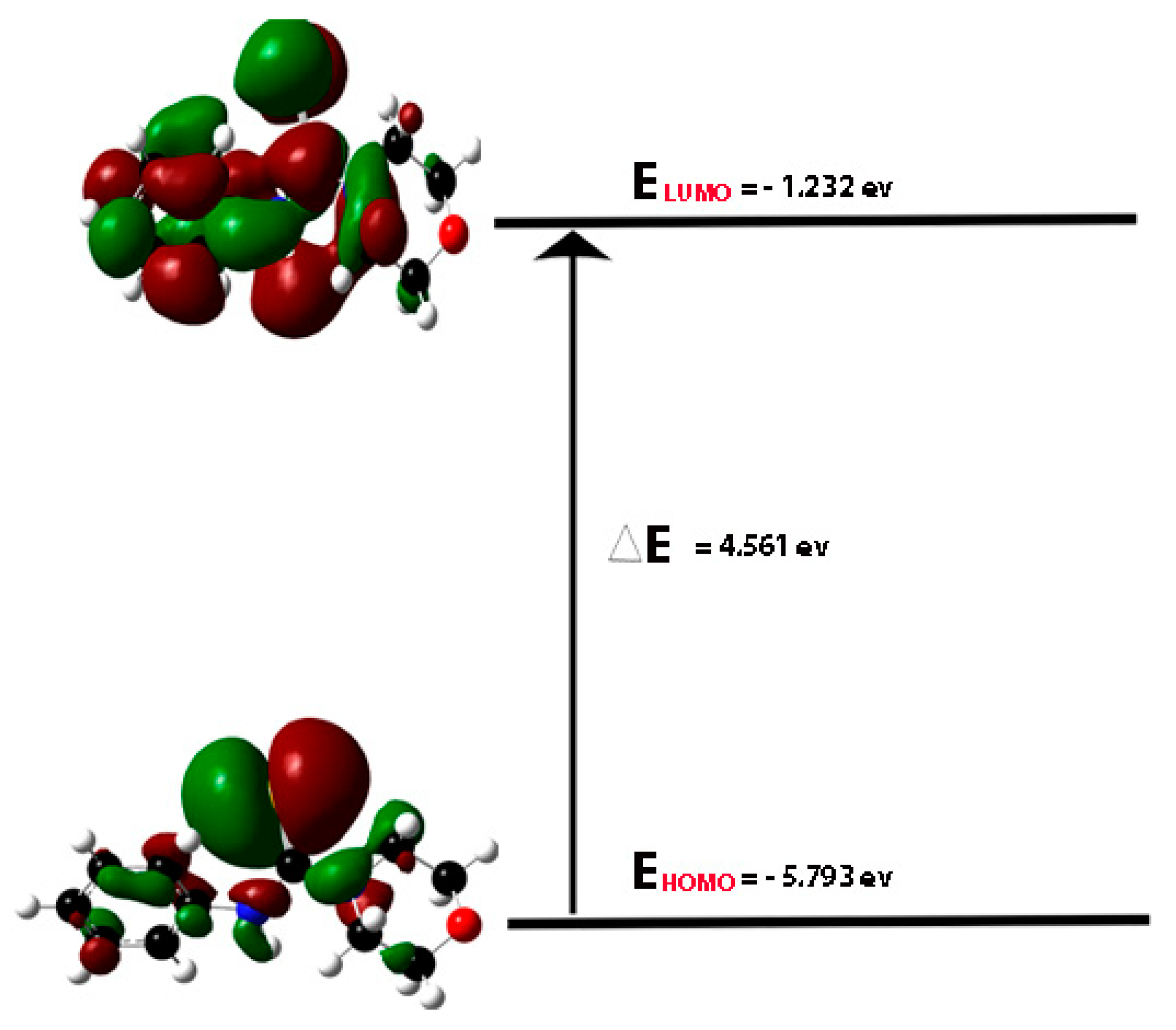
2.9. DFT Data Analysis
2.9.1. Geometry Optimization of HPMCT
2.9.2. Vibration Analysis
3. Experimental Part
3.1. Instrumental and Materials
3.2. Preparation of N-Phenylmorpholine-4-Carbothioamide (HPMCT)
3.3. Synthesis of Complexes
3.3.1. General Procedure for the Synthesis of [MCl2(κ1S-HPMCT)2] (1–4)
3.3.2. Synthesis of [Ni(PMCT)2] (5–11)
3.4. Antibacterial Studies
3.5. Anti-Cancer Studies
Cell Culture Conditions
3.6. Molecular Docking
3.6.1. Preparation of Targeted Proteins
3.6.2. Preparation of Tested Ligands and Receptor
3.7. DFT Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faihan, A.S.; Al-Jibori, S.A.; Hatshan, M.R.; Al-Janabi, A.S. Antibacterial, spectroscopic and X-ray crystallography of newly prepared heterocyclic thiourea dianion platinum (II) complexes with tertiary phosphine ligands. Polyhedron 2022, 212, 115602. [Google Scholar] [CrossRef]
- Faihan, A.S.; Al-Jibori, S.A.; Al-Janabi, A.S. Novel base-free dianion complexes of Pt (II) and Pd (II) derived from heterocyclic thiourea and tertiary phosphine ligands. J. Mol. Struct. 2022, 1251, 131966. [Google Scholar] [CrossRef]
- Bharati, P.; Bharti, A.; Maiti, M.K.B.; Butcher, R.J.; Singh, N.K. Square planar Ni(II) complexes of pyridine-4-carbonyl-hydrazine carbodithioate, 1-phenyl-3-pyridin-2-yl-isothiourea and 4-(2-methoxyphenyl)piperazine-1-carbodithioate involving N–S bonding: An approach to DFT calculation and thermal studies. Polyhderon 2013, 63, 156–166. [Google Scholar] [CrossRef]
- Al-Jibori, S.A.; Dayaaf, N.A.; Mohammed, M.Y.; Merzweiler, K.; Wagner, C.; Hogarth, G.; Richmond, M.G. cis–trans Isomerism at Square-Planar MN2S2 Centers (M = Pd, Pt): Crystal Structures of N-Phenyl-N-(2-thiazoyl)thiourea Complexes trans-Pd(S2N3C10H8)2 and cis-Pt(S2N3C10H8)2 and Density Functional Calculations. J. Chem. Crystallogr. 2013, 43, 365–372. [Google Scholar] [CrossRef]
- Yousef, T.A.; Alduaij, O.K.; El-Reash, G.M.A.; El Morshedy, R.M. Semiempirical studies, spectral analysis, in vitro antibacterial and DNA degradation studies of heterocyclic thiosemicarbazone ligand and its metal complexes. J. Mol. Liq. 2016, 222, 762–776. [Google Scholar] [CrossRef]
- Yousef, T.A.; El-Reash, G.M.A. Synthesis, and biological evaluation of complexes based on thiosemicarbazone ligand. J. Mol. Struct. 2020, 1201, 127180. [Google Scholar] [CrossRef]
- Al-Hayaly, L.J.; Abdullah, B.H.; Aldulaimi, A.A.N.; Al-Jibori, S.A. Palladium(II) and Platinum(II) complexes containing the mixed ligands N-phenyl-N-(2-pyridyl or 2-methylpyridyl) thiourea and diphosphines Ph2P(CH2)nPPh2 (n = 1–4). Orient. J. Chem. 2008, 24, 381–388. [Google Scholar]
