Electrochemical Impedance Spectroscopy (EIS) Characterization of Water/Sodium Bis(2-Ethylhexyl) Sulfosuccinate-HDEHP/n-Dodecane Reverse Micelles for Electroextraction of Neodymium
Abstract
:1. Introduction
2. Materials and Methods
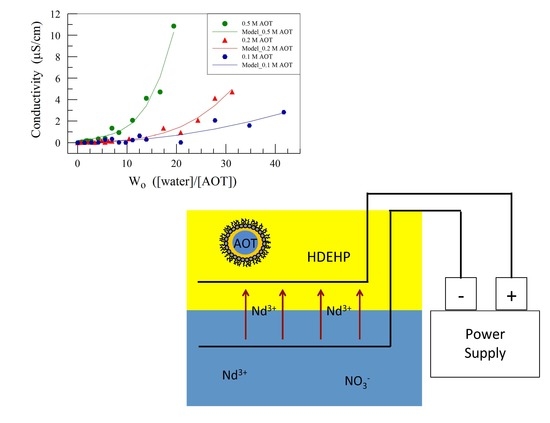
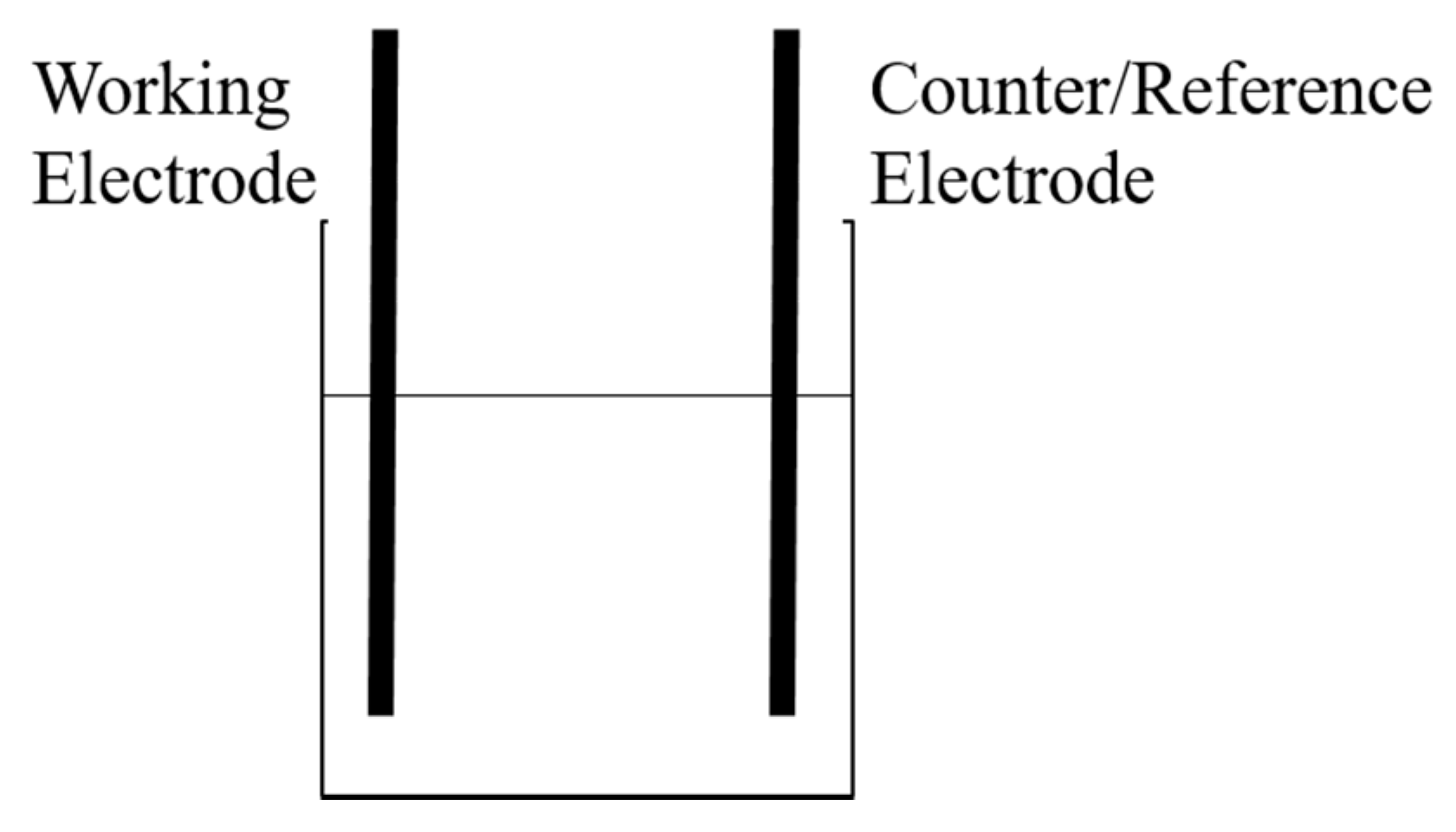
2.1. Electrochemical Impedance Spectroscopy for Electrochemical Extraction
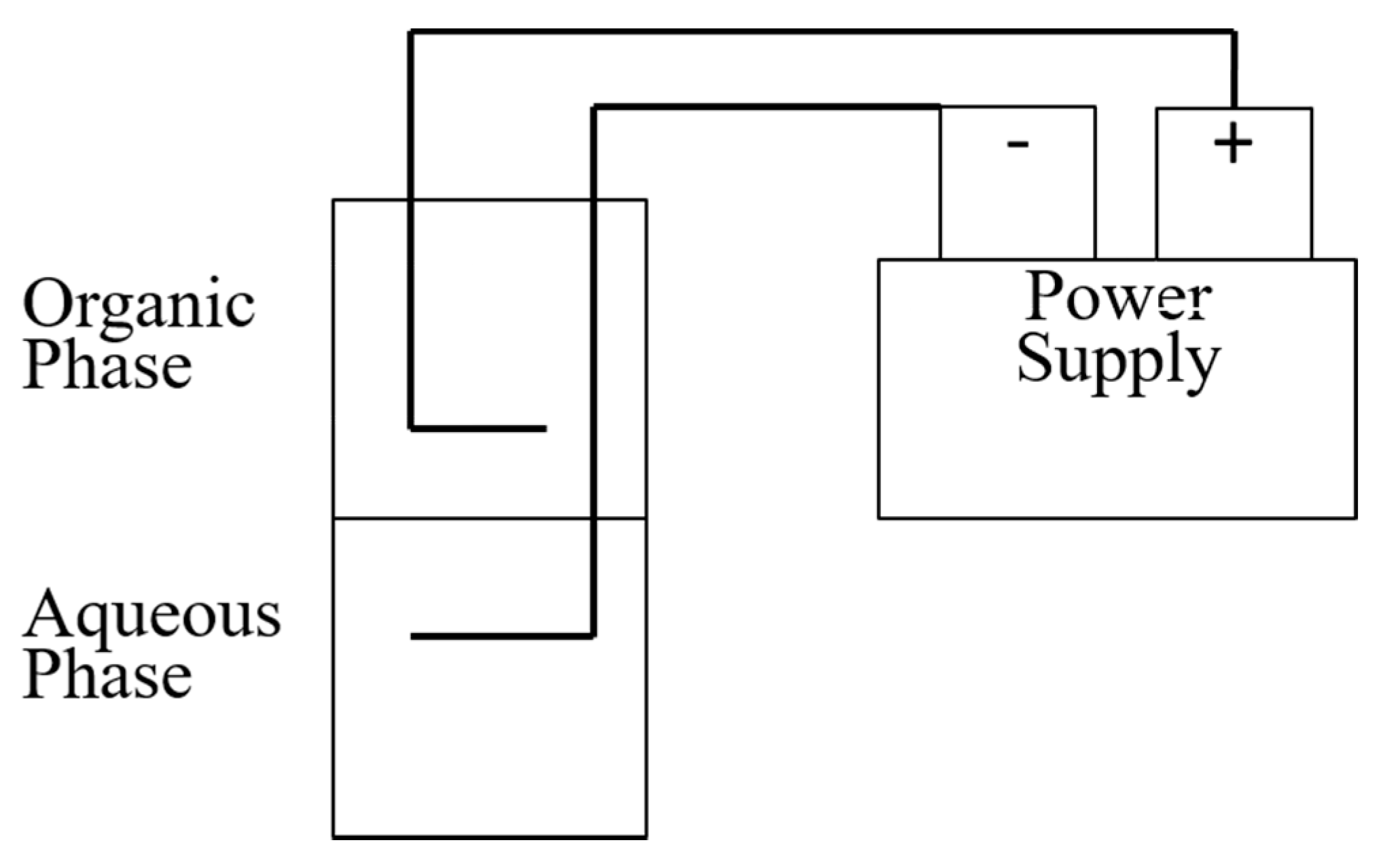
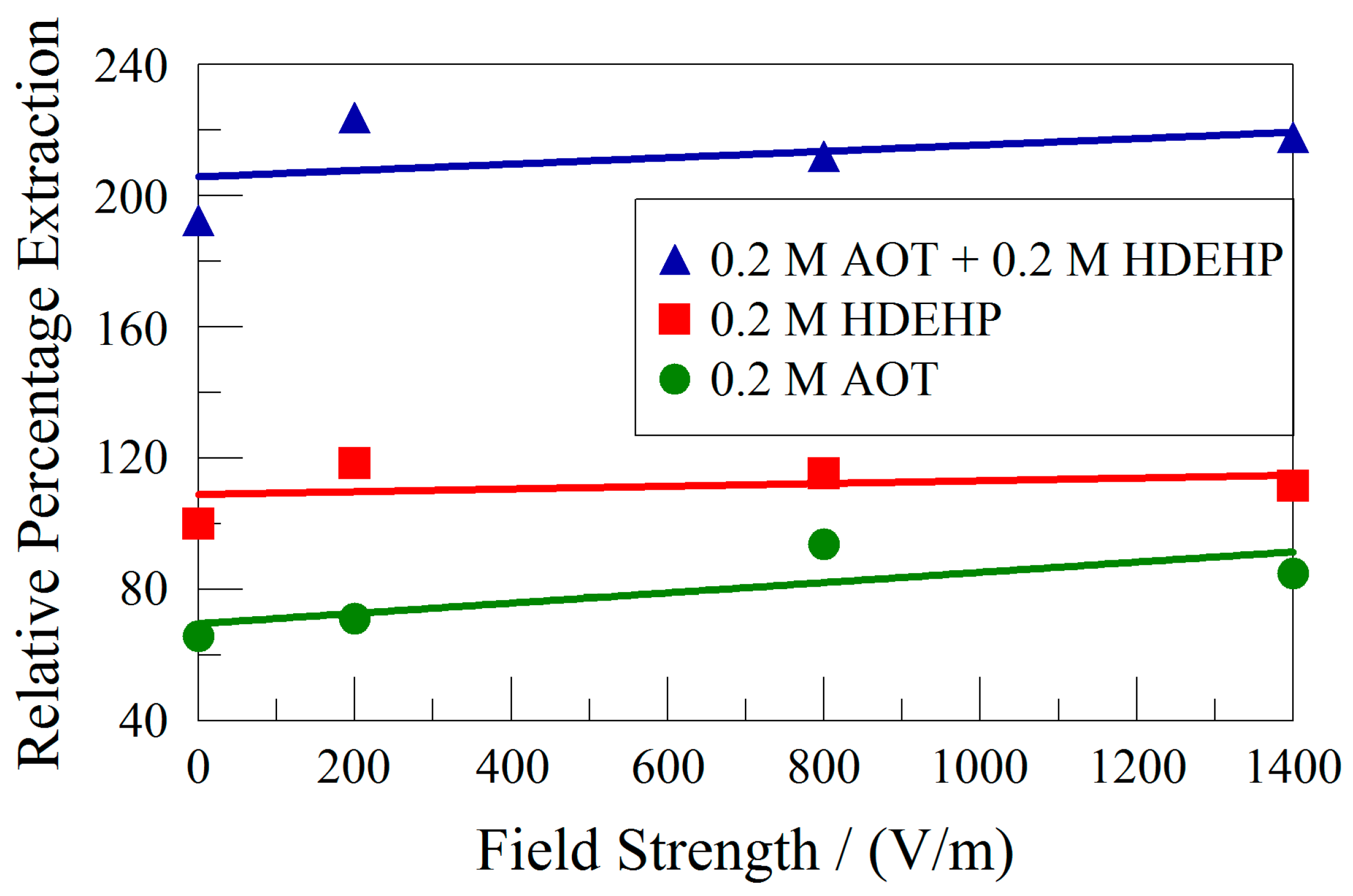
2.2. Effect of Electric Field Strength
3. Results and Discussion
3.1. Electrochemical Impedance Spectroscopy for Electrochemical Extraction
3.1.1. Effect of AOT Concentration on EIS Analysis of Organic Solution Conductivity
3.1.2. Effect of AOT + HDEHP on EIS Analysis of the Conductivity of Organic Solution
3.1.3. Effect of Temperature on EIS Analysis of Organic Solution Conductivity
3.2. Effect of Electric Field Strength
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoogerstraete, T.V.; Onghena, B.; Binnemans, K. Homogeneous liquid-liquid extraction of rare earths with the betaine-betainium bis(trifluoromethylsulfonyl)imide ionic liquid system. Int. J. Mol. Sci. 2013, 14, 21353–21377. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Binnemans, K. Separation of rare earths by split-anion extraction. Hydrometallurgy 2015, 156, 206–214. [Google Scholar] [CrossRef]