- Fun, H.K.; Razak, I.A.; Pakawatchai, C.; Khaokong, C.; Chantrapromma, S.; Sajthong, S. Tris(N,N′-diethylthiourea-S)iodocopper(I) and Tris(N,N′-diethylthiourea-S)iodosilver(I). Acta Cryst. C 1998, 54, 453–456. [Google Scholar] [CrossRef]
- Henderson, W.; Nicholson, B.K.; Dinger, M.B.; Bennett, R.L. Thiourea monoanion and dianion complexes of rhodium(III) and ruthenium(II). Inorg. Chim. Acta 2002, 338, 210. [Google Scholar] [CrossRef]
- Bodensieck, U.; Carraux, Y.; Stoeckli-Evans, H.; Suss-Fink, G. Tris(N,N′-diphenylthioureato)-chromium(III). Inorg. Chem. Acta 1992, 195, 135–137. [Google Scholar] [CrossRef]
- Kitagawa, S.; Munakata, M.; Shimono, H.; Matsuyama, S. Synthesis and crystal structure of hexanuclear copper(I) complexes of µ3-pyridine-2-thionate. Dalton Trans. 1990, 2105. [Google Scholar] [CrossRef]
- Kodomari, M.; Suzuki, M.; Tanigawa, K.; Aoyama, T. A convenient and efficient method for the synthesis of mono- and N,N-disubstituted thioureas. Tetrahedron Lett. 2005, 46, 5841–5843. [Google Scholar] [CrossRef]
- Faihan, A.S.; Hatshan, M.R.; Alqahtani, A.S.; Nasr, F.A.; Al-Jibori, S.A.; Al-Janabi, A.S. New divalent metal ion complexes with 1,8-diaminonapthalene-2-thione: Synthesis, Spectroscopic, anti-bacterial and anticancer activity studies. J. Mol. Struct. 2022, 1247, 131291. [Google Scholar] [CrossRef]
- Veale, E.B.; Tocci, G.M.; Gunnlaugsson, T. Demonstration of bidirectional photoinduced electron transfer (PET) sensing in 4-amino-1,8-naphthalimide based thiourea anion sensors. Org. Biomol Chem. 2009, 7, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Gopiraman, M.; Selvakumaran, N.; Kesavan, D.; Kim, I.S.; Karvembu, R. Chemical and Physical Interactions of 1-Benzoyl-3,3-Disubstituted Thiourea Derivatives on Mild Steel Surface: Corrosion Inhibition in Acidic Media. Ind. Eng. Chem. Res. 2012, 51, 7910–7922. [Google Scholar] [CrossRef]
- Ren, J.S.; Diprose, J.; Warren, J.; Esnouf, R.M.; Bird, L.E.; Ikemizu, S.; Slater, M.; Milton, J.; Balzarini, J.; Stuart, D.L.; et al. Phenylethylthiazolylthiourea (PETT) Non-nucleoside Inhibitors of HIV-1 and HIV-2 Reverse Transcriptases: STRUCTURAL AND BIOCHEMICAL ANALYSES. J. Biol. Chem. 2000, 275, 5633–5639. [Google Scholar] [CrossRef] [PubMed]
- Sayala, C.; Willemse, W.P.; Cras, J.A. Nickel(II) complexes of deprotonated thiourea; Crystal structure of Ni[(C6H5)NC(S)N{(C2H4)2O}]2. Recl. J. Neth. Chem. Soc. 1980, 99, 356–369. [Google Scholar]
- Yuen, H.Y.; Henderson, W.; Oliver, A.G. Nickel(II) complexes of di- and tri-substituted thiourea mono- and di-anions. Inorg. Chim. Acta 2011, 368, 1–5. [Google Scholar] [CrossRef]
- Faihan, A.S.; Hatshan, M.R.; Kadhim, M.M.; Alqahtani, A.S.; Nasr, F.A.; Saleh, A.M.; Al-Jibori, S.A.; Al-Janabi, A.S. Synthesis, spectroscopic, crystallographic, quantum and molecular docking investigations of cis-4,5-diphenylimidazolidine-2-thione. J. Mol. Struct. 2022, 1252, 132198. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81. [Google Scholar] [CrossRef]
- Rahman, F.U.; Bibi, M.; Altaf, A.A.; Tahir, M.N.; Farhat-Ullah, D.; Ur-Rehman, Z.; Khan, E.J. Zn, Cd and Hg complexes with unsymmetric thiourea derivatives; syntheses, free radical scavenging and enzyme inhibition essay. Mol. Struct. 2020, 1211, 128096. [Google Scholar] [CrossRef]
- Patterson, A. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Bai, W.J.; Ji, J.X.; Huang, Q.; Wei, W. Synthesis and evaluation of new thiourea derivatives as antitumor and antiangiogenic agents. Tetrahedron Lett. 2020, 61, 152366. [Google Scholar] [CrossRef]
- Tan, C.H.; Sim, D.S.Y.; Heng, M.P.; Lim, S.H.; Low, Y.Y.; Kam, T.S.; Sim, K.S. Evaluation of DNA Binding and Topoisomerase I Inhibitory Activities of 16’-Decarbomethoxydihydrovoacamine from Tabernaemontana corymbosa. Chem. Sel. 2020, 5, 14839–14843. [Google Scholar] [CrossRef]
- Hagras, M.; El Deeb, M.A.; Elzahabi, H.S.; Elkaeed, E.B.; Mehany, A.B.; Eissa, I.H.J.J.O.E.I. Discovery of new quinolines as potent colchicine binding site inhibitors: Design, synthesis, docking studies, and anti-proliferative evaluation. J. Enzym. Inhib. Med. Chem. 2021, 36, 640–658. [Google Scholar] [CrossRef]
- Eissa, H.; Dahab, M.A.; Ibrahim, M.K.; Alsaif, N.A.; Alanazi, A.; Eissa, S.I.; Mehany, A.B.; Beauchemin, A.M.J.B.C. Design and discovery of new antiproliferative 1, 2, 4-triazin-3 (2H)-ones as tubulin polymerization inhibitors targeting colchicine binding site. Bioorg. Chem. 2021, 112, 104965. [Google Scholar] [CrossRef]
- Alanazi, M.M.; Eissa, I.H.; Alsaif, N.A.; Obaidullah, A.J.; Alanazi, W.A.; Alasmari, A.F.; Albassam, H.; Elkady, H.; Elwan, A.J.J.O.E.I. Design, synthesis, docking, ADMET studies, and anticancer evaluation of new 3-methylquinoxaline derivatives as VEGFR-2 inhibitors and apoptosis inducers. J. Enzym. Inhib. Med. Chem. 2021, 36, 1760–1782. [Google Scholar] [CrossRef]
- Yousef, R.G.; Sakr, H.M.; Eissa, I.H.; Mehany, A.B.; Metwaly, A.M.; Elhendawy, M.A.; Radwan, M.M.; ElSohly, M.A.; Abulkhair, H.S.; El-Adl, K.J.N.J.O.C. New quinoxaline-2 (1 H)-ones as potential VEGFR-2 inhibitors: Design, synthesis, molecular docking, ADMET profile and anti-proliferative evaluations. New J. Chem. 2021, 45, 16949–16964. [Google Scholar] [CrossRef]
- Xia, X.; Maliski, E.G.; Gallant, P.; Rogers, D.J. Classification of kinase inhibitors using a Bayesian model. J. Med. Chem. 2004, 47, 4463–4470. [Google Scholar] [CrossRef]
- BIOVIA. QSAR, ADMET and Predictive Toxicology. Available online: https://www.3dsbiovia.com/products/collaborative-science/biovia-discovery-studio/qsar-admet-and-predictive-toxicology.html (accessed on 23 July 2022).