- Onghena, B.; Jacobs, J.; Meervelt, L.V.; Binnemans, K. Homogeneous liquid–liquid extraction of neodymium(III) by choline hexafluoroacetylacetonate in the ionic liquid choline bis(trifluoromethylsulfonyl)imide. Dalton Trans. 2014, 43, 11566–11578. [Google Scholar] [CrossRef] [PubMed]
- Riaño, S.; Binnemans, K. Extraction and separation of neodymium and dysprosium from used NdFeB magnets: An application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 2015, 17, 2931–2942. [Google Scholar] [CrossRef]
- Saleh, M.I.; Bari, M.F.; Saad, B. Solvent extraction of lanthanum(III) from acidic nitrate-acetato medium by Cyanex 272 in toluene. Hydrometallurgy 2002, 63, 75–84. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Marin, T.W.; Jensen, M.P. Ionic liquid based separations of trivalent lanthanide and actinide ions. Ind. Eng. Chem. Res. 2014, 53, 3641–3653. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Horwitz, E.P.; McAlister, D.R.; Dietz, M.L. Extraction chromatography versus solvent extraction: How similar are they? Separ. Sci. Technol. 2006, 41, 2163–2182. [Google Scholar] [CrossRef]
- Mocko, V.; Taylor, W.A.; Nortier, F.M.; Engle, J.W.; Barnhart, T.E.; Nickles, R.J.; Pollington, A.D.; Kunde, G.J.; Rabin, M.W.; Birnbaum, E.R. Isolation of 163Ho from dysprosium target material by HPLC for neutrino mass measurements. Radiochim. Acta 2015, 103, 577–585. [Google Scholar] [CrossRef]