- Venkatapathy, R.; Wang, N.C.Y.; Martin, T.M.; Harten, P.F.; Young, D. Structure–Activity Relationships for Carcinogenic Potential. Gen. Appl. Syst. Toxicol. 2009, 234, 209–221. [Google Scholar] [CrossRef]
- Goodrnan, G.; Wilson, R. Comparison of the dependence of the TD50 on maximum tolerated dose for mutagens and nonmutagens. Risk Anal. 1992, 12, 525–533. [Google Scholar] [CrossRef]
- National Research Council (US). Correlation between carcinogenic potency and the maximum tolerated dose: Implications for risk assessment. In Issues in Risk Assessment; National Academies Press (US): Washington, DC, USA, 1993. [Google Scholar]
- Diaza, R.G.; Manganelli, S.; Esposito, A.; Roncaglioni, A.; Manganaro, A.; Benfenati, E. Comparison of in silico tools for evaluating rat oral acute toxicity. SAR QSAR Environ. Res. 2015, 26, 1–27. [Google Scholar] [CrossRef]
- Pizzo, F.; Benfenati, E. Silico Methods for Predicting Drug Toxicity; Springer: Berlin/Heidelberg, Germany, 2016; Volume 163. [Google Scholar]
- Venkatapathy, R.; Moudgal, C.J.; Bruce, R.M. Assessment of the oral rat chronic lowest observed adverse effect level model in TOPKAT, a QSAR software package for toxicity prediction. J. Chem. Inf. Comput. Sci. 2004, 44, 1623. [Google Scholar] [CrossRef]
- Alesawy, M.S.; Elkaeed, E.B.; Alsfouk, A.A.; Metwaly, A.M.; Eissa, I.H. In silico screening of semi-synthesized compounds as potential inhibitors for SARS-CoV-2 papain-like protease: Pharmacophoric features, molecular docking, ADMET, toxicity and DFT studies. Molecules 2021, 26, 6593. [Google Scholar] [CrossRef]
- Eissa, I.H.; Alesawy, M.S.; Saleh, A.M.; Elkaeed, E.B.; Alsfouk, B.A.; El-Attar, A.-A.M.; Metwaly, A.M. Ligand and structure-based in silico determination of the most promising SARS-CoV-2 nsp16-nsp10 2′-o-Methyltransferase complex inhibitors among 3009 FDA approved drugs. Molecules 2022, 27, 2287. [Google Scholar] [CrossRef]
- Okuniewski, A.; Chojnacki, J.; Becker, B. N, N′-Diphenylthiourea acetone monosolvate. Acta Cryst. E 2011, 67, o55. [Google Scholar] [CrossRef]
- Creighton, J.R.; Gardiner, D.S.; Grovin, A.C.; Gutteridge, C.; Jackson, A.R.W.; Raper, E.S.; Sherwood, P.M.A. Copper (I) halide complexes of imidazole thiones: Crystal structure of dimeric monochloro bis (1-methylimidazoline-2-thione) copper (I). Inorg. Chim. Acta 1985, 103, 195. [Google Scholar] [CrossRef]
- Bauer, A.W. Single-disk antibiotic-sensitivity testing of staphylococci: An analysis of technique and results. Arch. Intern. Med. 1959, 104, 208. [Google Scholar] [CrossRef]
- Abbass, E.M.; Khalil, A.K.; Mohamed, M.M.; Eissa, I.H.; El-Naggar, A.M.J.B.C. Design, efficient synthesis, docking studies, and anticancer evaluation of new quinoxalines as potential intercalative Topo II inhibitors and apoptosis inducers. Bioorg. Chem. 2020, 104, 104255. [Google Scholar] [CrossRef]