- Ali, A.; Abbasi, Y.A.; Khan, M.H.; Saeed, M.M. Liquid-liquid extraction of Nd(III) and Eu(III) using nalidixic acid in dichloromethane. Radiochim. Acta 2010, 98, 513–517. [Google Scholar] [CrossRef]
- Gelis, A.V.; Lumetta, G.J. Actinide lanthanide separation process—ALSEP. Ind. Eng. Chem. Res. 2014, 53, 1624–1631. [Google Scholar] [CrossRef]
- Abreu, R.D.; Morais, C.A. Study on separation of heavy rare earth elements by solvent extraction with organophosphorus acids and amine reagents. Miner. Eng. 2014, 61, 82–87. [Google Scholar] [CrossRef]
- Nilsson, M.; Nash, K.L. Trans-lanthanide extraction studies in the TALSPEAK system: Investigating the effect of acidity and temperature. Solvent Extr. Ion Exch. 2009, 27, 354–377. [Google Scholar] [CrossRef]
- Yin, S.; Li, S.; Zhang, B.; Peng, J.; Zhang, L. Extraction kinetics of neodymium(III) from chloride medium in the presence of two complexing agents by D2EHPA using a constant interfacial area cell with laminar flow. Hydrometallurgy 2016, 161, 160–165. [Google Scholar] [CrossRef]
- Afonin, M.A.; Kopyrin, A.A.; Shvalbe, A.I.; Baulin, A.A.; Petrov, A.S. Chemical and electrochemical oscillatory extraction of F-elements—New procedure development. J. Alloy Compd. 2004, 374, 426–430. [Google Scholar] [CrossRef]
- Ozawa, M.; Ikegami, T. Electrochemical Separation of Rare Metal Fission Products from High-Level Liquid Waste of Spent Nuclear Fuel. Available online: http://www.oecd-nea.org/pt/docs/iem/jeju02/session2/SessionII-14.pdf (accessed on 15 June 2017).