- El-Adl, K.; Ibrahim, M.-K.; Alesawy, M.S.; Eissa, I.H. [1,2,4]Triazolo [4,3-c]quinazoline and bis([1,2,4]triazolo)[4,3-a:4′,3′-c]quinazoline derived DNA intercalators: Design, synthesis, in silico ADMET profile, molecular docking and anti-proliferative evaluation studies. Bioorg. Med. Chem. 2021, 30, 115958. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Johnson, B.G.; Frisch, M.J. Analytic second derivatives of the gradient-corrected density functional energy. Effect of quadrature weight derivatives. Chem. Phys. Lett. 1993, 216, 133. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular Orbital Methods. 9. Extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1975, 54, 724. [Google Scholar] [CrossRef]
- Schafer, A.; Huber, C.; Ahlrichs, R.J. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. Chem. Phys. 1994, 100, 5829. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639. [Google Scholar] [CrossRef]
- Ulic, S.E.; Vedova, C.O.D.; Hermann, A.; Mack, H.-G.; Oberhammer, H.J. Preparation and Properties of Trifluorothioacetic Acid-S-(trifluoromethyl) ester, CF3C(O)SCF3. Phys. Chem. A 2008, 112, 6211. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F.J. Natural bond orbital analysis of near-Hartree–Fock water dimer. Chem. Phys. 1983, 78, 4066. [Google Scholar] [CrossRef]
- Available online: http://www.chemcraftprog.com (accessed on 23 July 2022).
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]








| Comps. | Color | Yield(%) | Mp.°C | Cond. | Elemental Analysis % Calc. (Found) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| HPMCT | White | 90 | 145–147 | -- | 59.43 (59.29) | 6.35 (6.33) | 12.60 (12.78) |
| 1 | Greenish yellow | 73 | 130–132 | 10 | 45.63 (45.66) | 4.87 (4.90) | 9.68 (9.71) |
| 2 | Dark red | 95 | 148–150 | 17 | 42.49 (42.52) | 4.54 (4.57) | 9.01 (9.04) |
| 3 | Dark yellow | 90 | 178–180 | 19 | 37.18 (37.21) | 3.97 (4.07) | 7.88 (7.91) |
| 4 | White | 74 | 188–190 | 2 | 36.90 (36.93) | 3.94 (3.97) | 7.82 (7.85) |
| 5 | Light olive | 79 | 175–178 | 3 | 52.71 (52.74) | 5.23 (5.26) | 11.18 (11.21) |
| 6 | Dark yellow | 77 | 158–161 | 4 | 52.21 (52.24) | 5.18 (5.21) | 11.07 (11.10) |
| 7 | Dark yellow | 76 | 120–123 | 8 | 48.13 (48.16) | 4.77 (4.80) | 10.20 (10.23) |
| 8 | Dark yellow | 91 | 142–144 | 8 | 41.44 (41.47) | 4.11 (4.14) | 8.79 (8.82) |
| 9 | White | 67 | 190–193 | 6 | 52.02 (52.05) | 5.16 (5.19) | 11.03 (11.06) |
| 10 | White | 70 | 192–194 | 12 | 47.61 (47.64) | 4.72 (4.75) | 10.09 (10.12) |
| 11 | Gray | 82 | 118–121 | 11 | 41.08 (41.11) | 4.07 (4.10) | 8.71 (8.74) |
| Comps. | νNH | ν(C-H) | δNH | νC=N νC=N | νC=C | νC-N | νC-O | νC=S | νM-S νM-N | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ar. | Aliph. | |||||||||
| HPMCT | 3176s | 3030s | 2918m 2868m | 1595s | -- | 1531s | 1211s | 1028s | 707s | -- |
| 1 | 3336b | 3049w | 2968w 2860w | 1626s | -- | 1489s | 1276s | 1031m | 694m | 495w |
| 2 | 3174b | 3055w | 2960w 2858w | 1597s | -- | 1558s | 1269s | 1028s | 696m | 503w |
| 3 | 3207s | 3059w | 2966s 2854s | 1645s | -- | 1583s | 1303s | 1028s | 694s | 493s |
| 4 | 3184b | 3057w | 2966w 2858w | 1597s | -- | 1558s | 1269s | 1026s | 684s | 482w |
| 5 | -- | 3024w | 2918w 2841w | -- | 1550s | 1481m | 1276m | 1026s | 692m | 418w 518w |
| 6 | -- | 3061w | 2960w 2848w | -- | 1529s | 1485m | 1267m | 1022s | 694m | 470w 515w |
| 7 | -- | 3053w | 2968w 2856w | -- | 1541s | 1487w | 1267m | 1028m | 696m | 420w 516w |
| 8 | -- | 3051w | 2964w 2852w | -- | 1541s | 1485m | 1269m | 1028s | 694m | 461w 516w |
| 9 | -- | 3028m | 2976m 2854m | -- | 1531s | 1491m | 1265m | 1028s | 665m | 472w 507w |
| 10 | -- | 3057w | 2962w 2854w | -- | 1519s | 1435m | 1269m | 1026s | 696m | 466w 516w |
| 11 | -- | 3051w | 2962m 2848w | -- | 1581s | 1485m | 1274m | 1024s | 686m | 489w 586w |
| Comps. | δNH | δH Phenyl | δH OCH2 | δH NCH2 |
|---|---|---|---|---|
| HPMCT | 9.29 (s, 1H) | 7.04–7.28 (m, 5H) | 3.84 (t, J8.00 Hz, 4H) | 3.61 (t, J8.00 Hz, 4H) |
| 2 | 10.28 (s, 2H) | 7.05–7.81 (m, 10H) | 3.91 (t, J7.80 Hz, 8H) | 3.75 (t, J7.80 Hz, 8H) |
| 3 | 9.59 (s, 2H) | 7.33–7.81 (m, 10H) | 3.92 (t, J7.90 Hz, 8H) | 3.66 (t, J7.90 Hz, 8H) |
| 4 | 10.37 (s, 2H) | 7.21–7.36 (m, 10H) | 3.88 (t, J8.00 Hz, 8H) | 3.71 (t, J8.00 Hz, 8H) |
| 7 | - | 7.62 (d, J8.00 Hz, 4H), 7.29–7.34 (m, 4H), 7.04 (t, J7.60 Hz, 2H) | 3.92 (t, J8.00 Hz, 8H) | 3.66 (t, J8.00 Hz, 8H) |
| 8 | - | 7.48–7.76 (m, 10H) | 3.56 (t, J7.60 Hz, 8H) | 3.38 (t, J7.60 Hz, 8H) |
| 9 | - | 7.20–7.67 (m, 10H) | 3.82 (t, J8.00 Hz, 8H) | 3.70 (t, J8.00 Hz, 8H) |
| 10 | - | 7.21–7.36 (m, 10H) | 3.88 (t, J7.80 Hz, 8H) | 3.71 (t, J7.80 Hz, 8H) |
| 11 | - | 6.87–7.24 (m, 10H) | 3.74 (t, J8.00 Hz, 8H) | 3.61 (t, J8.00 Hz, 8H) |
| Comps. | C=S | δC Phenyl | δC OCH2 | δC NCH2 |
|---|---|---|---|---|
| HPMCT | 182.35 | 141.45, 128.56, 125.97, 124.92 | 66.30 | 48.96 |
| 2 | 176.78 | 139.41, 126.23, 124.34, 122.10 | 64.44 | 51.45 |
| 3 | 177.31 | 137.26, 126.11, 124.09, 122.45 | 63.71 | 50.61 |
| 4 | 174.80 | 139.59, 129.59, 127.14, 126.77 | 65.86 | 50.52 |
| 7 | 168.51 | 136.02, 127.81, 124.75, 121.93 | 66.52 | 48.22 |
| 8 | 167.37 | 137.34, 127.63, 124.93, 121.81 | 67.01 | 47.39 |
| 11 | 168.89 | 136.02, 128.94, 125.01, 122.13 | 66.34 | 46.19 |
| Compounds | IC50 (μM) |
|---|---|
| HPMCT | 80.30 ± 1.905 |
| [PdCl2(HPMCT)2] | 28.93 ± 1.105 |
| [PtCl2(HPMCT)2] | 12.72 ± 0.576 |
| cis-platin | 3.19 ± 0.097 |
| Ligand | RMSD Value (Å) | Docking Score (kcal/mol) | Interaction | |
|---|---|---|---|---|
| H.B | π | |||
| HPMCT | 1.35 | −7.01 | 2 | 5 |
| 1 | 1.97 | −6.11 | - | - |
| 2 | 1.69 | −6.15 | 2 | 5 |
| 3 | 2.10 | −6.32 | 1 | 5 |
| 4 | 2.40 | −5.86 | 1 | 2 |
| 5 | 1.23 | −5.20 | 1 | 1 |
| 6 | 1.26 | −5.10 | 1 | 1 |
| 7 | 1.13 | −5.13 | 2 | 5 |
| 8 | 1.75 | −5.56 | 1 | - |
| 9 | 1.72 | −5.36 | - | 3 |
| 10 | 2.03 | −4.02 | 1 | 4 |
| 11 | 1.32 | −5.20 | 2 | 6 |