- Kuipa, P.K.; Hughes, M.A. Influence of high voltage electric fields applied across a horizontal liquid-liquid interface on the rate of metal extraction using a rotating diffusion cell. Sep. Sci. Technol. 1999, 34, 2643–2661. [Google Scholar] [CrossRef]
- Luo, G.S.; Pan, S.; Liu, J.G.; Dai, Y.Y. Liquid-liquid phase equilibrium under external electric fields. Sep. Sci. Technol. 2007, 36, 2799–2809. [Google Scholar] [CrossRef]
- Laity, R.W. Diffusion of ions in an electric field. J. Phys. Chem. 1963, 67, 671–676. [Google Scholar] [CrossRef]
- Li, Q.; Li, T.; Wu, J. Electrical conductivity of water/sodium bis(2-ethylhexyl) sulfosuccinate/n-heptane and water/sodium bis(2-ethylhexyl) phosphate/n-heptane systems: The influences of water content, bis(2-ethylhexyl) phosphoric acid, and temperature. J. Colloid. Interf. Sci. 2001, 239, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Novikov, G.F.; Voilov, D.N.; Osipova, M.A.; Chernov, I.A. The impedance of solutions of AOT/water micelles in hexane. Russ. J. Phys. Chem. A 2007, 81, 2030–2034. [Google Scholar] [CrossRef]
- Lin, T.; Kodger, T.E.; Weitz, D.A. Transport of charged colloids in a nonpolar solvent. Soft Matter 2013, 9, 5173–5177. [Google Scholar] [CrossRef]
- Beunis, F.; Strubbe, F.; Karvar, M.; Drobchak, O.; Brans, T.; Neyts, K.; Verschueren, A.R.M. Electric charging of inverse micelles in a nonpolar liquid with surfactant. Colloid Surf. A 2014, 440, 10–19. [Google Scholar] [CrossRef]
- Gao, S.; Shen, X.H.; Chen, Q.D.; Gao, H.C. Solvent extraction of thorium(IV) using W/O microemulsion. Sci. China Chem. 2012, 55, 1712–1718. [Google Scholar] [CrossRef]
- Kubota, F.; Shinohara, K.; Shimojo, K.; Oshima, T.; Goto, M.; Furusaki, S.; Hano, T. Extraction of rare earth metals by calix[4]arene solubilized in AOT reversed micellar solution. Sep. Purif. Technol. 2001, 24, 93–100. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1989. [Google Scholar]