| Comp. | A-Log p | Mwt. | HBA | HBD | Rotatable Bonds | Rings | Aromatic Rings | MFPSA | Minimum Distance | Is Similar |
|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 4.407 | 549.017 | 6 | 0 | 4 | 6 | 2 | 0.185 | 1.81736 | TRUE |
| 1 | 4.691 | 579.065 | 6 | 2 | 8 | 4 | 2 | 0.147 | 1.82746 | TRUE |
| 5 | 4.407 | 501.291 | 6 | 0 | 4 | 6 | 2 | 0.197 | 1.82961 | TRUE |
| 8 | 4.407 | 637.675 | 6 | 0 | 4 | 6 | 2 | 0.184 | 1.89913 | TRUE |
| 11 | 2.735 | 222.307 | 2 | 1 | 3 | 2 | 1 | 0.243 | 1.90748 | TRUE |
| Reference | 1.915 | 313.389 | 1 | 1 | 3 | 3 | 2 | 0.074 | - | Reference |
| 6 | 6.004 | 508.159 | 8 | 0 | 4 | 6 | 2 | 0.186 | 2.27628 | FALSE |
| 10 | 4.567 | 555.008 | 8 | 0 | 4 | 6 | 2 | 0.228 | 2.18775 | FALSE |
| 9 | 4.567 | 508.006 | 8 | 0 | 4 | 6 | 2 | 0.232 | 2.17541 | FALSE |
| 2 | 4.807 | 625.971 | 4 | 4 | 8 | 4 | 2 | 0.168 | 2.01066 | FALSE |
| 4 | 4.691 | 716.109 | 6 | 2 | 8 | 4 | 2 | 0.144 | 1.9746 | FALSE |
| 3 | 4.691 | 710.597 | 6 | 2 | 8 | 4 | 2 | 0.146 | 1.97191 | FALSE |
| Comp. | BBB Level a | Solubility Level b | Absorption Level c | Hepatotoxicity | CYP2D6 Prediction d | PPB Prediction e |
|---|---|---|---|---|---|---|
| 10 | 1 | 1 | 0 | False | false | true |
| 6 | 0 | 1 | 0 | False | false | true |
| 5 | 0 | 2 | 0 | False | false | true |
| 7 | 0 | 2 | 0 | False | false | true |
| 8 | 0 | 2 | 0 | False | false | true |
| 9 | 1 | 1 | 0 | False | false | true |
| 1 | 1 | 2 | 0 | False | false | true |
| 4 | 1 | 2 | 0 | False | false | true |
| 2 | 1 | 3 | 0 | False | true | false |
| 3 | 1 | 2 | 0 | False | false | true |
| 11 | 2 | 3 | 0 | False | false | false |
| Comp. | FDA Rodent Carcinogenicity (Male, Mouse) | Carcinogenic Potency TD50 (Rat) a | Rat Maximum Tolerated Dose (Feed) b | Developmental Toxicity Potential | Rat Oral LD50 b | Rat Chronic LOAEL b | Ocular Irritancy (Rat) | Skin Irritancy (Rat) |
|---|---|---|---|---|---|---|---|---|
| 10 | Non-Carcinogen | 0.599 | 00.00750 | Non-Toxic | 00.07350 | 00.01560 | None | Mild |
| 6 | Non-Carcinogen | 00.6550 | 00.01850 | Non-Toxic | 00.06980 | 00.00990 | Moderate | Mild |
| 5 | Non-Carcinogen | 04.1340 | 00.01270 | Non-Toxic | 00.09350 | 00.02980 | None | Mild |
| 7 | Non-Carcinogen | 03.0130 | 00.00680 | Non-Toxic | 00.10710 | 00.01920 | Moderate | Mild |
| 8 | Non-Carcinogen | 03.1360 | 00.00270 | Non-Toxic | 0.0998 | 0.0201 | Moderate | Mild |
| 9 | Non-Carcinogen | 0.581 | 0.0120 | Non-Toxic | 0.0756 | 0.0151 | None | Mild |
| 1 | Single-Carcinogen | 5.481 | 0.0225 | Non-Toxic | 0.5264 | 0.0075 | Mild | None |
| 4 | Single-Carcinogen | 5.721 | 0.0054 | Non-Toxic | 0.4634 | 0.0079 | Mild | None |
| 2 | Non-Carcinogen | 11.41 | 0.0306 | Non-Toxic | 01.17706 | 0.0050 | Severe | Mild |
| 3 | Single-Carcinogen | 5.716 | 0.0057 | Non-Toxic | 0.4662 | 0.0079 | Mild | None |
| 11 | Single-Carcinogen | 231.5 | 0.0666 | Non-Toxic | 0.3213 | 0.0249 | Mild | None |
| Parameters | [Ni(PMCT)2] | [Pd(HPMCT)2] | [Pd(PMCT)2] | [Pt(HPMCT)2] | [Pt(PMCT)2] |
|---|---|---|---|---|---|
| Etotal (Kcal/mol) | −1,374,759.30 | −1,926,136.774 | −1,347,751.571 | −1,920,786.055 | −1,342,397.088 |
| EHOMO,ev | −5.365 | −5.829 | −5.413 | −5.805 | −5.128 |
| ELUMO,ev | −1.497 | −2.419 | −1.815 | −1.807 | −1.211 |
| Eg,ev (ELUMO-EHOMO) | 3.868 | 3.41 | 3.598 | 3.998 | 3.917 |
| Hardness (½(ELUMO-EHOMO)) | 1.934 | 1.705 | 1.799 | 1.999 | 1.9585 |
| Dipole Moment (debye) | 0.000735 | 2.291495 | 0.003571 | 0.004176 | 0.001631 |
| LE (-EHOMO ev) | 5.365 | 5.829 | 5.413 | 5.805 | 5.128 |
| EA (-ELUMO ev) | 1.497 | 2.419 | 1.815 | 1.807 | 1.211 |
| Bond Length | [PdCl2(HPMCT)2] | [PtCl2(HPMCT)2] | Bond Length | [Ni(PMCT)2] | [Pd(PMCT)2] | [Pt(PMCT)2] |
| S23-M | 2.402 | 2.377 | S23-M | 2.277 | 2.399 | 2.405 |
| ----- | ----- | ----- | N7-M | 1.913 | 2.071 | 2.079 |
| ------ | ----- | ----- | N31-M | 1.913 | 2.399 | 2079 |
| S31-M | 2.379 | 2.377 | S29-M | 2.277 | 2.071 | 2.405 |
| C34-N37 | 1.365 | 1.360 | C8-N7 | 1.334 | 1.333 | 1.335 |
| N38-C34 | 1.354 | 1.366 | N9-C8 | 1.365 | 1.366 | 1.365 |
| C34-S23 | 1.738 | 1.727 | C8-S23 | 1.766 | 1.769 | 1.769 |
| C22-S31 | 1.723 | 1.727 | C30-S29 | 1.766 | 1.769 | 1.769 |
| Bond Angles | [PdCl2(HPMCT)2] | [PtCl2(HPMCT)2] | Bond Length | [Ni(PMCT)2] | [Pd(PMCT)2] | [Pt(PMCT)2] |
| S23-M-S31 | 178.966 | 179.994 | S23-M-S29 | 179.994 | 179.986 | 179.995 |
| Cl60-M-Cl61 | 177.999 | 179.996 | N7-M-N31 | 179.995 | 179.993 | 179.991 |
| S23-M-Cl60 | 85.741 | 87.216 | S23-M-N7 | 73.928 | 69.254 | 68.497 |
| S23-M-Cl61 | 93.792 | 92.781 | N31-M-S29 | 73.930 | 69.248 | 68.499 |
| S31-M-Cl60 | 93.405 | 92.790 | S23-M-N31 | 106.076 | 110.739 | 111.50 |
| S31-M-Cl61 | 87.043 | 87.213 | N7-M-S29 | 106.066 | 110.759 | 111.505 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Halbosy, A.T.F.; Hamada, A.A.; Faihan, A.S.; Saleh, A.M.; Yousef, T.A.; Abou-Krisha, M.M.; Alhalafi, M.H.; Al-Janabi, A.S.M. Thiourea Derivative Metal Complexes: Spectroscopic, Anti-Microbial Evaluation, ADMET, Toxicity, and Molecular Docking Studies. Inorganics 2023, 11, 390. https://doi.org/10.3390/inorganics11100390
Al-Halbosy ATF, Hamada AA, Faihan AS, Saleh AM, Yousef TA, Abou-Krisha MM, Alhalafi MH, Al-Janabi ASM. Thiourea Derivative Metal Complexes: Spectroscopic, Anti-Microbial Evaluation, ADMET, Toxicity, and Molecular Docking Studies. Inorganics. 2023; 11(10):390. https://doi.org/10.3390/inorganics11100390
Chicago/Turabian StyleAl-Halbosy, Ahmed T. F., Adnan A. Hamada, Ahmed S. Faihan, Abdulrahman M. Saleh, Tarek A. Yousef, Mortaga M. Abou-Krisha, Mona H. Alhalafi, and Ahmed S. M. Al-Janabi. 2023. "Thiourea Derivative Metal Complexes: Spectroscopic, Anti-Microbial Evaluation, ADMET, Toxicity, and Molecular Docking Studies" Inorganics 11, no. 10: 390. https://doi.org/10.3390/inorganics11100390