- Abdollahi, Z.; Darestani, M.T.; Ghasemi, S.; Gomes, V.G. Characterizing colloidal behavior of non-ionic emulsifiers in non-polar solvents using electrical impedance spectroscopy. Colloid Polym. Sci. 2014, 292, 2695–2705. [Google Scholar]
- Yu, Z.-J.; Zhou, N.-F.; Neuman, R.D. The role of water in the formation of reversed micelles: An antimicellization agent. Langmuir 1992, 8, 1885–1888. [Google Scholar] [CrossRef]
- Steytler, D.C.; Jenta, T.R.; Robinson, B.H. Structure of reversed micelles formed by metal salts of bis(ethylhexyl) phosphoric acid. Langmuir 1996, 12, 1483–1489. [Google Scholar] [CrossRef]
- Anderson, S.P. Development of Novel and Effective Method for the Extraction of Neodymium Using Sodium Bis(2-ethylhexyl) Sulfosuccinate Reverse Micelles. Ph.D. Thesis, Florida A&M University, Tallahassee, FL, USA, 2017. [Google Scholar]
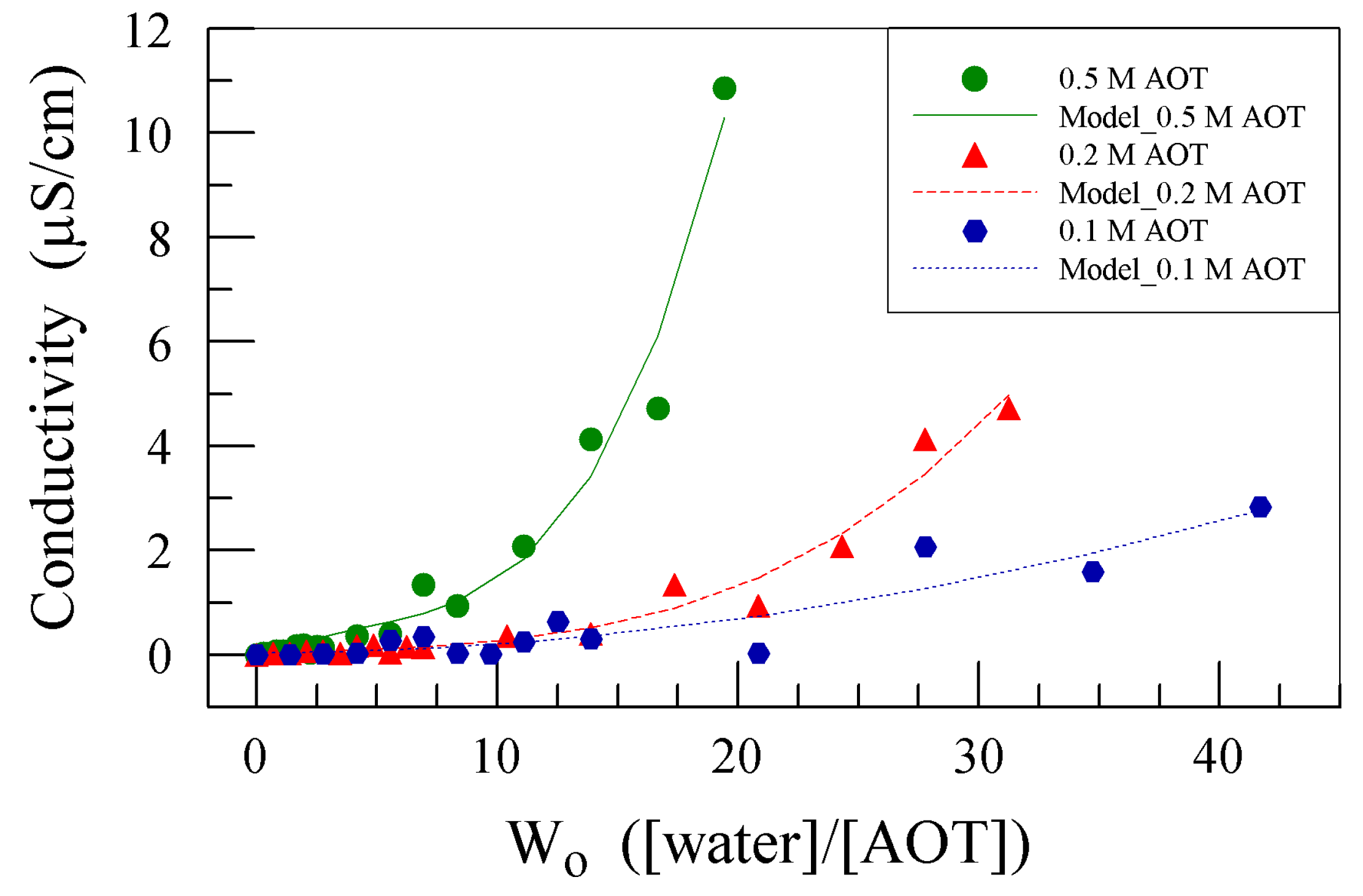
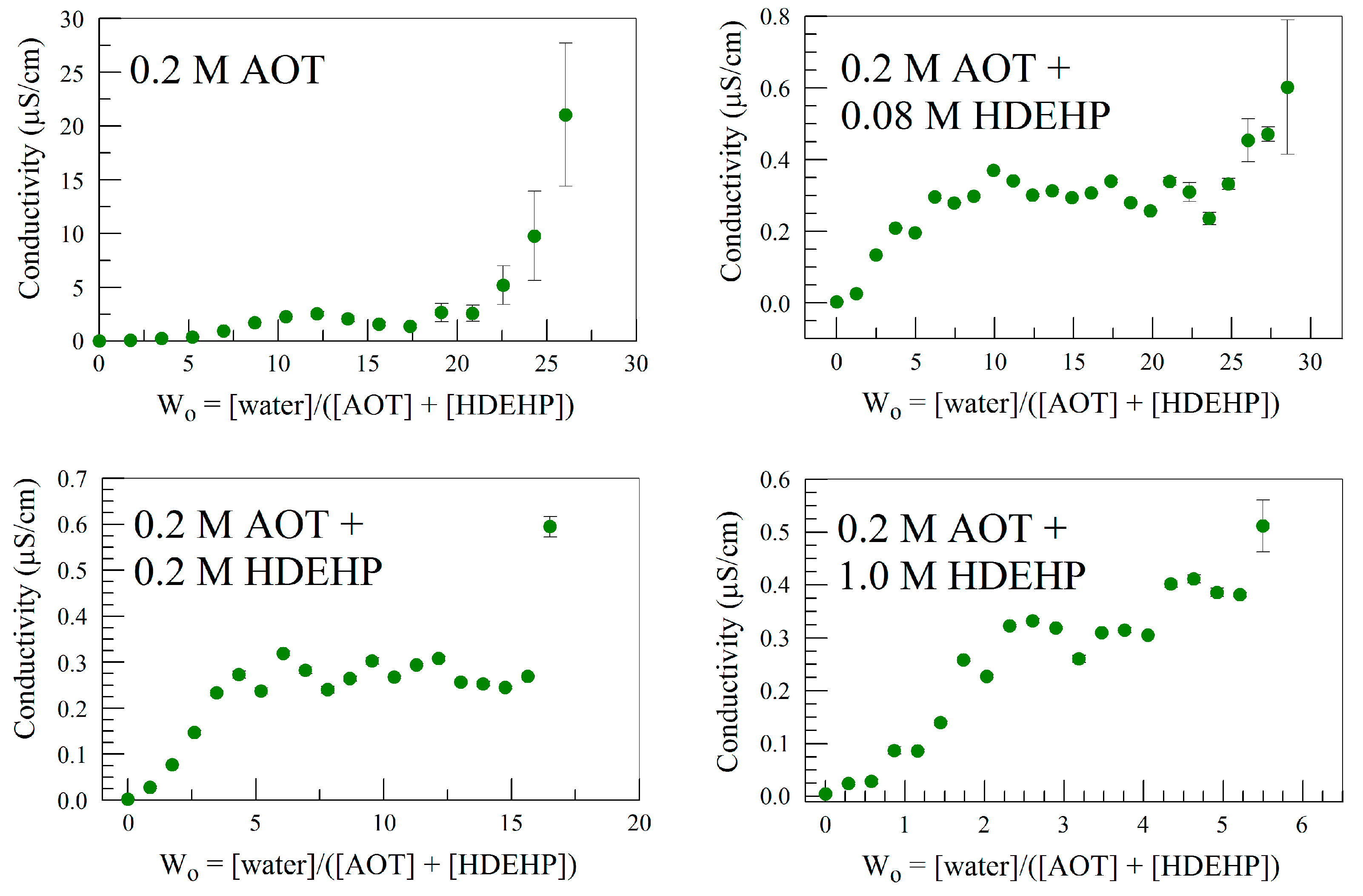
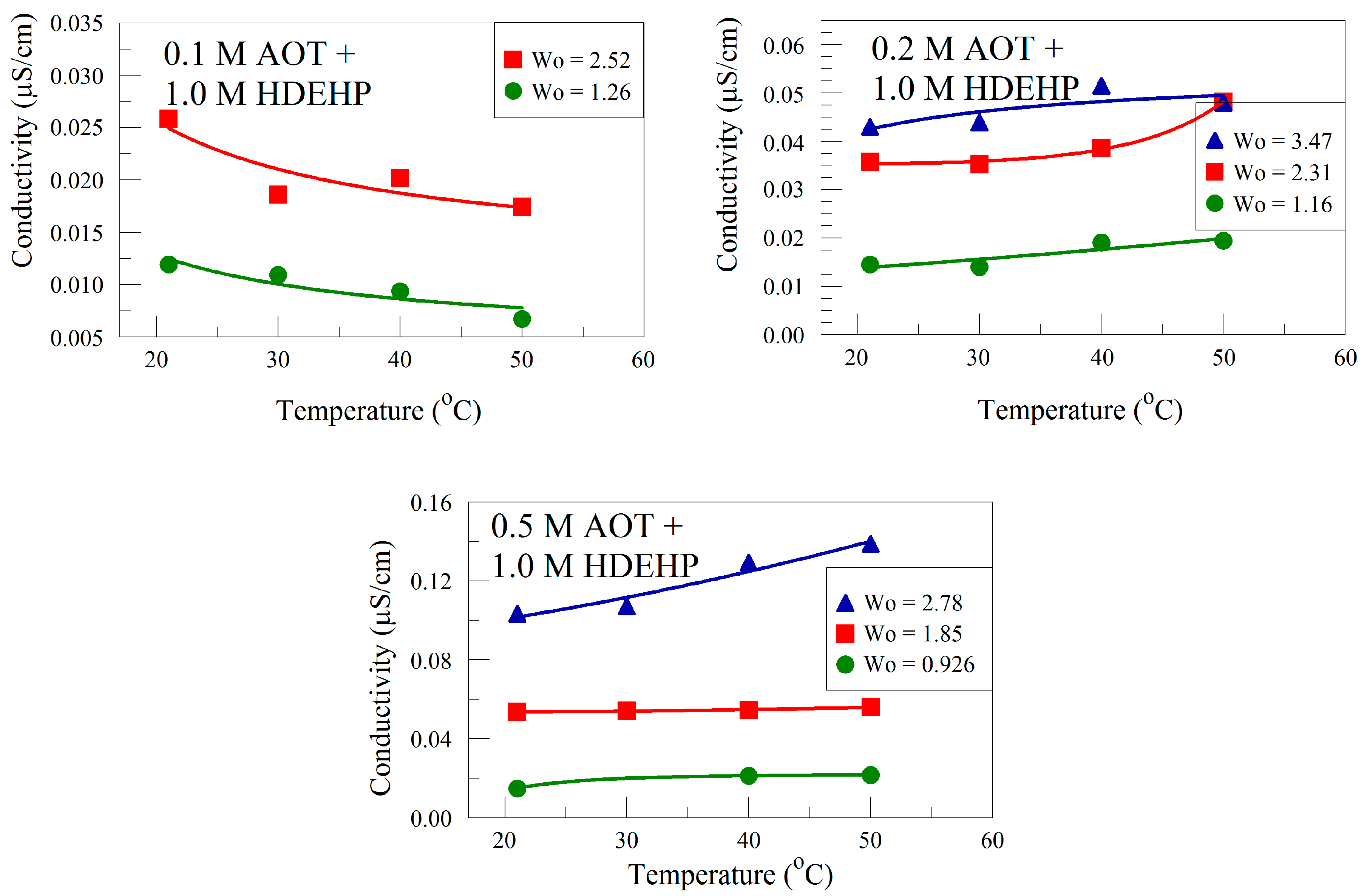
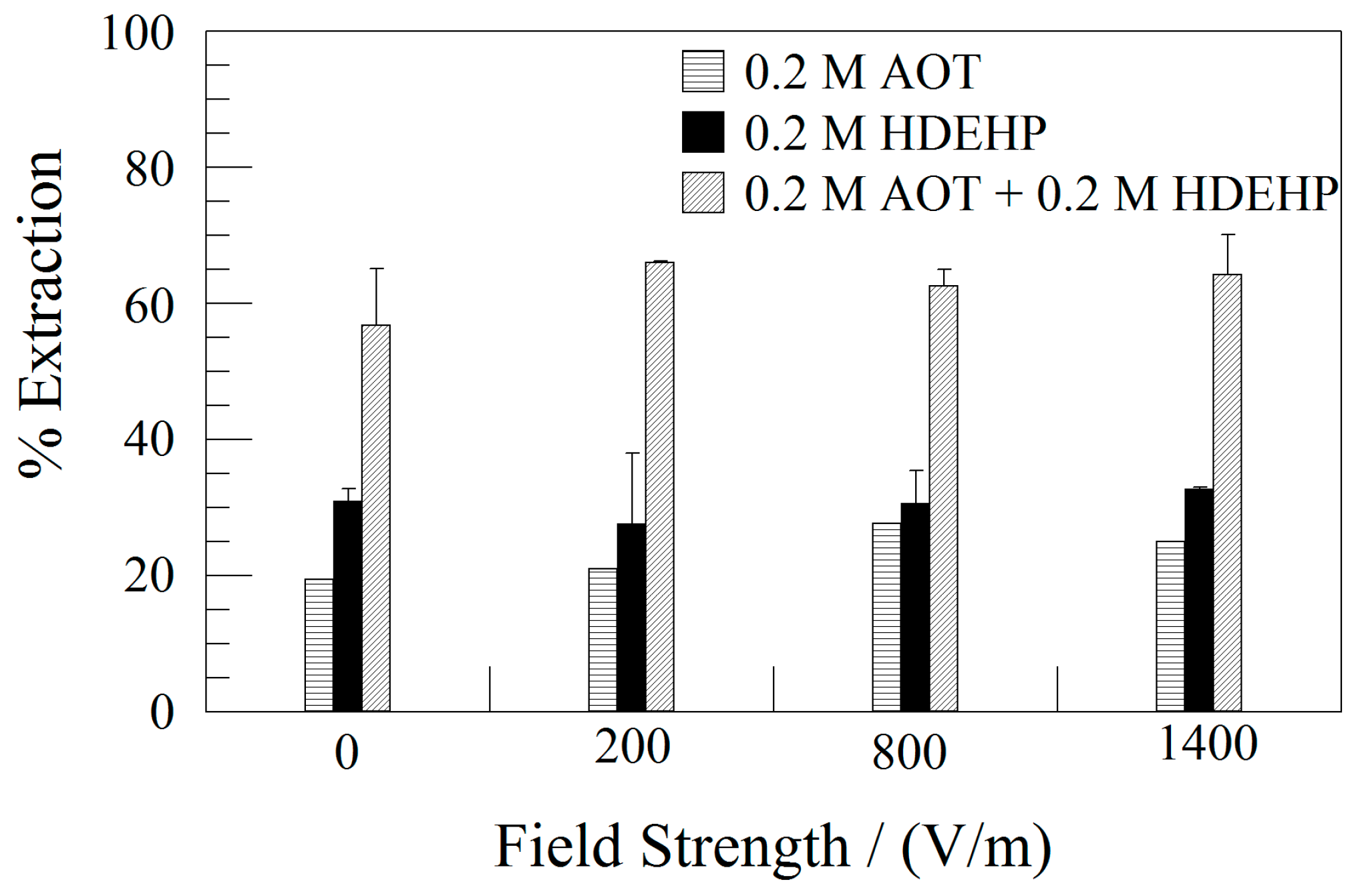
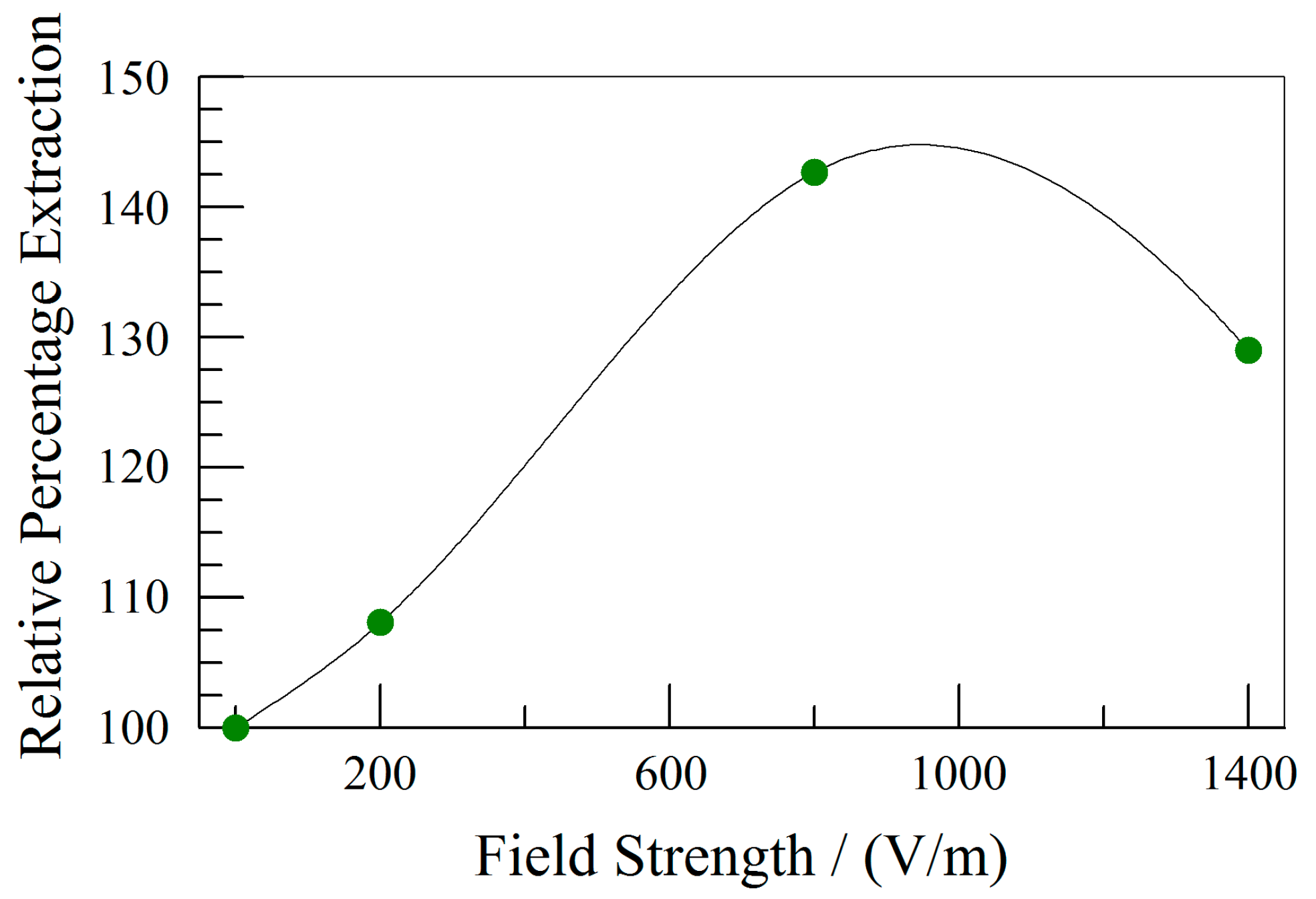
| [AOT] | Equation | Correlation |
|---|---|---|
| 0.5 M | σ = 0.002680 × Wo3 − 0.03911 × Wo2 + 0.2870 × Wo − 0.2087 | 98.67% |
| 0.2 M | σ = 0.0002087 × Wo3 − 0.002359 × Wo2 + 0.02918 × Wo + 0.003058 | 98.41% |
| 0.1 M | σ = −0.000001473 × Wo3 + 0.001628 × Wo2 + 0.0002050 × Wo + 0.03963 | 92.79% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, S.; Nilsson, M.; Kalu, E.E. Electrochemical Impedance Spectroscopy (EIS) Characterization of Water/Sodium Bis(2-Ethylhexyl) Sulfosuccinate-HDEHP/n-Dodecane Reverse Micelles for Electroextraction of Neodymium. ChemEngineering 2017, 1, 3. https://doi.org/10.3390/chemengineering1010003
Anderson S, Nilsson M, Kalu EE. Electrochemical Impedance Spectroscopy (EIS) Characterization of Water/Sodium Bis(2-Ethylhexyl) Sulfosuccinate-HDEHP/n-Dodecane Reverse Micelles for Electroextraction of Neodymium. ChemEngineering. 2017; 1(1):3. https://doi.org/10.3390/chemengineering1010003
Chicago/Turabian StyleAnderson, Shannon, Mikael Nilsson, and Egwu Eric Kalu. 2017. "Electrochemical Impedance Spectroscopy (EIS) Characterization of Water/Sodium Bis(2-Ethylhexyl) Sulfosuccinate-HDEHP/n-Dodecane Reverse Micelles for Electroextraction of Neodymium" ChemEngineering 1, no. 1: 3. https://doi.org/10.3390/chemengineering1010003
APA StyleAnderson, S., Nilsson, M., & Kalu, E. E. (2017). Electrochemical Impedance Spectroscopy (EIS) Characterization of Water/Sodium Bis(2-Ethylhexyl) Sulfosuccinate-HDEHP/n-Dodecane Reverse Micelles for Electroextraction of Neodymium. ChemEngineering, 1(1), 3. https://doi.org/10.3390/chemengineering1010003